Published online Nov 14, 2009. doi: 10.3748/wjg.15.5364
Revised: August 31, 2009
Accepted: September 7, 2009
Published online: November 14, 2009
Peutz-Jeghers syndrome (PJS), a rare autosomal dominant inherited disorder, is characterized by hamartomatous gastrointestinal polyps and mucocutaneous pigmentation. Patients with this syndrome have a predisposition to a variety of cancers in multiple organs. Mutations in the serine/threonine kinase 11 (STK11) gene have been identified as a major cause of PJS. Here we present the clinical and molecular findings of two unrelated Thai individuals with PJS. Mutation analysis by Polymerase Chain Reaction-sequencing of the entire coding region of STK11 revealed two potentially pathogenic mutations. One harbored a single nucleotide deletion (c.182delG) in exon 1 resulting in a frameshift leading to premature termination at codon 63 (p.Gly61AlafsX63). The other carried an in-frame 9-base-pair (bp) deletion in exon 7, c.907_915del9 (p.Ile303_Gln305del). Both deletions were de novo and have never been previously described. This study has expanded the genotypic spectrum of the STK11 gene.
-
Citation: Ausavarat S, Leoyklang P, Vejchapipat P, Chongsrisawat V, Suphapeetiporn K, Shotelersuk V. Novel mutations in the
STK11 gene in Thai patients with Peutz-Jeghers syndrome. World J Gastroenterol 2009; 15(42): 5364-5367 - URL: https://www.wjgnet.com/1007-9327/full/v15/i42/5364.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5364
| Primer sequences (5’ to 3’) | Annealing temperature (°C) | |
| STK11 cDNA | F GTCGGAACACAAGGAAGGAC | 60 |
| R AACCGGCAGGAAGACTGAGG | ||
| STK11 Exon 1 | F GTCGGAACACAAGGAAGGAC | 57 |
| R CAGAACCATCAGCACCGT GA | ||
| STK11 Exon 7 | F ATGTCCCAGGAGTGGAGTGG | 60 |
| R ACAGGACACTGCCCA GAGAC |
Peutz-Jeghers syndrome (PJS) is an autosomal dominant disorder with predisposition for cancer (OMIM 175200), and is characterized by the occurrence of multiple gastrointestinal hamartomatous polyps and pigmentation of the lips, buccal mucosa and digits[1]. PJS patients also have a significantly increased risk for developing benign and malignant tumors of multiple organs both in the gastrointestinal tract and in extragastrointestinal sites[2]. Cancers of the lung, pancreas, breast, ovary and testis have been observed[3-6]. Germline mutations in the serine/threonine kinase 11 (STK11) gene at 19p13.3 have been demonstrated to be responsible for most PJS cases[7,8]. The STK11 gene consists of nine coding exons and translates into a protein of 433 amino acids[8]. STK11 is proposed to function as a tumor suppressor that acts as an early gatekeeper[9], regulates cell polarity[10], and controls cell cycle arrest, cell proliferation and apoptosis[11].
PJS is the first cancer susceptibility syndrome caused by inactivation of the serine/threonine kinase. Previous findings suggested that the combination of germline mutations and a defect of the second allele causing loss of heterozygosity in somatic cells was responsible for the phenotypic manifestations of PJS[8].
At least 200 different disease-causing mutations in the STK11 gene have been described with the majority being missense/nonsense mutations. The splice-junction alterations, insertions, and nucleotide or whole gene deletions have also been reported (http://www.hgmd.cf.ac.uk, accessed July 2009). Most STK11 mutations affect the catalytic kinase domain and result in inactivation or loss of protein function.
In this study, we report the clinical and molecular characterization of two unrelated Thai PJS patients. Mutation analysis revealed that both patients carried de novo mutations. Both mutations were also novel. This is the first report of molecularly-confirmed PJS in Thai patients.
A 14-year-old Thai boy was referred for further management of intestinal obstruction. The patient developed abdominal pain with distension and melena 3 d after receiving appendectomy. He was diagnosed with intussusception for which he underwent surgical resection. Physical examination revealed hyperpigmentation on the lower lip, buccal mucosa, and digits. Endoscopic examination revealed multiple polyps in the stomach, small bowel and colon. No other family members had any features consistent with PJS.
A 14-year-old Thai girl was referred for treatment of intestinal polyps. She presented with hematochezia and prolapse of a polypoid mass. Characteristic mucocutaneous pigmentation was also observed. Endoscopic examination demonstrated multiple polyps throughout the gastrointestinal tract. She underwent polypectomy three times. The resected polyps were found to have characteristic features of Peutz-Jeghers polyps. There was no history of gastrointestinal problems in either of her parents.
Peripheral blood samples were obtained from the probands and their available parents after written informed consent. Total RNA and genomic DNA were extracted from peripheral blood using Qiagen RNA and DNA extraction kits according to manufacturer’s instructions, respectively (Qiagen, Valencia, CA, USA). Reverse transcription was performed using ImProm-IITM reverse transcriptase (Promega, Madison, WI, USA), according to the manufacturer’s instructions. PCR amplification of the entire coding sequence of the STK11 gene was performed using primers as shown in Table 1. In brief, we used 50 ng of cDNA, 1XPCR buffer (Promega), 1.5 mmol/L MgCl2, 0.2 mmol/L dNTPs, 0.2 μmol/L of each primer and 0.5 U Taq DNA polymerase (Promega) in a volume of 20 μL using the following parameters: 35 cycles of 1 min at 94°C, 1 min at 60°C and 1 min 30 s at 72°C. PCR products were treated with ExoSAP-IT (USP Corporation, Cleveland, OH) according to the manufacturer’s recommendations, and sent for direct sequencing (Macrogen Inc., Seoul, Korea). Sequence data were analyzed using Sequencher (version 4.2; Gene Codes Corporation, Ann Arbor, MI, USA). Mutations found in both patients were confirmed by direct sequencing of the genomic DNA using a set of primers and parameters according to their mutation sites (Table 1). The nucleotide position is in accordance with the STK11 mRNA (Genbank Accession No. NM_000455). The available parents were also tested for the identified mutation by PCR-sequencing.
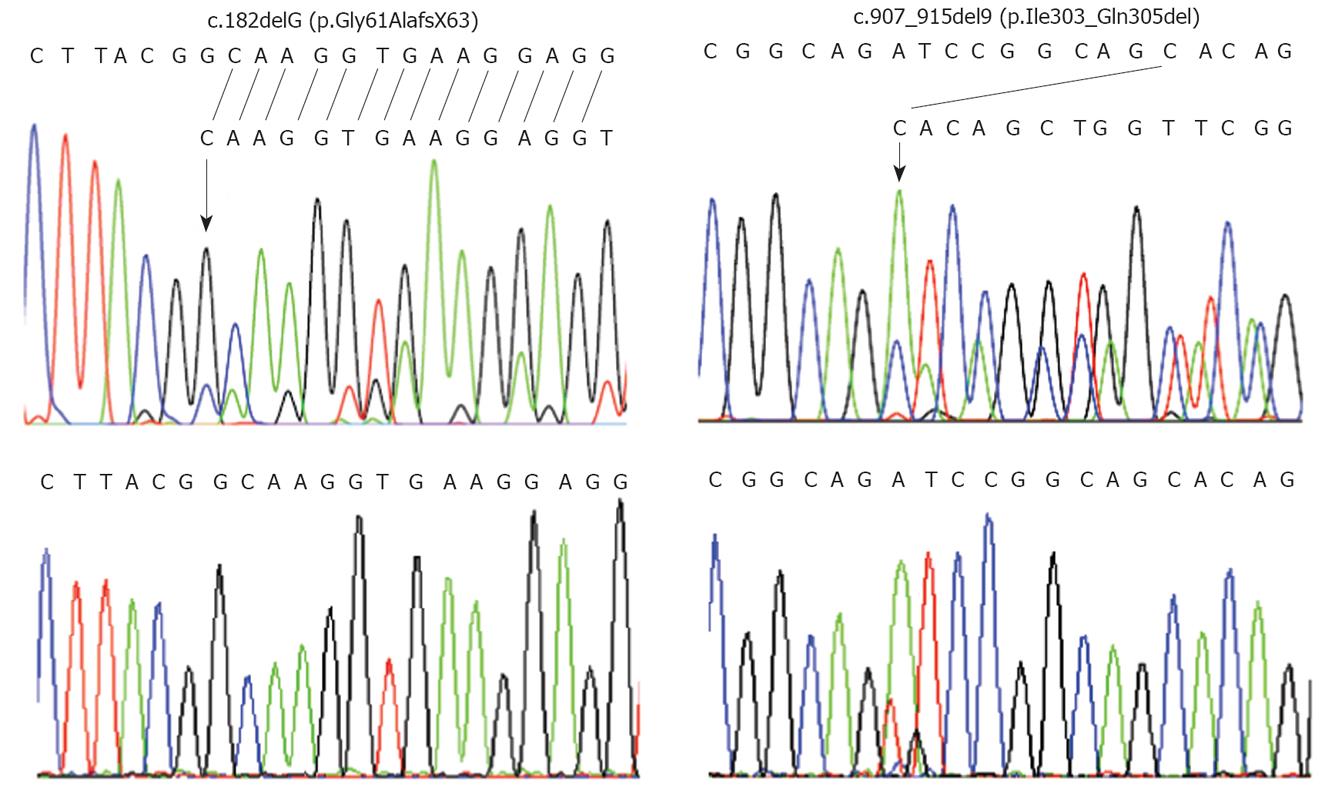
PCR-sequencing analysis of the entire coding sequence of STK11 revealed that patient 1 was heterozygous for a novel deletion of a guanine at nucleotide position 182 (c.182delG) in exon 1 of the STK11 gene (Figure 1, left upper panel). The loss of a guanine is expected to lead to a frameshift starting at codon 61 and introduce a premature stop codon at position 63. Sequencing his genomic DNA confirmed the presence of the c.182delG (Gly61AlafsX63) mutation. This mutation was not found in either parent (Figure 1, left lower panel).
Patient 2 was heterozygous for a novel in-frame 9-base-pair (bp) deletion (c.907_915del9) in exon 7 (Figure 1, right upper panel). This identified mutation was confirmed by sequencing of the patient’s genomic DNA. The 9-bp deletion results in absence of three amino acids in the kinase domain of the STK11 protein. The c.907_915del9 was not detected in her mother. Her clinically unaffected father was unavailable for mutation analysis.
Both Thai patients fulfilled the clinical diagnostic criteria for PJS[12]. These include the presence of hamartomatous polyps and characteristic mucocutaneous pigmentation. Inactivating mutations in the STK11 gene have been detected in 50%-94% of PJS patients. The highest mutation detection rate could be achieved if only patients who met the clinical criteria were considered and all mutation screening methods were used[12-15]. In this study, two different germline mutations were identified in the STK11 gene and were found to be de novo as evidenced by their clinically unaffected parents as well as molecular findings. Both mutations have never been previously described.
A novel heterozygous 1-bp deletion (c.182delG; p.Gly61AlafsX63) in exon 1 of the STK11 gene was detected in patient 1. It resulted in a frameshift leading to premature termination of the codon at position 63. The mutation was not detected in his parents. Since codons 49-309 encode the catalytic kinase domain[16], this truncated protein is expected to result in incomplete catalytic kinase domain leading to inactivation of the kinase activity as well as a complete loss of the C-terminal domain.
The second patient was heterozygous for a novel in-frame 9-bp deletion in exon 7 (c.907_915del9; p.Ile303_Gln305del). The 9-bp deletion results in absence of three amino acids in the kinase domain of the STK11 protein. This alteration was close to another 9-bp deletion that has been previously described in PJS patients from different populations (c.908_916del9; p.Ile303Asn; Arg304_His306del) and involved similar residues. In addition, they were located at the conserved catalytic core of the kinase domain[7,17]. These findings confirmed an important role of these critical residues.
Several studies have been performed to analyze genotype-phenotype correlations in PJS. One such study showed that PJS patients with predominantly truncating mutations had breast carcinomas, whereas in-frame deletions in the ATP binding domain were rarely associated with cancers in PJS patients[18]. However, an analysis of the spectrum of cancers and the risk of these cancers with different types of STK11 mutations in 419 PJS individuals suggested that there was no significant correlation between the type or site of mutation and the cancer risk[19]. Considering the correlation between time to the onset of gastrointestinal symptoms and the mutation status, individuals with missense mutations of STK11 had a later time to onset of the symptoms compared with those with truncating mutations[20]. A subsequent study showed a trend toward earlier age of intussusception onset in individuals with STK11 truncating mutations; however, the difference was not statistically significant[21]. Our patient with a truncating mutation, c.182delG, in the STK11 gene developed intussusception at the age of 14 years, close to the median time to onset of intussusception in those with STK11 truncating mutations from both studies[20,21]. To strengthen the genotype-phenotype correlations, larger and well-designed studies are still required.
Identification of the STK11 mutation remains essential for a correct diagnosis of PJS and has important clinical implications. Genetic counseling and surveillance strategies which are of value in disease management could be provided for patients with PJS and their at risk family members regardless of their mutations identified.
In conclusion, we report on the clinical and molecular characterization of two unrelated Thai patients with PJS. Two novel potential pathogenic mutations, c.182delG (p.Gly61AlafsX63) and c.907_915del9 (p.Ile303_Gln305del) were identified expanding the mutational spectrum of STK11. This study demonstrates that the STK11 gene is responsible for PJS across different populations and emphasizes the important role of genetic testing for definite diagnosis as well as appropriate genetic counseling.
Peer reviewers: Dr. Takayoshi Kiba, Translational Research Informatics Center, Foundation for Biomedical Research and Innovation, Hyogo 650-0047, Japan; Dr. Ferenc Sipos, 2nd Department of Internal Medicine, Semmelweis University, Budapest 1088, Hungary
S- Editor Li LF L- Editor Webster JR E- Editor Tian L
| 1. | Jeghers H, Mckusick VA, Katz KH. Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits; a syndrome of diagnostic significance. N Engl J Med. 1949;241:1031-1036. |
| 2. | Giardiello FM, Welsh SB, Hamilton SR, Offerhaus GJ, Gittelsohn AM, Booker SV, Krush AJ, Yardley JH, Luk GD. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987;316:1511-1514. |
| 3. | Bowlby LS. Pancreatic adenocarcinoma in an adolescent male with Peutz-Jeghers syndrome. Hum Pathol. 1986;17:97-99. |
| 4. | Burdick D, Prior JT. Peutz-Jeghers syndrome. A clinicopathologic study of a large family with a 27-year follow-up. Cancer. 1982;50:2139-2146. |
| 5. | Christian CD, Mcloughlin TG, Cathcart ER, Eisenberg MM. Peutz-Jeghers syndrome associated with functioning ovarian tumor. JAMA. 1964;190:935-938. |
| 6. | Wilson DM, Pitts WC, Hintz RL, Rosenfeld RG. Testicular tumors with Peutz-Jeghers syndrome. Cancer. 1986;57:2238-2240. |
| 7. | Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Höglund P. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184-187. |
| 8. | Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, Müller O, Back W, Zimmer M. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38-43. |
| 9. | Gruber SB, Entius MM, Petersen GM, Laken SJ, Longo PA, Boyer R, Levin AM, Mujumdar UJ, Trent JM, Kinzler KW. Pathogenesis of adenocarcinoma in Peutz-Jeghers syndrome. Cancer Res. 1998;58:5267-5270. |
| 10. | Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, Clevers HC. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457-466. |
| 11. | Fan D, Ma C, Zhang H. The molecular mechanisms that underlie the tumor suppressor function of LKB1. Acta Biochim Biophys Sin (Shanghai). 2009;41:97-107. |
| 13. | Boardman LA, Couch FJ, Burgart LJ, Schwartz D, Berry R, McDonnell SK, Schaid DJ, Hartmann LC, Schroeder JJ, Stratakis CA. Genetic heterogeneity in Peutz-Jeghers syndrome. Hum Mutat. 2000;16:23-30. |
| 14. | Lim W, Hearle N, Shah B, Murday V, Hodgson SV, Lucassen A, Eccles D, Talbot I, Neale K, Lim AG. Further observations on LKB1/STK11 status and cancer risk in Peutz-Jeghers syndrome. Br J Cancer. 2003;89:308-313. |
| 15. | Aretz S, Stienen D, Uhlhaas S, Loff S, Back W, Pagenstecher C, McLeod DR, Graham GE, Mangold E, Santer R. High proportion of large genomic STK11 deletions in Peutz-Jeghers syndrome. Hum Mutat. 2005;26:513-519. |
| 16. | Yoo LI, Chung DC, Yuan J. LKB1--a master tumour suppressor of the small intestine and beyond. Nat Rev Cancer. 2002;2:529-535. |
| 17. | Lim W, Olschwang S, Keller JJ, Westerman AM, Menko FH, Boardman LA, Scott RJ, Trimbath J, Giardiello FM, Gruber SB. Relative frequency and morphology of cancers in STK11 mutation carriers. Gastroenterology. 2004;126:1788-1794. |
| 18. | Schumacher V, Vogel T, Leube B, Driemel C, Goecke T, Möslein G, Royer-Pokora B. STK11 genotyping and cancer risk in Peutz-Jeghers syndrome. J Med Genet. 2005;42:428-435. |
| 19. | Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJ, Keller JJ, Westerman AM, Scott RJ, Lim W. Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006;12:3209-3215. |
| 20. | Amos CI, Keitheri-Cheteri MB, Sabripour M, Wei C, McGarrity TJ, Seldin MF, Nations L, Lynch PM, Fidder HH, Friedman E. Genotype-phenotype correlations in Peutz-Jeghers syndrome. J Med Genet. 2004;41:327-333. |
| 21. | Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJ, Keller JJ, Westerman AM, Scott RJ, Lim W. STK11 status and intussusception risk in Peutz-Jeghers syndrome. J Med Genet. 2006;43:e41. |