Published online Nov 21, 2009. doi: 10.3748/wjg.15.5409
Revised: August 26, 2009
Accepted: September 2, 2009
Published online: November 21, 2009
AIM: To investigate the level of gastric ghrelin in stomach mucosa of dyspeptic patients in relation to Helicobacter pylori (H pylori) infection, bacterial cytotoxicity, topography and gender.
METHODS: The study comprised 40 premenopausal women (19 H pylori positive) and 48 men (17 H pylori positive) with functional dyspepsia. All gastric biopsy specimens revealed normal mucosa or non-atrophic gastritis. Gastric ghrelin concentration was determined by Enzyme linked immunosorbent assay. The cagA and vacA strains of bacterial DNA were identified by multiplex polymerase chain reaction.
RESULTS: In general, infection with H pylori caused an increase in gastric ghrelin level regardless of gender and stomach topography. Significantly more hormone was present in both, non-infected and H pylori positive female samples, as compared to males. The distribution of bacterial strains showed cagA(+) vacA s1m1 and cagA(-) vacA s2m2 genotypes as the most common infections in the studied population. A tendency to higher ghrelin levels was observed in less cytotoxic (cagA negative) strain-containing specimens from the antrum and corpus of both gender groups (without statistical significance).
CONCLUSION: An increase in gastric ghrelin levels at the stage of non-atrophic gastritis in H pylori positive patients, especially in those infected with cagA(-) strains, can exert a gastroprotective effect.
-
Citation: Stec-Michalska K, Malicki S, Michalski B, Peczek L, Wisniewska-Jarosinska M, Nawrot B. Gastric ghrelin in relation to gender, stomach topography and
Helicobacter pylori in dyspeptic patients. World J Gastroenterol 2009; 15(43): 5409-5417 - URL: https://www.wjgnet.com/1007-9327/full/v15/i43/5409.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5409
Ghrelin is a peptide hormone that plays an important role in food intake, energy homeostasis and body-weight regulation. It is expressed in multiple tissues and exerts both, endocrine and paracrine/autocrine effects. This 28-amino acid peptide was discovered by Kojima et al[1] in 1999. Approximately 70% of the body pool of this hormone is produced by gastric X/A cells located in the corpus and fundus of the stomach and is secreted into the extracellular matrix and into the blood. Normal ghrelin levels in serum range from 145-160 fmol/mL and rapidly drop after gastrectomy[2]. Ghrelin stimulates growth hormone (GH) release via the GHRH-dependent (growth hormone releasing hormone) mechanism acting on the growth hormone secretagogue receptor (GHS-R). Released GH and its mediator, insulin-like growth factor 1, affect a range of physiological processes[3]. It has been suggested that ghrelin exerts a gastroprotective role. Its level is significantly lower in gastric tumours than in normal gastric mucosa[4].
Ghrelin possesses anti-proliferative effects on breast, lung and thyroid cell lines and exerts protective actions on the gastric mucosa[5]. In the alimentary tract, ghrelin increases acid secretion and stimulates gastric emptying via vagal activation[6]. Ghrelin levels change significantly depending on the body’s energy requirements[7]. It is known that exogenous ghrelin significantly inhibits the activation of nuclear factor (NF)-κB and plasma tumor necrosis factor-α[8]. It is well known that Helicobacter pylori (H pylori)-mediated gastric inflammation critically depends on the efficient recruitment and activation of macrophages, with sufficient NF-κB activation[9]. This transcription factor plays an important role in activation of expression of proinflammatory cytokines. Studies are being conducted to determine the effect of sociological and environmental factors on ghrelin release.
There is a great deal of interest in the role of H pylori infection in oncogenesis. H pylori infection always induces chronic active gastritis, which, in the presence of other factors, can lead to the development of gastric cancer (GC). Thus, based on evidence that infections increase the risk of GC, H pylori was categorized as a class I carcinogen more than 10 years ago[10]. Only 15% of those colonized develop the disease, and the pathogenesis depends upon strain virulence, host genetic susceptibility, and environmental cofactors. Virulent factors include the cag pathogenicity island (PAI), which induces proinflammatory and proliferative epithelial cell signaling. The expression of various products encoded in the cag PAI is known to be involved in inducing inflammation, ulceration and carcinogenesis. Each H pylori strain possesses one vacA gene, which due to its genetic variability, can be either of type s1 or s2 in the polymorphic signal region or type m1 or m2 in the polymorphic mid-region[11]. Another PAI-associated gene is the cagA gene, expressed by the majority of H pylori strains, irrespective of the geographic origin and clinical diagnosis[12]. H pylori vacA s1m1 cagA virulent strains are significantly more frequent in patients with GC[13]. An increased predisposition to GC is thought to be associated with the presence of H pylori cagA strains[14].
The aim of this study was to determine whether there is any correlation between gastric ghrelin level and H pylori infection in relation to bacterial strain cytotoxicity, patients’ gender and stomach topography.
The study comprised 88 patients with functional dyspepsia (classification according to Rome III criteria)[15]. Exclusion criteria included chronic or psychiatric illness, pregnancy, drug and alcohol abuse - defined as consumption of more than two alcoholic drinks per day and NSAIDs or PPIs administration during the 14 d prior to the study. Patients with histopathological changes higher than those of non-atrophic gastritis (according to Sydney System Scale update[16]) and with a body mass index (BMI) below 18 and above 29 kg/m2 were excluded from the study. The study comprised 40 premenopausal women (mean age 42 ± 13 years), of whom 19 were H pylori positive and 48 men (mean age 35 ± 10 years), of whom 17 were H pylori positive. Initially, all the selected subjects had their BMI calculated (as body weight in kilograms divided by the square of their height in meters)[17]. The physical examinations were performed at fasting in the morning. Subjects underwent a routine endoscopic evaluation at enrolment (a gastroscopy with biopsy). From each patient, five biopsy specimens from the antrum and five from the corpus were taken. One of the samples was used to perform a urease test, and a second sample was used for histological assessment. Another sample was used to determine the bacterial strain, and the remaining two specimens, taken from the anterior and posterior wall of the antrum or corpus were used for ghrelin level determination.
Histological assessments were performed using haematoxylin-eosin and Giemsa staining. Two paraffin-embedded tissue blocks (from the antrum and corpus) were used for microscopic section preparation. From each paraffin block, two sections were obtained. The microscopic gastric mucosa assessment was based on criteria according to the updated Sydney System Classification[16].
Biopsy specimens were frozen in physiological saline at -20°C. DNA, RNA and protein fractions were extracted using a TriPure Isolation Reagent (Roche Diagnostic GmbH, Mannheim, Germany). Protein concentrations were assessed with the Biorad Protein Assay (BIO-RAD Laboratories, Hercules, CA, USA). Purified protein fractions were used to evaluate ghrelin levels in biopsy specimens by the immunoenzymatic enzyme linked immunosorbent assay (ELISA) method.
Multiplex PCR reactions were performed with the use of specific primers for amplification of the cagA gene and subtypes of the vacA (s1/2 and m1/2) gene in biopsy specimens[18]. The tissue samples were suspended in lysis buffer (10 mmol/L Tris-HCl, 0.1 mol/L EDTA, 5% SDS, 100-200 μg/mL proteinase K). The mixture was incubated at 50°C for 2 h, and then DNA was isolated by extraction with a mixture of phenol, chloroform and isoamyl alcohol and precipitated with ethanol. PCR products of the lengths: 350 base pairs (bp) (cagA), 259 bp (vacA s1), 286 bp (vacA s2), 567 bp (vacA m1) and 642 bp (vacA m2) were subjected to electrophoretic analysis on a 2% agarose gel.
Ghrelin levels in gastric biopsy specimens were measured using the indirect ELISA “sandwich” test. A 96-well MaxiSorp plate (NUNC Thermo Fisher Scientific, Roskilde, Denmark) was coated with chicken monoclonal antibodies against the C-terminal domain of human ghrelin (Abcam Ltd., Cambridge, UK) in carbonate buffer (pH 9.6). Plate wells were rinsed 5 times with PBS buffer and blocked with 1% BSA-PBS (SERVA Electrophoresis GmbH, Heidelberg, Germany) and protein fraction was added to the wells at a final amount of 1 mg per well. The plates were incubated at room temperature for 2 h. The wells were then rinsed five times with PBS, and rabbit polyclonal IgG antibodies against the N-terminal end of human ghrelin were added. The plates were incubated overnight at 4°C. HRP goat polyclonal antibodies against rabbit IgG (Abcam) were applied. The reaction was revealed by ABTS substrate (BIOMOL GmbH, Hamburg, Germany) at a 1 mg/mL concentration in phosphate-citrate buffer with H2O2 (Chempur, Piekary Slaskie, Poland) for 20 min in darkness. Then, the absorbance was measured at 414 nm by the Synergy HT (BioTek, Winooski, VT, USA) plate reader. The measurements allowed us to obtain the values of ghrelin absorbance after subtraction of the background (the absorbance value of the well without gastric biopsy proteins). Test normalization was performed by analyzing a model curve obtained using commercially available hormone. The mean value of ghrelin levels determined in two specimens, taken from the anterior and posterior wall of the antrum or corpus of each patient was taken for further evaluations.
The study was conducted in accordance with the Declaration of Helsinki and with principles of the Good Clinical Practice. These studies were approved by the Ethical Commission of the Medical University of Lodz, Poland. Each patient before being enrolled into the research program was acquainted with the aim of the study and gave conscious, written consent to participate in the study.
The Shapiro-Wilks’W test was used to analyze the normality of the distribution. The differences between median values of gastric ghrelin in two independent groups were calculated by the use of the nonparametric Mann-Whitney U test. In order to test the statistical significance of differences between the three groups we used the non-parametric Kruskal-Willis test. The gastric ghrelin level is shown as a box around the midpoint (median) which represents the 25th and 75th percentiles. The whiskers outside of the box represent the non outlier data. Outlier data points are also plotted. The statistical significance of the difference for each test was identified by a two-tailed probability (P). P values of less than 0.05 were considered significant.
A total of 88 patients with functional dyspepsia, having normal mucosa or non-atrophic gastritis were enrolled in the study. Full characteristics of the patients including the mean age, BMI and alcohol consumption are given in Table 1.
| Hp(-) | Hp(+) | |||||
| Number of patients (female/male) | 21/31 | 19/17 | ||||
| Age (yr, female/male) | 41.3 ± 15.0/36.4 ± 11.5 | 45.0 ± 11.6/38.4 ± 13.8 | ||||
| BMI (female/male) | 23.9 ± 3.2/25.1 ± 2.6 | 23.3 ± 3.0/24.0 ± 3.4 | ||||
| Alcohol drinking habit (% of group population, female/male) | ND (45.0/28.6) | 1/m (50.0/50.0) | 1/w (5.0/21.4) | ND (57.0/33.3) | 1/m (43.0/44.4) | 1/w (0.0/22.3) |
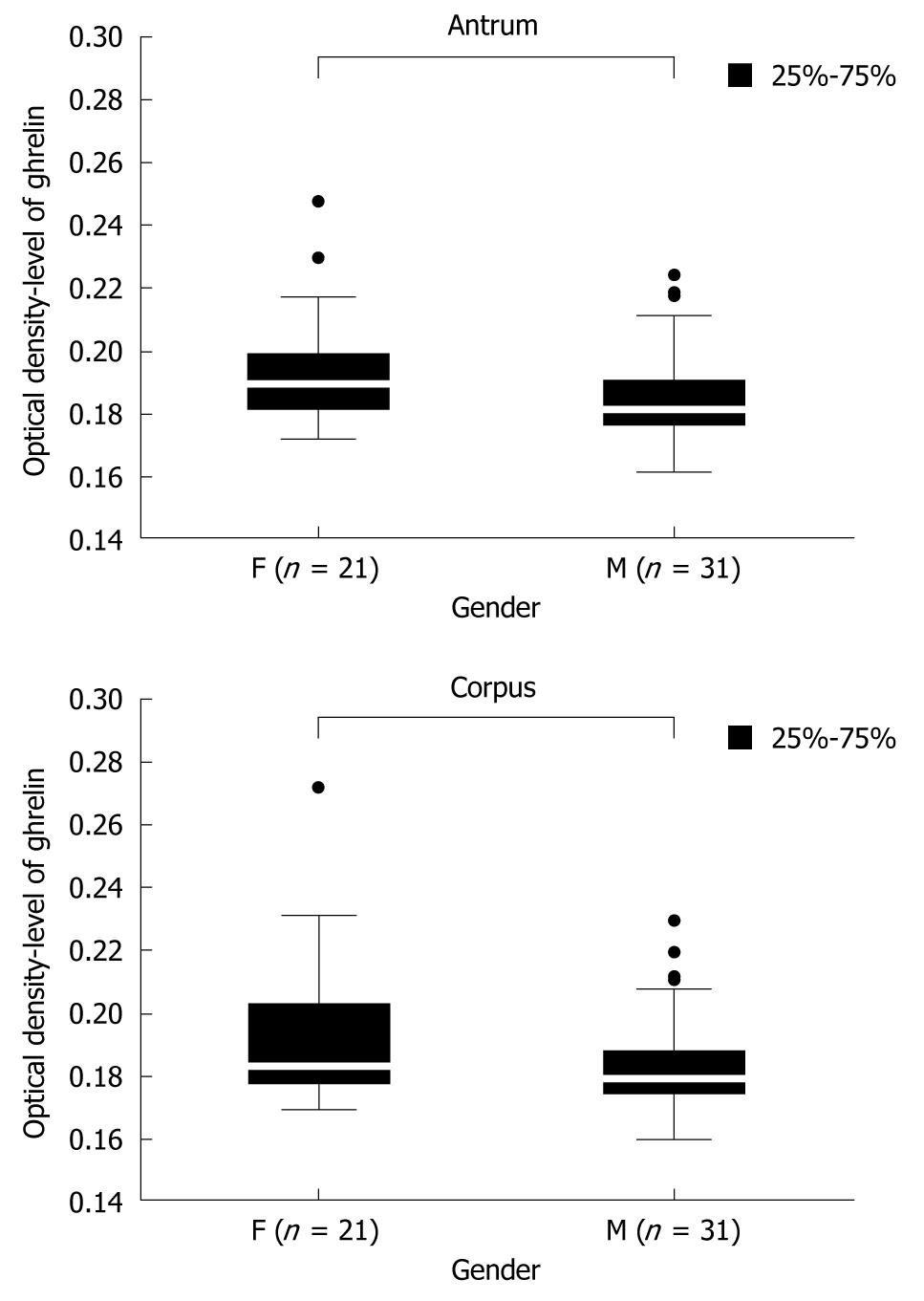
There are no clear data concerning the dependence of gastric ghrelin level on patients’ gender. Therefore the first groups analyzed were the controls, without H pylori infection, consisting of 21 premenopausal women (mean age 41 ± 13 years) and 31 men (mean age 36 ± 11 years). The levels of gastric ghrelin in the antrum and corpus were determined for each patient and then the mean values were calculated for the analyzed groups of patients. The results are presented in Figure 1 and show statistically significant [antrum: 0.189250 (F) vs 0.181000 (M), P = 0.004610 and corpus: 0.183000 (F) vs 0.179000 (M), P = 0.017149] higher levels of this hormone in female samples (F) compared to male samples (M), independent of gastric topography.
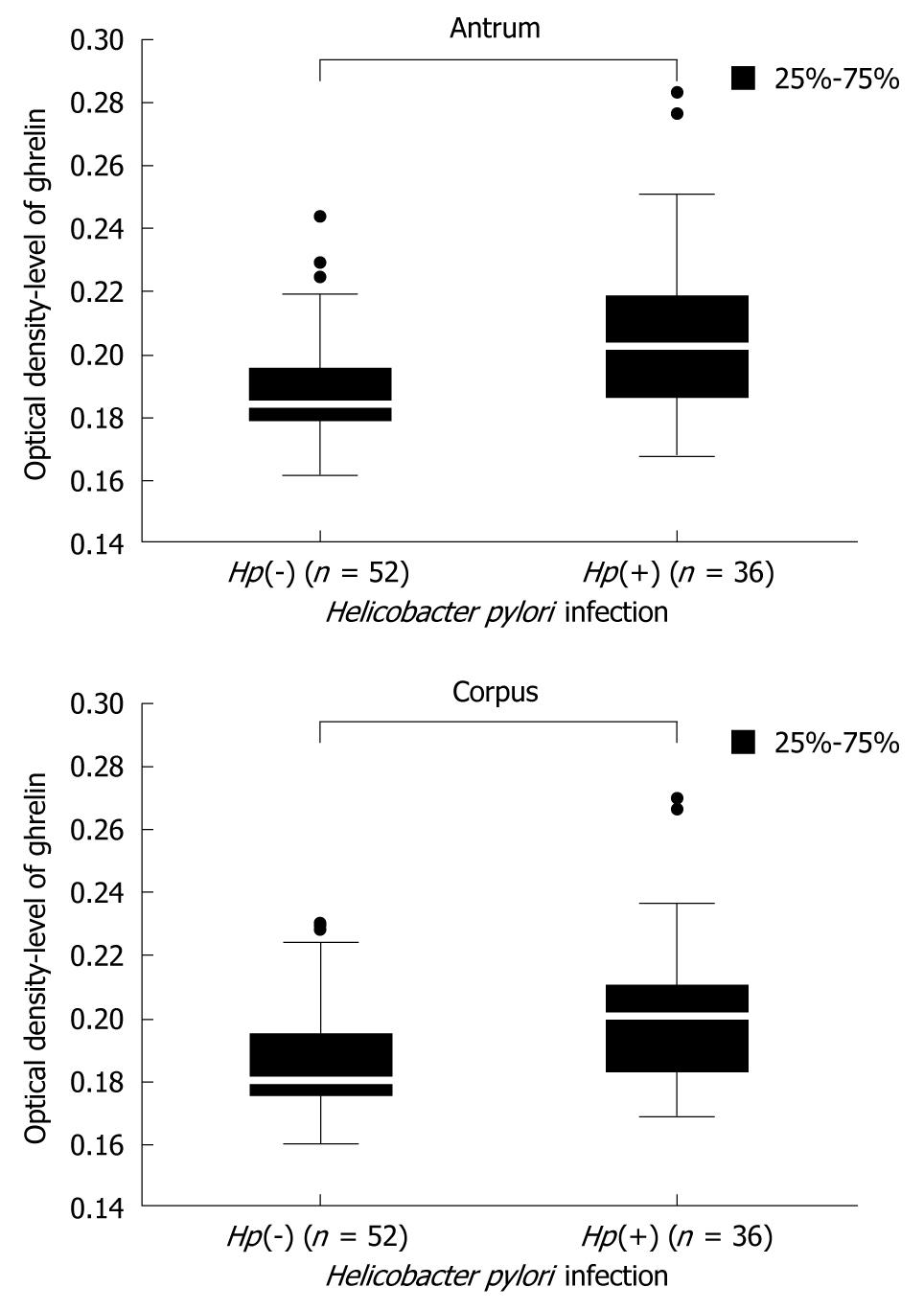
The first question was to clarify the influence H pylori infection on the level of gastric ghrelin. Data concerning this issue are not consistent and therefore we compared the levels of gastric ghrelin in samples from the antrum and corpus of H pylori negative [Hp(-)] and H pylori positive [Hp(+)] patients. These results are shown in Figure 2 and demonstrate that, independent of stomach topography, the levels of this hormone were elevated in infected specimens. Moreover, the observed differences between infected and non-infected groups of patients were statistically significant {antrum: 0.184000 [Hp(-)] vs 0.202500 [Hp(+)], P = 0.000000 and corpus: 0.178000 [Hp(-)] vs 0.201000 [Hp(+)], P = 0.000000} (Figure 2).
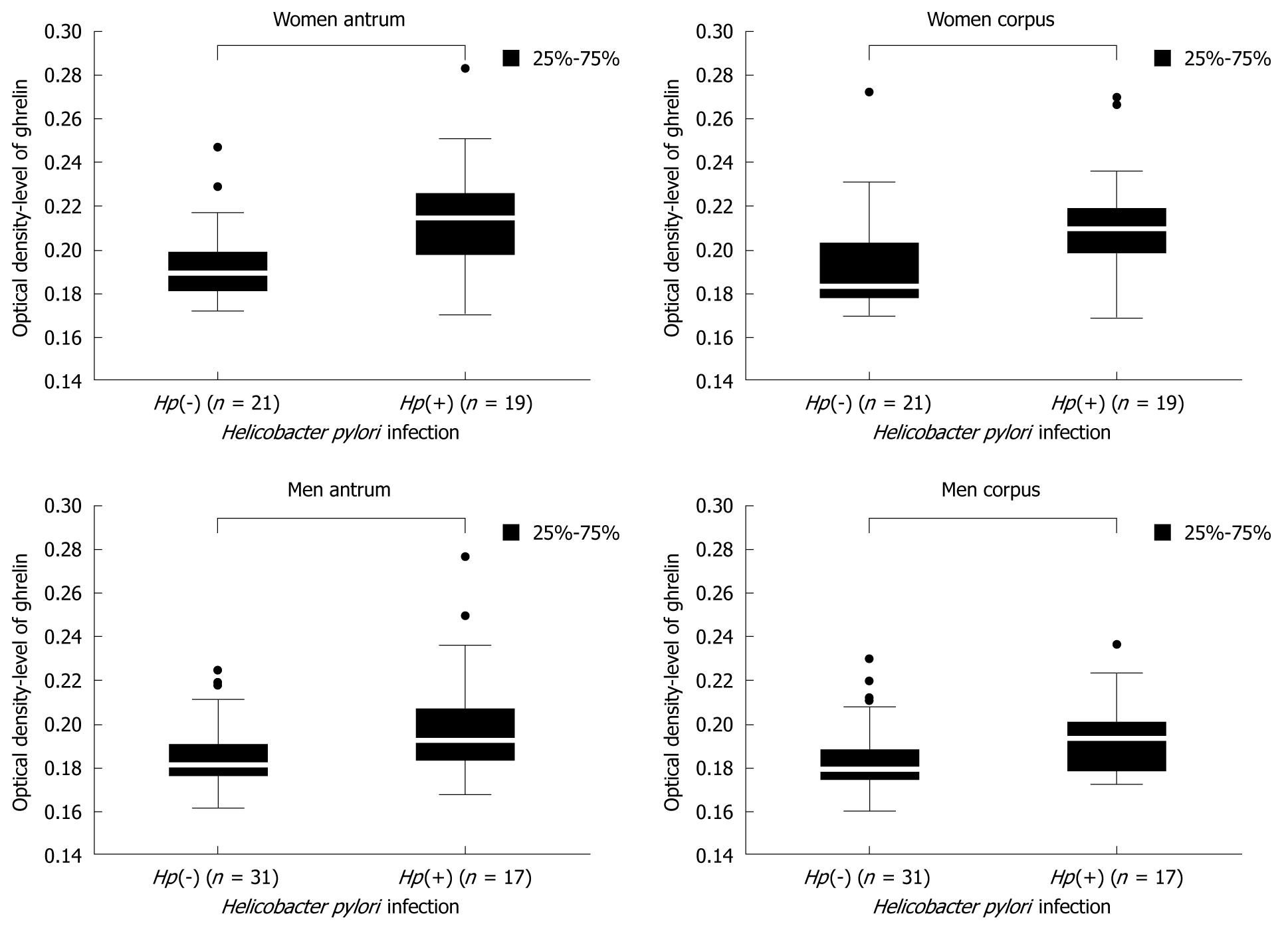
Because the level of gastric ghrelin was higher in the stomach mucosa of women than in men, we performed a detailed analysis of ghrelin levels depending on H pylori infection and sex. As shown in Figure 3, in both men and women, the levels of gastric ghrelin were higher, as expected, in H pylori positive biopsy specimens {women antrum: 0.189250 [Hp(-)] vs 0.214667 [Hp(+)], P = 0.000002; women corpus: 0.183000 [Hp(-)] vs 0.209250 [Hp(+)], P = 0.000214; men antrum: 0.181000 [Hp(-)] vs 0.192250 [Hp(+)], P = 0.000664; men corpus: 0.179000 [Hp(-)] vs 0.193000 [Hp(+)], P = 0.001514}, regardless of gastric topography. However, the differences in hormone levels between H pylori negative and H pylori positive patients were more prominent in female samples.
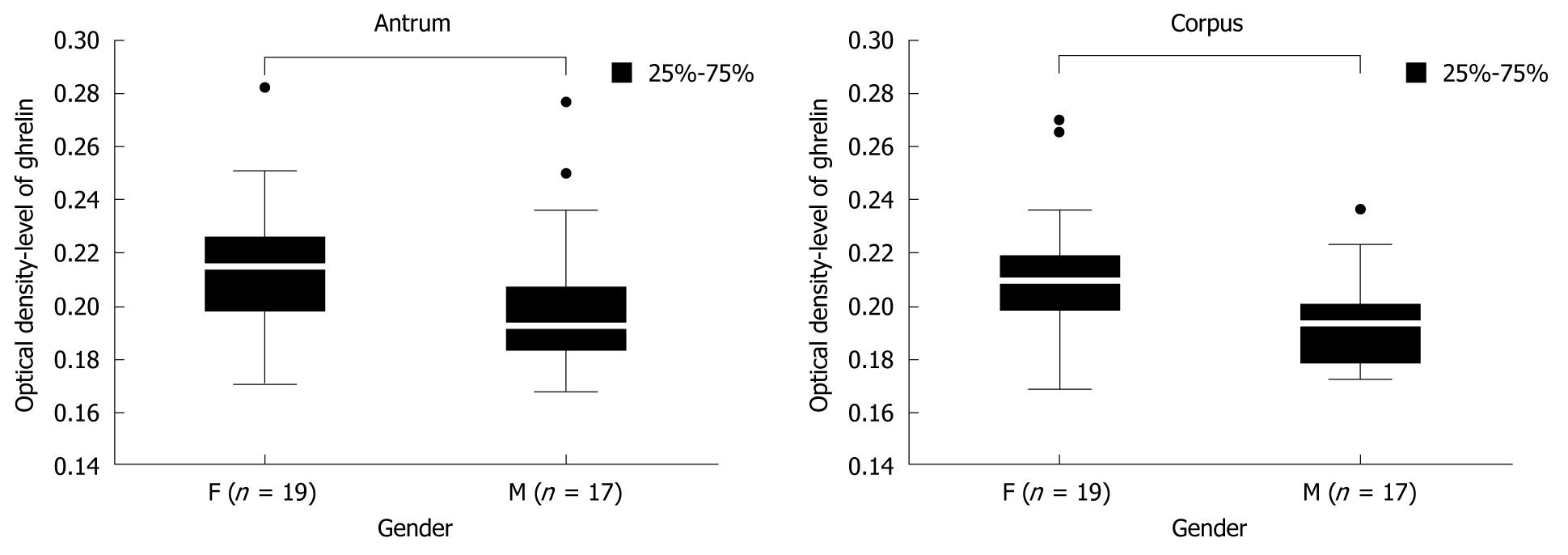
Next, we compared the levels of gastric ghrelin in H pylori positive female and male samples. The screened groups (19 women and 17 men) were sufficient to obtain statistically significant data, and indicated that in female samples the levels of hormone were higher that in male samples [antrum: 0.214667 (F) vs 0.192250 (M), P = 0.000375 and corpus: 0.209250 (F) vs 0.193000 (M), P = 0.000292] independent of stomach topography (antrum vs corpus) (Figure 4).
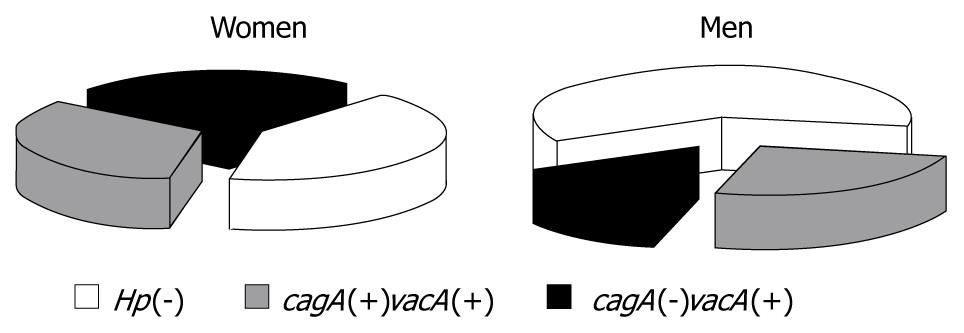
The distribution of selected bacterial strains vagA and cagA in the study population was evaluated by multiplex PCR amplification of bacterial DNA (Table 2). The most common infections, with cagA(+) vacA s1m1 and cagA(-) vacA s2m2 strains, were observed in specimens from the antrum and from the corpus of both study groups. In contrast, the cagA(+) vacA s1m2 and cagA(+) vacA s2m2 strains were present in a minor number of patients only. Interestingly, in one case (a female patient), two different strains were detected: cagA(-) vacA s2m2 (in the corpus) and cagA(+) vacA s2m2 (in the antrum). Because of the varied distribution of H pylori strains and the small number of patients within these groups, H pylori infected subjects were combined into two subgroups: those without cytotoxin CagA gene [cagA(-)], and those with cytotoxin CagA(+) gene [cagA(+)].
| H pylori genotype | No. of patients (female/male) | |
| Antrum | Corpus | |
| cagA(-) vacA s2 m2 | 8 (5/3) | 9 (6/3) |
| cagA(+) vacA s1 m1 | 17 (11/6) | 17 (11/6) |
| cagA(+) vacA s1 m2 | 1 (1/0) | 1 (1/0) |
| cagA(+) vacA s2 m2 | 2 (1/1) | 1 (0/1) |
| cagA(+) vacA(+) genotype ndt | 3 (0/3) | 3 (0/3) |
| cagA(-) vacA(+) genotype ndt | 5 (1/4) | 5 (1/5) |
As expected, all the evaluated H pylori strains expressed vacA but differed with respect to the subtype combinations of s1/s2 and m1/m2. The percentage distribution of all studied subgroups (regardless of the subtypes) is presented in Figure 5. The data showed that the biggest group of subjects was composed of those without H pylori infection. The percentage distribution of patients according to genotype varied according to gender. In men, the least common H pylori strain was cagA(-)vacA(+), whereas in women, the percentage distribution of all the genotypes was similar.
Due to the observed differences in ghrelin levels in the samples from women and men, the relationship between the hormone levels and H pylori cytotoxicity was evaluated separately for each gender.
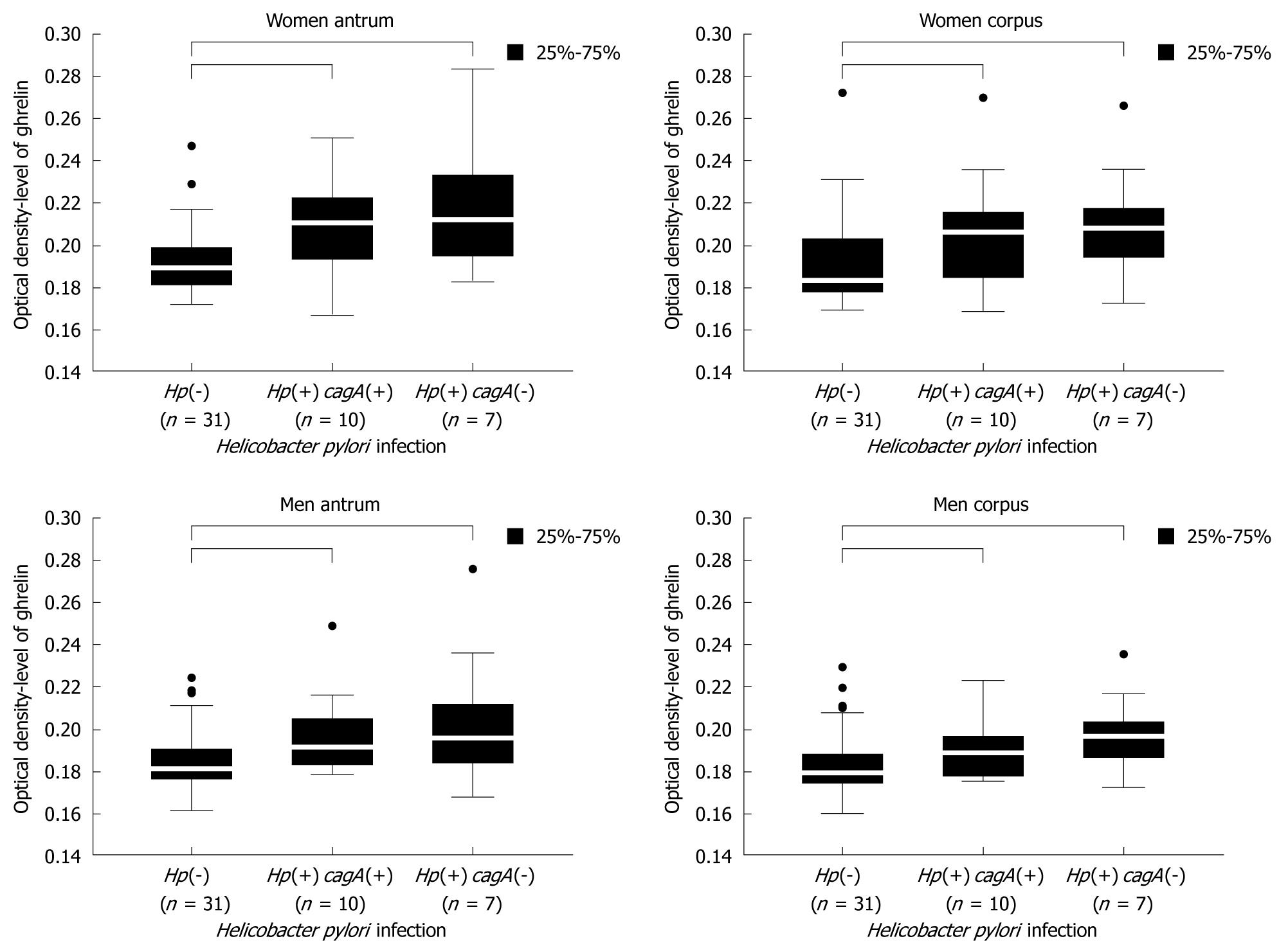
Female samples: The results obtained for female samples (Figure 6) showed that ghrelin levels were higher in women infected with both, cagA(+)vacA(+) and cagA(-)vacA(+) strains compared to women without H pylori infection {women antrum: 0.189250 [Hp(-)] vs 0.210500 [Hp(+) cagA(+)] vs 0.212000 [Hp(+) cagA(-)], P = 0.0002 and women corpus: 0.183000 [Hp(-)] vs 0.206000 [Hp(+) cagA(+)] vs 0.208000 [Hp(+) cagA(-)], P = 0.0027}. No statistical significance was noted for differences in ghrelin levels in relation to strain cytotoxicity, although a tendency for higher amounts of hormone in the samples infected with cagA(-)vacA(+) strains was observed.
Male samples: In H pylori infected men the observed changes in gastric ghrelin levels were similar to the changes observed in female samples, but varied depending on gastric topography. Statistically significant increases in ghrelin levels were noted in the antrum {men antrum: 0.181000 [Hp(-)] vs 0.191250 [Hp(+) cagA(+)] vs 0.195500 [Hp(+) cagA(-)], P = 0.0028 and men corpus: 0.179000 [Hp(-)] vs 0.188500 [Hp(+) cagA(+)] vs 0.196333 [Hp(+) cagA(-)], P = 0.0023}, however, the differences between ghrelin levels in relation to strain cytotoxicity were not significant (Figure 6). In corpus samples, an increase in ghrelin level was only caused by cagA(-)vacA(+), however, this increase was not statistically significant. Moreover, the differences between hormone levels in samples carrying both studied H pylori genotypes did not reach P < 0.05.
The data available in the literature concerning both, the effects of H pylori infection and patients’ gender on ghrelin secretion are not consistent. Some authors claim that the level of circulating ghrelin is higher in women than in men[19], but others maintain that it is similar in both sexes[20] and does not vary from childhood to adulthood[21] or undergo an age-related decrease[22]. In our studies we clearly demonstrated that in the case of gastric ghrelin, the hormone level was much higher in pre-menopausal women than in men (Figure 1). It was interesting to note that this relationship could also be seen in H pylori infected patients (Figure 4), where the amount of ghrelin was higher in female specimens. Studies by Gualillo et al[23] performed on rat stomach showed that gonadal hormones did not alter ghrelin mRNA, and suggested that ghrelin level was age- rather than gender-dependent. Therefore, one might conclude that higher hormone level in female mucosa might originate from altered hormone release rather than from a change in level of expression. Higher ghrelin level in pre-menopausal women positively correlates with lower testosterone level when compared to that in postmenopausal women[19], in whom testosterone level is higher. We speculate that higher gastric ghrelin levels in the premenopausal study group could exert a protective effect on gastric mucosa. GC incidence increases with age, and this malignant tumour is relatively rare in males and females under 45 years. In general, the incidence rates in females at a given age are equivalent to the rates in males at an age 10 years younger. The consistency of these differences has never been adequately explained although theories have been proposed that sex-specific hormones in females play a protective role against this malignancy[24].
The influence of H pylori infection on gastric ghrelin level requires discussion. Most studies show that H pylori infection leads to decreased ghrelin secretion[25-27]. However, other reports suggest that serum ghrelin levels are similar in infected and non-infected patients[25]. In our studies, H pylori infected mucosa had more ghrelin than non-infected mucosa, regardless of gender and stomach topography (Figures 2 and 3). Moreover, we determined whether H pylori cytotoxicity could influence the level of ghrelin in gastric mucosa, since there is only very limited published information on the changes in ghrelin levels in relation to virulence of H pylori strains[28]. It is well known that the strains with higher virulence produce both CagA and vacuolating cytotoxin VacA. CagA protein is the product of cagA gene expression, which belongs to the group of genes defined as the “cytotoxicity island”. The cagB, cagC, picA, and picB genes also belong to this group. The cagA gene is present in about 60%-70% of H pylori strains. It is associated with prominent inflammatory and atrophic changes and increases the risk (3-6 times) of developing a peptic ulcer, gastric adenocarcinoma and lymphoma[29-32]. H pylori infection results in an increase in proliferative activity and affects the apoptotic processes of the glandular epithelium of the gastric mucosa. Although there are a variety of proposed mechanisms by which H pylori infection increases the risk of GC, it is thought that the primary mode of action involves the induction of long-term chronic inflammation[33].
Because of the varied distribution of H pylori strains and the small number of patients with vacA s1m2 and s2m1 genotype in our studies (Table 2), the analysis comprised two groups of subjects - those infected with H pylori strains of cagA(-)vacA(+) genotype and those of cagA(+)vacA(+) genotype. We showed that bacterial strains of both genotypes increased ghrelin secretion in infected women and men compared to non-infected subjects (Figure 6), however, infection with cagA(-)vacA(+) strains showed a tendency to increase gastric ghrelin level more than infection with cagA(+)vacA(+) strains, although no statistical significance was reached.
It is worth noting that the results of the study discussed above and the conclusions drawn from them apply only to patients without advanced histopathological changes in the gastric mucosa. It is likely that H pylori infection leads to the early stage of inflammatory changes described as non-atrophic gastritis, which causes activation of protective mechanisms in the mucosa and results in an increase in gastric ghrelin expression. This increase was more prominent in the gastric mucosa of both male and female patients infected with cagA(-)vacA(+) bacterial strains of lower cytotoxicity (Figure 6).
From the literature it can be concluded that ghrelin levels in the gastric mucosa significantly decrease with more advanced histopathological changes, especially after the atrophic gastritis stage[34,35]. It has been suggested that the level of this hormone can be considered as one of the markers of gastric mucosal change progression and, at the same time, can be one of the indications for gastroscopy.
The results of the study performed by Osawa et al[36] showed a significant decrease in gastric preproghrelin mRNA level 12 wk after H pylori eradication. These observations are inconsistent with our results. The discrepancies between our results (higher gastric ghrelin level in H pylori positive patients) and the previously published data[36-38] could have arisen due to different stages of gastric mucosa inflammatory changes (chronic, severe atrophic gastritis, peptic ulcer) in subjects enrolled in the study. Moreover, earlier reports did not analyze results for women and men separately, which, as our study suggests, is an important factor influencing gastric ghrelin level. The study on the impact of H pylori infection on ghrelin level is part of our research on the relationship between these bacteria and biosynthesis of the proteins possessing proapoptotic and antiproliferative activities, which are important in the process of carcinogenesis[39-41].
In conclusion, based on the results obtained in our study, we may assume that an increase in gastric ghrelin levels at the stage of non-atrophic gastritis in H pylori positive patients, especially those infected with cagA negative strains can exert a gastroprotective effect. Future studies are needed to delineate the role of gastric ghrelin in pathological processes induced by H pylori.
Ghrelin is a peptide hormone that plays an important role in food intake, energy homeostasis and body-weight regulation. Ghrelin possesses anti-proliferative effects on breast, lung and thyroid cell lines and exerts a protective action on gastric mucosa. Its level is significantly lower in gastric tumors than in normal gastric mucosa. The published data on the effects of sociological and environmental factors on gastric ghrelin level are not consistent. One of these factors is Helicobacter pylori (H pylori) infection which always induces chronic active gastritis, and in the presence of other factors, can lead to the development of gastric cancer.
Investigation on the mechanisms involved in oncogenesis in the digestive tract-environmental factors related to bacterial infection.
The authors demonstrate, for the first time, that premenopausal women have higher gastric ghrelin levels than men. The level of this appetite hormone increases in H pylori infection, and was higher in female samples. More cytotoxic H pylori strains expressing the cagA(+) gene had less of an effect on gastric ghrelin level. Expression of this hormone was also dependent on histopathological changes, and at the stage of non-atrophic gastritis in H pylori positive patients, especially those infected with cagA negative strains, may exert a gastroprotective effect.
The data discussed in this paper are of value since they univocally demonstrate the influence of selected environmental and sociological factors on the level of expression of gastric ghrelin. The results may be used in future research to correlate the level of gastric ghrelin with plasma ghrelin and to discuss the effect of selected factors on the hormone release process. Future studies are needed to delineate the role of gastric ghrelin in pathological processes induced by H pylori.
The authors demonstrate the increased protein levels of ghrelin in gastric biopsy specimens of H pylori-positive subjects compared to H pylori-negative subjects, particularly in virulent types of H pylori-carrying patients, and suggest a protective role of ghrelin. The authors also demonstrate the gender difference in this respect. The findings are interesting, but this reviewer strongly suggests that the authors should provide additional data on mRNA expression of ghrelin in gastric specimens or plasma levels of ghrelin, since increased ghrelin concentration in the stomach will be produced by either increased synthesis or decreased secretion from the stomach.
Peer reviewer: Akio Inui, MD, PhD, Professor, Department of Behavioral Medicine, Kagoshima University Graduate School of Medical and Dental Sciences, 8-35-1 Sakuragaoka, Kagoshima 890-8520, Japan
S- Editor Tian L L- Editor Webster JR E- Editor Zheng XM
| 1. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. |
| 2. | Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753-4758. |
| 3. | Fuller SJ, Mynett JR, Sugden PH. Stimulation of cardiac protein synthesis by insulin-like growth factors. Biochem J. 1992;282:85-90. |
| 4. | An JY, Choi MG, Noh JH, Sohn TS, Jin DK, Kim S. Clinical significance of ghrelin concentration of plasma and tumor tissue in patients with gastric cancer. J Surg Res. 2007;143:344-349. |
| 5. | Locatelli V, Bresciani E, Bulgarelli I, Rapetti D, Torsello A, Rindi G, Sibilia V, Netti C. Ghrelin in gastroenteric pathophysiology. J Endocrinol Invest. 2005;28:843-848. |
| 6. | Dornonville de la Cour C, Lindström E, Norlén P, Håkanson R. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept. 2004;120:23-32. |
| 8. | Konturek PC, Brzozowski T, Walter B, Burnat G, Hess T, Hahn EG, Konturek SJ. Ghrelin-induced gastroprotection against ischemia-reperfusion injury involves an activation of sensory afferent nerves and hyperemia mediated by nitric oxide. Eur J Pharmacol. 2006;536:171-181. |
| 9. | Yanai A, Maeda S, Shibata W, Hikiba Y, Sakamoto K, Nakagawa H, Ohmae T, Hirata Y, Ogura K, Muto S. Activation of IkappaB kinase and NF-kappaB is essential for Helicobacter pylori-induced chronic gastritis in Mongolian gerbils. Infect Immun. 2008;76:781-787. |
| 10. | Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177-240. |
| 11. | Atherton JC, Cao P, Peek RM Jr, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771-17777. |
| 12. | Wirth T, Wang X, Linz B, Novick RP, Lum JK, Blaser M, Morelli G, Falush D, Achtman M. Distinguishing human ethnic groups by means of sequences from Helicobacter pylori: lessons from Ladakh. Proc Natl Acad Sci USA. 2004;101:4746-4751. |
| 13. | Miehlke S, Kirsch C, Agha-Amiri K, Günther T, Lehn N, Malfertheiner P, Stolte M, Ehninger G, Bayerdörffer E. The Helicobacter pylori vacA s1, m1 genotype and cagA is associated with gastric carcinoma in Germany. Int J Cancer. 2000;87:322-327. |
| 14. | Ferreira AC, Isomoto H, Moriyama M, Fujioka T, Machado JC, Yamaoka Y. Helicobacter and gastric malignancies. Helicobacter. 2008;13 Suppl 1:28-34. |
| 15. | Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377-1390. |
| 16. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. |
| 17. | Weigle DS, Cummings DE, Newby PD, Breen PA, Frayo RS, Matthys CC, Callahan HS, Purnell JQ. Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J Clin Endocrinol Metab. 2003;88:1577-1586. |
| 18. | Chattopadhyay S, Patra R, Ramamurthy T, Chowdhury A, Santra A, Dhali GK, Bhattacharya SK, Berg DE, Nair GB, Mukhopadhyay AK. Multiplex PCR assay for rapid detection and genotyping of Helicobacter pylori directly from biopsy specimens. J Clin Microbiol. 2004;42:2821-2824. |
| 19. | Greenman Y, Rouach V, Limor R, Gilad S, Stern N. Testosterone is a strong correlate of ghrelin levels in men and postmenopausal women. Neuroendocrinology. 2009;89:79-85. |
| 20. | Chuang CH, Sheu BS, Yang HB, Lee SC, Kao AW, Cheng HC, Chang WL, Yao WJ. Gender difference of circulating ghrelin and leptin concentrations in chronic Helicobacter pylori infection. Helicobacter. 2009;14:54-60. |
| 21. | Bellone S, Rapa A, Vivenza D, Castellino N, Petri A, Bellone J, Me E, Broglio F, Prodam F, Ghigo E. Circulating ghrelin levels as function of gender, pubertal status and adiposity in childhood. J Endocrinol Invest. 2002;25:RC13-RC15. |
| 22. | Broglio F, Benso A, Castiglioni C, Gottero C, Prodam F, Destefanis S, Gauna C, van der Lely AJ, Deghenghi R, Bo M. The endocrine response to ghrelin as a function of gender in humans in young and elderly subjects. J Clin Endocrinol Metab. 2003;88:1537-1542. |
| 23. | Gualillo O, Caminos JE, Kojima M, Kangawa K, Arvat E, Ghigo E, Casanueva FF, Diéguez C. Gender and gonadal influences on ghrelin mRNA levels in rat stomach. Eur J Endocrinol. 2001;144:687-690. |
| 24. | Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (M/F) pattern: etiologic hypothesis. Gastric Cancer. 2002;5:213-219. |
| 25. | Plonka M, Bielanski W, Konturek SJ, Targosz A, Sliwowski Z, Dobrzanska M, Kaminska A, Sito E, Konturek PC, Brzozowski T. Helicobacter pylori infection and serum gastrin, ghrelin and leptin in children of Polish shepherds. Dig Liver Dis. 2006;38:91-97. |
| 26. | Isomoto H, Ueno H, Saenko VA, Mondal MS, Nishi Y, Kawano N, Ohnita K, Mizuta Y, Ohtsuru A, Yamashita S. Impact of Helicobacter pylori infection on gastric and plasma ghrelin dynamics in humans. Am J Gastroenterol. 2005;100:1711-1720. |
| 27. | Méndez-Sánchez N, Pichardo-Bahena R, Vásquez-Fernández F, Lezama-Mora JI, León-Canales AL, Barredo-Prieto B, González-Avila D, Ponciano-Rodríguez G, Uribe M. Effect of Helicobacter pylori infection on gastric ghrelin expression and body weight. Rev Gastroenterol Mex. 2007;72:359-364. |
| 28. | Isomoto H, Nishi Y, Ohnita K, Mizuta Y, Kohno S, Ueno H, Nakazato M. The Relationship between Plasma and Gastric Ghrelin Levels and Strain Diversity in Helicobacter pylori Virulence. Am J Gastroenterol. 2005;100:1425-1427. |
| 29. | Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297-301. |
| 30. | Konturek PC, Konturek SJ, Starzynska T, Marlicz K, Bielanski W, Pierzchalski P, Karczewska E, Hartwich A, Rembiasz K, Lawniczak M. Helicobacter pylori-gastrin link in MALT lymphoma. Aliment Pharmacol Ther. 2000;14:1311-1318. |
| 31. | Wu AH, Crabtree JE, Bernstein L, Hawtin P, Cockburn M, Tseng CC, Forman D. Role of Helicobacter pylori CagA+ strains and risk of adenocarcinoma of the stomach and esophagus. Int J Cancer. 2003;103:815-821. |
| 32. | Takeuchi K, Ohno Y, Tsuzuki Y, Ando T, Sekihara M, Hara T, Kuwano H. Helicobacter pylori infection and early gastric cancer. J Clin Gastroenterol. 2003;36:321-324. |
| 33. | Naumann M, Crabtree JE. Helicobacter pylori-induced epithelial cell signalling in gastric carcinogenesis. Trends Microbiol. 2004;12:29-36. |
| 34. | di Mario F, Cavallaro LG. Non-invasive tests in gastric diseases. Dig Liver Dis. 2008;40:523-530. |
| 35. | Checchi S, Montanaro A, Pasqui L, Ciuoli C, Cevenini G, Sestini F, Fioravanti C, Pacini F. Serum ghrelin as a marker of atrophic body gastritis in patients with parietal cell antibodies. J Clin Endocrinol Metab. 2007;92:4346-4351. |
| 36. | Osawa H, Kita H, Ohnishi H, Nakazato M, Date Y, Bowlus CL, Ishino Y, Watanabe E, Shiiya T, Ueno H. Changes in plasma ghrelin levels, gastric ghrelin production, and body weight after Helicobacter pylori cure. J Gastroenterol. 2006;41:954-961. |
| 37. | Osawa H, Nakazato M, Date Y, Kita H, Ohnishi H, Ueno H, Shiiya T, Satoh K, Ishino Y, Sugano K. Impaired production of gastric ghrelin in chronic gastritis associated with Helicobacter pylori. J Clin Endocrinol Metab. 2005;90:10-16. |
| 38. | Osawa H. Ghrelin and Helicobacter pylori infection. World J Gastroenterol. 2008;14:6327-6333. |
| 39. | Stec-Michalska K, Antoszczyk S, Klupinska G, Nawrot B. Loss of FHIT expression in gastric mucosa of patients with family histories of gastric cancer and Helicobacter pylori infection. World J Gastroenterol. 2005;11:17-21. |
| 40. | Stec-Michalska K, Peczek L, Krakowiak A, Michalski B, Chojnacki J, Knopik-Dabrowicz A, Klupinska G, Nawrot B. Expression of somatostatin receptor subtype 3 in the gastric mucosa of dyspeptic patients in relation to Helicobacter pylori infection and a family history of gastric cancer. J Gastroenterol Hepatol. 2008;23:424-429. |
| 41. | Stec-Michalska K, Peczek L, Michalski B, Wisniewska-Jarosinska M, Krakowiak A, Nawrot B. Helicobacter pylori infection and family history of gastric cancer decrease expression of FHIT tumor suppressor gene in gastric mucosa of dyspeptic patients. Helicobacter. 2009;14:126-134. |