Published online Oct 28, 2009. doi: 10.3748/wjg.15.5020
Revised: September 9, 2009
Accepted: September 16, 2009
Published online: October 28, 2009
AIM: To compare hepatitis C virus (HCV) titers in patients with chronic hepatitis C with and without exposure to 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins).
METHODS: Medical records were reviewed for 6463 patients with documented HCV infection at a single center between March 2004 and September 2006. Patients with confirmed viremia and meeting inclusion criteria were assigned to one of three groups: Group A (n = 50), dyslipidemic patients with statin usage during HCV RNA polymerase chain reaction (PCR) determination; Group B (n = 49), dyslipidemic patients with prior or future statin usage but not at the time of HCV RNA PCR determination; and Group C (n = 102), patients without statin usage during the study period. The primary analysis explored the effect of statin therapy on HCV viremia. Secondary analyses assessed class effect, dose response, and effect of other lipid-lowering therapies on HCV viral titers.
RESULTS: Median HCV RNA titers did not significantly differ among the three groups (Group A: 4 550 000 IU/mL, Group B: 2 850 000 IU/mL, Group C: 3 055 000 IU/mL). For those subjects with longitudinal assessment of HCV viremia prior to and while on statins, there were no significant differences between pre- and post-HCV viral titers. Additionally, no differences in HCV titers were observed at any dose level of the most prescribed statin, simvastatin. However, hypertriglyceridemia independently correlated with HCV titers, and niacin exposure was associated with significantly lower viral titers (P < 0.05).
CONCLUSION: There was no apparent effect of statins on HCV viral replication in this analysis. Further investigation is warranted to explore the possible antiviral properties of triglyceride-lowering agents and their potential role as adjuncts to standard HCV therapy.
- Citation: Forde KA, Law C, O’Flynn R, Kaplan DE. Do statins reduce hepatitis C RNA titers during routine clinical use? World J Gastroenterol 2009; 15(40): 5020-5027
- URL: https://www.wjgnet.com/1007-9327/full/v15/i40/5020.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5020
| Variables | Median (range) | P-value | ||||
| Group A (n = 50) | Group B (n = 49) | Group C (n = 102) | All | Group A vs Group B | Group A vs Group C | |
| Gender (male/female) | 50/0 | 49/0 | 100/2 | 0.38 | ||
| Race (white/black/other/unknown) | 15/28/0/7 | 11/31/0/7 | 18/64/0/20 | 0.74 | ||
| Ethnicity (hispanic/non-hispanic/unknown) | 2/44/4 | 1/38/3 | 3/78/21 | 0.15 | ||
| Prior interferon-alpha therapy (%) | 14 | 8 | 3 | 0.042 | 0.32 | 0.015 |
| HCV genotype (1/2/3/not-typed) | 34/4/1/1 | 32/5/0/0 | 70/5/0/0 | 0.26 | ||
| Age (yr) | 56 (47-87) | 55 (42-80) | 56 (41-83) | 0.60 | ||
| Body mass index | 29.1 (18.9-47.8) | 28.1 (20.8-43.6) | 26.8 (17.1-41.8) | 0.0021 | 0.24 | 0.001 |
| Albumin (g/dL) | 4 (3-5) | 4 (3-4) | 4 (2-5) | 0.37 | ||
| ALT (U/L) | 46 (11-388) | 40 (12-207) | 53 (15-345) | 0.09 | 0.19 | 0.035 |
| AST (U/L) | 37 (18-199) | 36 (16-136) | 53 (22-304) | < 0.0001 | 0.24 | 0.002 |
| Alkaline phosphatase (U/L) | 74 (28-215) | 79 (44-162) | 77 (40-1106) | 0.23 | ||
| Total bilirubin (mg/dL) | 0 (0-2) | 0 (0-1) | 0 (0-2) | 0.01 | 0.40 | 0.060 |
| Creatinine (mg/dL) | 1 (0-1) | 1 (0-1) | 1 (0-2) | 0.24 | ||
| International normalized ratio | 1 (0-2) | 0 (0-1) | 1 (0-3) | 0.48 | ||
| Platelets (× 1000/mL) | 242 (129-758) | 242 (122-450) | 208 (55-606) | 0.020 | 0.97 | 0.024 |
| Total cholesterol (mg/dL) | 177 (110-304) | 187 (71-277) | 161 (75-260) | 0.0002 | 0.21 | 0.038 |
| HDL cholesterol (mg/dL) | 40 (25-70) | 42 (26-69) | 41 (21-110) | 0.16 | ||
| LDL cholesterol (mg/dL) | 114 (45-222) | 122 (64-187) | 93 (22-173) | < 0.0001 | 0.29 | 0.006 |
| Triglycerides (mg/dL) | 120 (35-384) | 114 (44-443) | 105 (39-467) | 0.31 | ||
Hepatitis C virus (HCV) infection affects approximately 1.8% of the United States population[1,2]. The burden of disease is markedly increased in the United States Veteran population with 4%-19% of veterans being seropositive for antibodies against the virus[3-5]. Pegylated interferon combined with ribavirin is the current standard therapy for HCV and is curative in approximately 40%-50% of patients. However, the adverse effects and contraindications to therapy limit the applicability and utilization of this regimen in many infected persons[6]. The potential sequelae of chronic HCV infection, the limitations of current therapy, and the large economic burden of this disease provide a critical impetus for the pursuit of novel therapeutic agents.
Recent studies using HCV replicon systems suggest a potential therapeutic role for the lipid-lowering agents 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, commonly referred to as statins, in chronic HCV infection. These studies are predicated on cardinal observations of the HCV life cycle. Firstly, HCV virions circulate in plasma in association with low density lipoprotein (LDL) particles. Secondly, the LDL receptor and the high density lipoprotein (HDL) scavenger receptor B1 putatively facilitate HCV entry into hepatocytes[7]. Thirdly, and perhaps more importantly, HCV replication depends on the formation of a “membranous web” replication complex[8,9]. Within this endoplasmic reticulum-based replication complex, host proteins are found to be closely associated with HCV nonstructural proteins. The process which links these host and HCV proteins, termed prenylation, appears to depend on two distinct host protein pools, farnesyl and geranylgeranyl, both protein products of the cholesterol synthesis pathway. Therefore, statins, agents which block the formation of the lipid precursors for prenylation, could theoretically interfere with viral replication[10].
Indeed, in vitro studies using HCV replicon-bearing hepatoma cell lines do suggest that statins inhibit HCV replication by disrupting the formation of viral replication complexes, an effect that can be reversed by the addition of mevalonate or geranylgeraniol, synthetic proteins in the cholesterol pathway[10-12]. Further, the combination of interferon-α and fluvastatin in experimental models exhibited strong synergistic inhibitory effects on HCV RNA replication suggesting that fluvastatin, in particular, but potentially other statins, could be useful as an adjunct to interferon-α based therapy[11]. While the findings from these studies have been invaluable, the applicability to human use remains in question.
The effect of statins on HCV replication in human subjects has been prospectively addressed in two small studies with mixed results[13,14]. O’Leary et al[13] found no reduction in HCV RNA titers at week 4 and week 12 relative to baseline levels. In contrast, another study identified a non-sustained, non-dose-related, reduction in HCV RNA titers in 50% of those treated[14]. Neither study, however, explicitly addressed the efficacy of individual statin drugs, viral genotype, or controlled for exposure to non-statin lipid-lowering agents. In order to examine a larger number of exposed subjects, to assess the relative efficacy of individual statin formulations, to control for potential confounders, and to determine whether further prospective trials might be warranted, we performed a cross-sectional and longitudinal analysis of HCV RNA viral loads in chronic HCV patients who received a statin for therapy of dyslipidemia.
This study protocol was reviewed and approved by the Institutional Review Board of the Philadelphia Veterans Affairs Medical Center (VAMC).
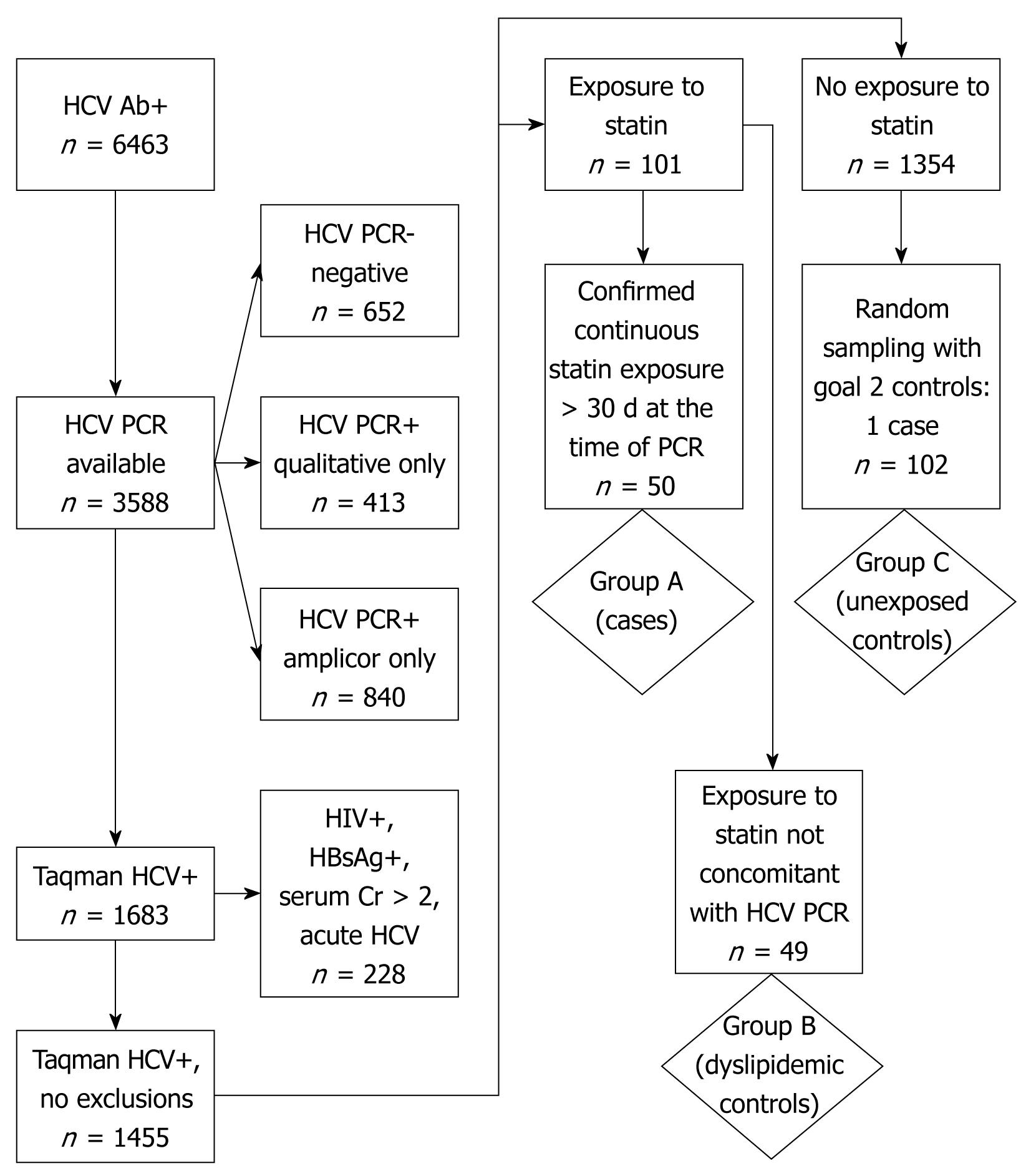
We performed a retrospective analysis of chronic HCV-infected patients who were seen at the Philadelphia VAMC from March 14, 2004 to September 14, 2006 and who had at least one quantitative HCV RNA polymerase chain reaction (PCR) test performed using the Taqman® assay (Roche Diagnostics, Indianapolis, IA). Screening for viral hepatitis is part of routine intake for all primary care clinic patients at the Philadelphia VAMC. Patient level data was extracted from the facility’s Hepatitis C Registry, an automated system that registers all patients with positive HCV antibody testing from clinical laboratory data and facilitates acquisition of additional clinical information from the Computerized Patient Record System (CPRS) and Veterans Health Information Systems and Technology Architecture databases. Charts of patients identified as HCV antibody-positive were queried as to the presence and type of confirmatory PCR testing. Those without confirmatory PCR testing and those without detectable viremia by quantitative Taqman® HCV PCR assay were excluded. Patients were also excluded if HIV antibody, HIV RNA and/or HBsAg were positive or if antiretroviral drugs were present in the medication profile. Patients with acute HCV, defined as seroconversion within 2 years of exposure, and patients with chronic kidney disease, defined as serum creatinine greater than 2 mg/dL, were also excluded.
Pharmacy records were then examined to identify the start and stop dates of HMG-CoA reductase inhibitors, niacin, clofibrate, ezetimibe, and interferon-α preparations. Interferon-α based therapy was confirmed through review of progress note documentation in the CPRS. HCV RNA titers obtained during interferon-α therapy were annotated. Utilizing refill data and chart review, patients in whom the HCV RNA determination date(s) occurred at least 30 d after initiation of HMG-CoA reductase inhibitor therapy, in whom exposure to the statin drug spanned at least 60 d, and in whom the duration of statin therapy included the date of HCV RNA determination were designated as Group A. Two control groups who also met the inclusion criteria were selected for comparison. Subjects with hypercholesterolemia for whom a statin medication was prescribed but either had HCV RNA titers drawn prior to the statin initiation date or in whom statin therapy was discontinued at least 60 d prior to the HCV RNA determination were selected as dyslipidemic controls Group B. Group C was chosen from a pool of HCV positive subjects without documented statin exposure during the evaluation period. Analysis of this group was performed with and without exclusion of subjects with total cholesterol levels greater than 200 mg/dL. Using the date of closest correlation to the HCV RNA titer obtained, laboratory data, including basic chemistries, liver associated enzymes, coagulation panels, and lipid profiles were extracted for each group. The longitudinal results of HCV RNA titers during ongoing statin therapy were also recorded for those patients in Group A with more than one HCV RNA determination within the study period.
Comparisons of frequency data were performed with χ2 or Fisher’s exact testing as appropriate. All group-wise comparisons were performed using non-parametric tests including Kruskal-Wallis or Mann-Whitney U tests. Regression analysis was conducted to explore the effect of potential confounders on the primary study endpoint, HCV RNA titers. All analyses were conducted with JMP 7 software (SAS Institute, Cary, NC) and/or STATA 9.2 (College Station, TX). P-values < 0.05 were considered significant.
A total of 6463 patients were found to be HCV antibody-positive (+). Fifty HCV-infected patients who met criteria for statin exposure with concomitant HCV RNA determination (Group A), 49 HCV-infected dyslipidemic patients not on a statin at the time of HCV RNA determination (dyslipidemic controls, Group B) and 102 statin-unexposed HCV-infected controls (Group C), were analyzed (Figure 1).
Patients in the three groups were similar in terms of age, gender, race, ethnicity, HCV genotype, serum albumin, alanine aminotransferase (ALT), alkaline phosphatase, total bilirubin, creatinine, international normalized ratio, platelets, HDL cholesterol, triglyceride levels and exposure to non-statin lipid-lowering agents (Table 1). Among Group A, the median duration from first statin exposure to HCV RNA determination was 288 d (range 34-1435, data not shown). Group A, when compared to Group C, had a significantly higher median body mass index (BMI) (29.1 vs 26.8, P < 0.01), platelet count (243 × 1000/mL vs 208 × 1000/mL, P < 0.05), total cholesterol level (177 mg/dL vs 161 mg/dL, P < 0.01), and LDL levels (114.3 mg/dL vs 93.4 mg/dL, P < 0.01). However, there were no significant differences between Group A and Group B. Group C had a significantly higher median aspartate aminotransferase level (54 U/L vs 37 U/L, P < 0.01) and ALT level (53 U/L vs 46 U/L, P < 0.05) when directly compared with Group A.
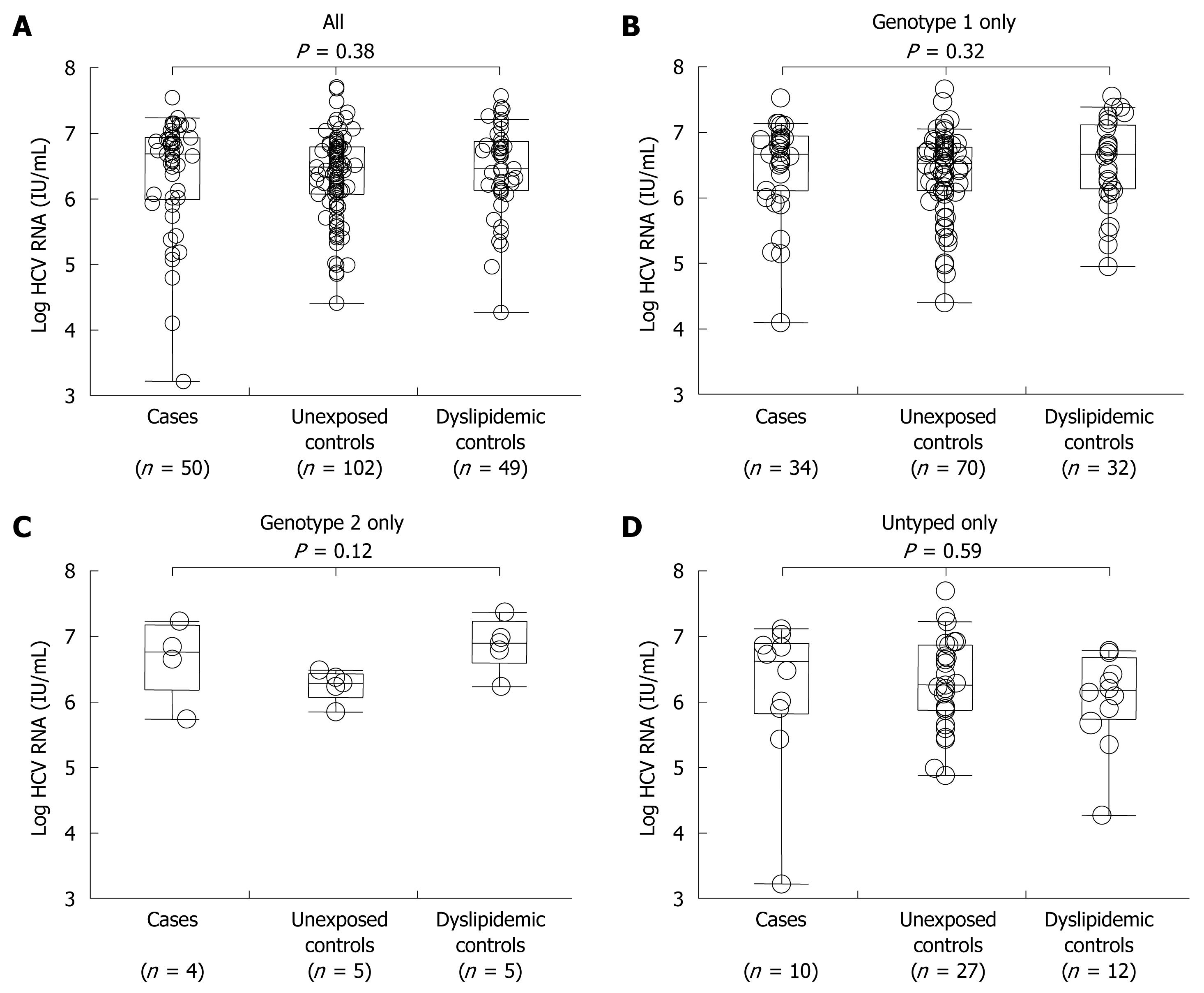
Median HCV RNA titer in Group A was 4 550 000 IU/mL vs 2 850 000 IU/mL in Group B vs 3 055 000 IU/mL in Group C (Figure 2A). The similarity in serum titers suggest that in this cohort there was no evidence that as a class HMG-CoA reductase inhibitors exhibit antiviral properties in vivo. To confirm that there was no genotype-specific effect of HMG-CoA reductase inhibitors, we analyzed each known HCV genotype separately for genotype 1, genotype 2 and untyped subjects in all groups. We again found no significant differences between these groups for any genotype (Figure 2B-D).
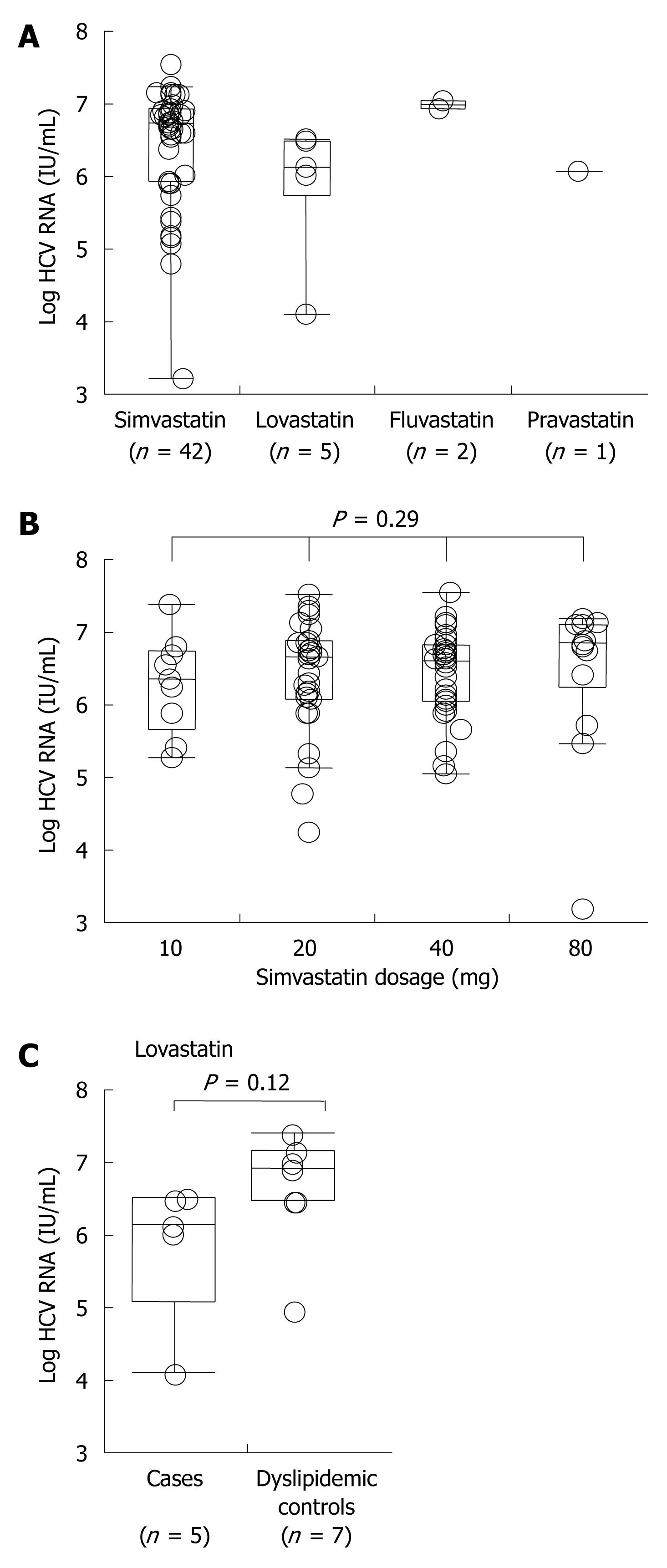
Among Group A, 42 (84%) received simvastatin, 5 (10%) lovastatin, 2 (4%) pravastatin and 1 (2%) fluvastatin. When comparing HCV RNA titers among patients receiving any of the four statin agents, there were no differences observed (Figure 3A). Furthermore, there was no apparent antiviral effect of simvastatin, the most prescribed statin in this cohort, at any dose level (Figure 3B). However, when comparing the two most commonly used agents in our cohort, there was a trend towards lower HCV RNA titers in subjects who received lovastatin relative to simvastatin. To further investigate whether or not the trend towards lower HCV RNA titers in lovastatin users might be significant, we compared HCV RNA titers in active lovastatin users in Group A and former or future lovastatin users in Group B. However, no significant trend towards a lower HCV viral load could be identified (Figure 3C). Thus, there was no class effect of HMG-CoA reductase inhibitors and further conclusions regarding specific statin formulations cannot be made.
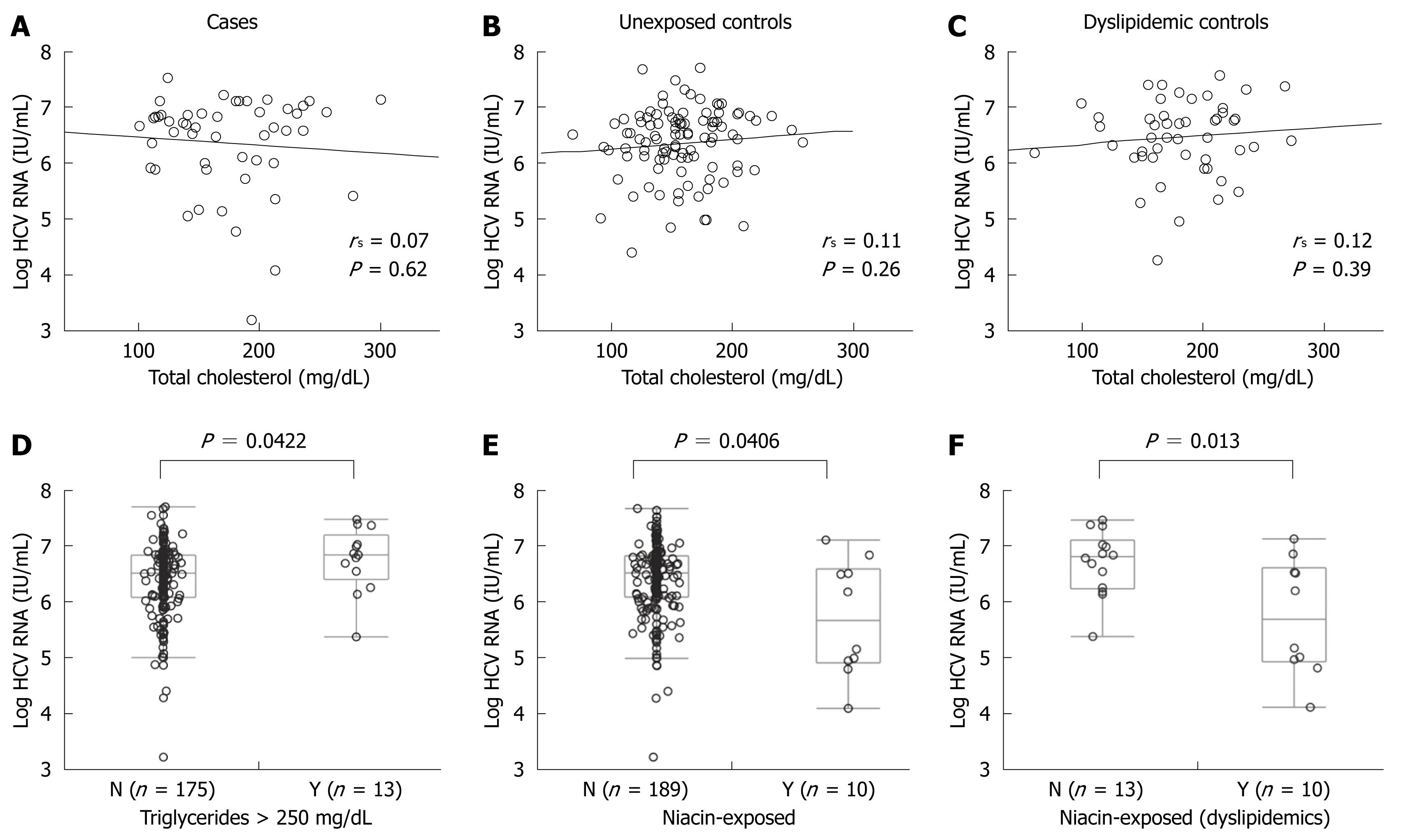
The lack of apparent anti-viral effect of statins theoretically could have resulted from neutralization from a pro-viral effect of hypercholesterolemia rather than a lack of effect of the statin in individual patients. To control for this potential confounder, we correlated viral titers and total cholesterol for Group A (Figure 4A), Group B (Figure 4B), and Group C subjects (Figure 4C) and found no evidence of a relationship between total serum cholesterol and HCV RNA titers in any group. Further, there was no correlation of HCV RNA titers and HDL, LDL, or triglyceride levels (data not shown). Thus, hypercholesterolemia itself did not appear to mediate a pro-viral effect or to be related to cholesterol metabolism in a fashion that may negate any anti-viral effect of statin preparations.
As noted above, Group A subjects had a significantly higher median BMI (29.1 vs 26.8, P < 0.01) than Group C, but no significant difference in BMI was found between Group A and B. To assess any potential interaction of BMI on HCV RNA titers, we performed regression analysis that demonstrated no association between BMI and HCV RNA titer in either Group A or Group C.
In order to assess serum triglyceride levels as a confounding variable, we analyzed the association between serum triglycerides as well as triglyceride-directed therapy on HCV RNA titers. As shown in Table 1, 6/50 in Group A, 2/49 in Group B and 5/102 in Group C received non-statin lipid lowering therapy at the time of HCV RNA determination. Of these, a total of 10 exposures were to niacin (5/6 in Group A, 2/2 in Group B, and 3/5 in Group C). After excluding patients on niacin and gemfibrozil, the presence of triglyceride levels greater than 250 mg/dL was associated with a higher median HCV RNA titer (> 250 mg/dL: 6 760 000 IU/mL vs < 250 mg/dL: 3 130 000 IU/mL, P < 0.05, Figure 4D) and triglycerides were weakly but significantly correlated with HCV viral titers (R2 = 0.023, P < 0.05, data not shown). After excluding two patients taking gemfibrozil, niacin-exposure, irrespective of statin exposure, was associated with lower median HCV RNA titers (exposed: median 835 000 IU/mL vs unexposed: 3 350 000 IU/mL, P < 0.05, Figure 4E). Compared to 13 patients with untreated hypertriglyceridemia (defined as triglycerides > 250 mg/dL), niacin-exposure was associated with a 1.16-log reduction in median HCV RNA titer (P < 0.05). Similar analyses for fibric acid derivatives or ezetimibe were not possible due to the limited number of exposed patients.
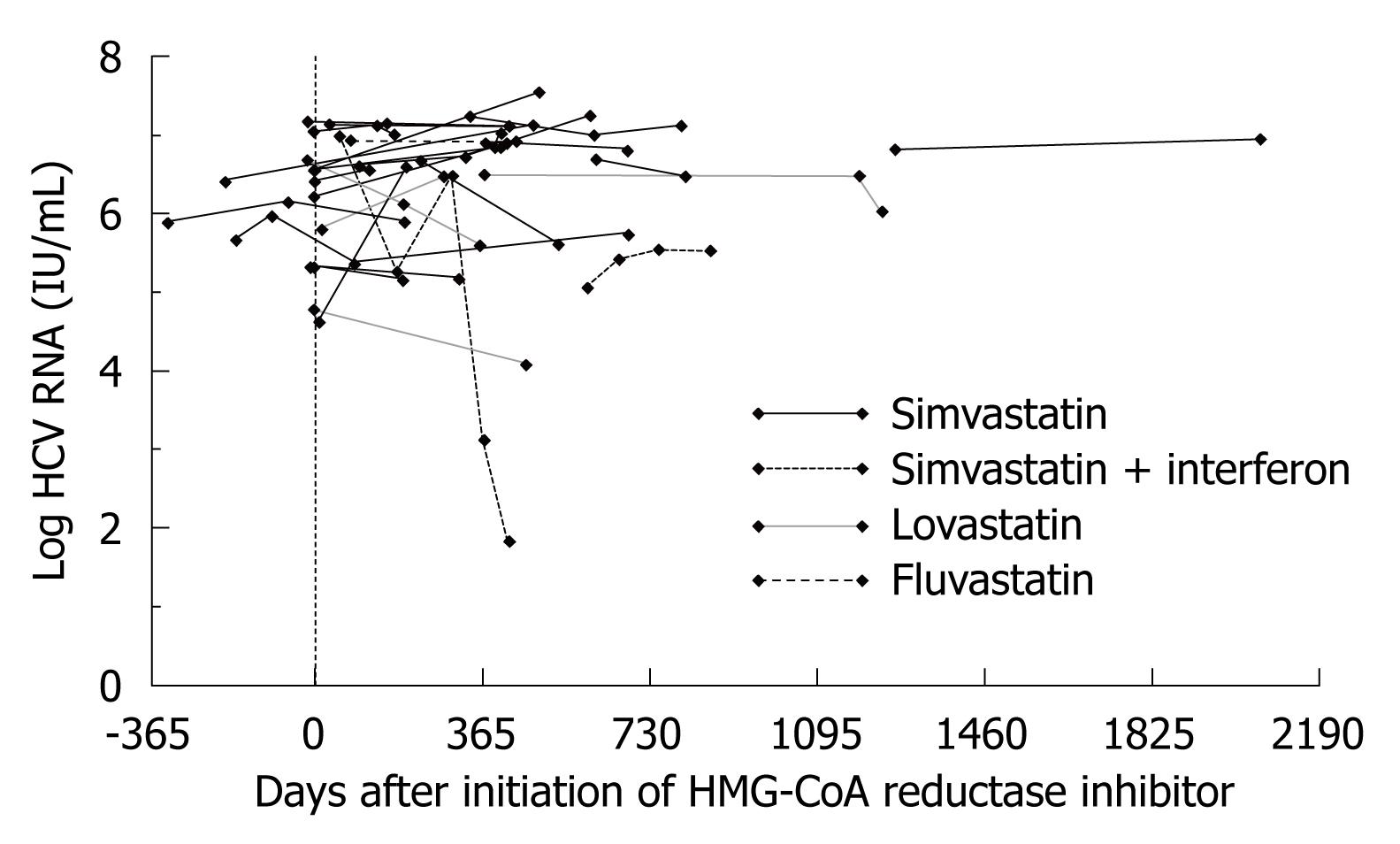
Twenty-eight Group A subjects (23 simvastatin, one fluvastatin and four lovastatin) had more than one determination of HCV RNA titer, allowing for longitudinal assessment of changes in HCV viremia during the duration of HMG-CoA reductase exposure. In the vast majority of non-interferon-treated subjects, there was either a modest increase or no change in HCV RNA titers during such therapy (Figure 5). In 11 of these 28 subjects, an HCV RNA determination was made prior to and after the institution of statin therapy. Within this subgroup, there was no significant difference between pre- and post-statin levels of HCV viremia. In two of three lovastatin-exposed subjects with initial HCV RNA testing within 1 mo (range: -15 to +17 d) of initiation of interferon, HCV RNA titers declined by 0.7-1.1 log between 9-16 mo. Only two subjects with repeated assessments were exposed to interferon during the observation period. These data imply that statin exposure in this cohort was not associated with significant inhibition of viral replication for individuals with longitudinal follow-up.
The sustained virologic response rate of chronic hepatitis C in registration trials with currently approved interferon-α based antiviral therapy ranges from 44%-54%[15,16], but varies widely by genotype, ethnicity and underlying histology. In practice, at centers similar to the study site, HCV treatment response rates range from 20% for genotype 1 to 43%-52% for genotypes 2-3[17]. Suboptimal response and significant toxicity continue to spur the development of novel anti-HCV therapies.
The capacity of HMG-CoA reductase inhibitors to impede HCV replicon replication in vitro in a dose-dependent fashion raised hope that this commonly prescribed and acceptably safe class of medications could serve as an adjunct to standard interferon-based therapy. Data from such in vitro studies further demonstrated a hierarchy of statin-induced viral inhibition, with the greatest effect demonstrated with fluvastatin followed by atorvastatin, simvastatin, and lovastatin, respectively. These in vitro findings prompted a pilot study in which 10 subjects with chronic hepatitis C and laboratory evidence of dyslipidemia were treated with atorvastatin 20 mg daily. However, no significant changes in HCV RNA titers were demonstrated at the atorvastatin dose administered though such subjects did have a significant lowering of LDL and total cholesterol[13]. This first study did not examine the effect of fluvastatin, the most potent statin identified in in vitro experiments. A more recent small, uncontrolled study of 22 patients given fluvastatin at doses ranging from 20-80 mg/d found transient, 0.5-log reductions in HCV RNA titers in 50% of treated subjects[14], but did not correlate these responses with the lipid lowering therapeutic effect of statins or specifically explore viral genotypes and other medications used.
In order to validate the need for further prospective study of the effect of statins on HCV viral replication in clinical practice, we performed a cross-sectional study of a larger number of subjects and controlled for potential confounders such as viral genotype, cholesterol levels, triglyceride levels and exposure to other lipid lowering medications. We found exposure to different statin preparations, primarily simvastatin, during routine clinical use, was not associated with a change in HCV viral titers. In a limited number of subjects with longitudinal measures of HCV viral load pre- and post-initiation of statin therapy, our data suggested that there was no evidence of clinically significant change in HCV RNA titers. Specifically for simvastatin, the statin for which we had the most data, we were unable to show a dose-dependent association with reduced HCV titers, contrasting with in vitro studies[11]. Given the small number of fluvastatin-exposed subjects at our institution (n = 2), we were unfortunately not able to analyze the effect of exposure to the most potent in vitro inhibitor.
A plausible explanation for the discrepancy between our in vivo and others’in vitro results may rest with the pharmacokinetic properties of statins. There is a significant first pass effect for all statins with the exception of pravastatin[18]. Intrahepatic concentrations of statins with routine use, however, have not been to our knowledge well documented in the literature. Serum levels of such agents after prolonged therapy are significantly lower than the statin concentrations that were used in replicon systems. For example, the maximal serum concentration of fluvastatin dosed at 40 mg daily is approximately 0.589 μmol/L[19], approximately 10-fold lower than effective statin concentrations for inducing viral inhibition in the replicon systems[10-12].
Additionally, replicon-bearing cell lines are highly adapted and behave quite differently from in vivo hepatocytes. For instance, Huh7 cells exposed to interferons are exquisitely responsive to the agent regardless of viral genotype[20]. This is in direct contrast to the disparate rates of sustained virologic response observed clinically with interferon therapy. It is possible that the adaptations that confer interferon sensitivity also confer statin sensitivity, explaining the in vitro results. Alternatively, the specific dependence on prenylation of nonstructural proteins for establishment of the viral replication complex might be a feature of cell-culture models for which alternative pathways may be present in vivo. The HCV might also develop resistance mutations under selection pressure induced by statin therapy, an effect that could be demonstrated via sampling at regular intervals early after initial statin exposure for early viral load changes and sequence evolution. Lastly, pro-viral effects of statins might occur via induction of LDL-receptor expression which may paradoxically facilitate viral uptake into uninfected hepatocytes[21].
Another possible explanation for the lack of difference between our study groups could be the presence of a significant confounding variable. For example, obesity, which is associated with non-response to interferon-based antiviral therapy[22], and hypercholesterolemia, were significantly more prevalent in Groups A and B than C. We, however, did not find within any of the groups an association of HCV RNA titers with height, weight or body-mass index (data not shown) nor with total cholesterol; therefore we do not believe that differences in these variables could explain our negative findings. Since hypertriglyceridemia was directly associated with viral titer, the excess of hypertriglyceridemia cases in Group A could contribute to a type II error. However, when we controlled for triglycerides in the regression analysis, statin exposure remained insignificantly associated with HCV RNA titer.
While not powered for an analysis of non-statin lipid lowering medications and their effect on HCV viral load, our analysis unexpectedly identified a possible direct association of triglycerides and viremia and a suggestion that niacin may have antiviral properties in vivo. Recent work with human serum[23] and with primary hepatocytes[24] suggests that HCV is co-secreted with VLDL, implicating triglyceride metabolism as an additional critical step in the viral lifecycle. Given these preliminary findings as well as a biologically plausible mechanism for the action of triglyceride lowering medications on HCV replication, these findings merit further investigation in a larger dataset and, if confirmed, in a prospective clinical trial.
There are several limitations of this analysis that we would like to acknowledge. First, the cross-sectional design inherently precludes determining causality and is inherently weaker than prospective evaluation. Further, given the limited number of subjects with measurements prior to and after the initiation of statin therapy, we cannot definitively rule out an association between statins and reduction in HCV viral replication. Secondly, the sample size remained relatively small after applying our exclusion criteria, raising the possibility of a type II error and specifically that of finding no association when one does indeed exist. Thirdly, there were no direct measurements of patient adherence with prescribed statin therapy, an important factor since poor adherence may make the medication in question appear to be less efficacious. Fourthly, the preferred statin agent on the formulary during the observation period was not the most highly active agent in vitro. Lastly, there is a small possibility that patients in Group B could have received a statin agent prescribed by a non-VA physician, which was not recorded in the VA electronic medical record. Since there is significant financial incentive for most veterans to obtain medications through the VA, we believe the impact of this factor is quite small.
In summary, in this single center, retrospective analysis, there was no evidence of an apparent effect of statins on HCV viral replication. Unexpectedly, we found that triglyceride lowering agents such as niacin may have HCV antiviral properties in vivo. Therefore, we suggest exploration of this result. Additionally, the potential antiviral efficacy of drugs such niacin in chronic hepatitis C as adjuncts to interferon-based therapies merit further investigation.
Interferon and ribavirin are the mainstay of treatment for chronic hepatitis C infection. Unfortunately, this combination is only effective in approximately 50% of all-comers. Therefore, novel therapies must be explored. Cardinal observations of the hepatitis C virus (HCV) life cycle have demonstrated that the lipid metabolism pathway is implicated in viral entry and replication within the hepatocyte. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, referred to commonly as statins, have been shown to not only disrupt lipid metabolism within the hepatocyte but also halt HCV viral replication in in vitro models of chronic HCV infection. Relatively few studies have explored the effect of statins on HCV viral replication in vivo.
Though in vitro studies using HCV replicon-bearing hepatoma cell lines do suggest that statins inhibit HCV replication, the applicability of use in humans with chronic hepatitis C infection remains in question. In this study, the authors attempt to, but are unsuccessful in, demonstrating a significant reduction in HCV viral titers with routine use of statins in a cohort of patients infected with hepatitis C.
The results did not suggest an effect of statins on HCV viral replication. However, the investigators did find preliminarily that triglyceride lowering agents may in fact lower levels of HCV viremia. Though significant, this finding was noted in a small number of patients and given that the study was not powered for this question, this should be viewed as hypothesis generating.
By continuing to explore potential adjunct therapies to current HCV anti-viral therapies, it is likely that some highly effective adjunct(s) will be discovered. This study, while not demonstrating a significant effect of statins in this cohort, does provide some preliminary data to support further investigation of lipid altering medications and their potential effect on HCV.
The process which links host and HCV proteins during intrahepatic viral replication is termed prenylation. Prenylation is dependent on two distinct host protein pools, farnesyl and geranylgeranyl, both protein products of the cholesterol synthesis pathway.
This is a well written manuscript. The study suffers from the limitations of a retrospective cohort study and may have not detected a difference in HCV viral titers because longitudinal assessment was not made for all subjects prior to and after initiation of statin therapy. The authors need to comment on these limitations in the discussion.
This material is based upon work supported in part by the Office of Research and Development, Department of Veterans Affairs and with the resources and the use of facilities at the Philadelphia VA Medical Center. We also thank Kyong-Mi Chang, MD for her review of the manuscript and for development of the hepatitis C registry at the Philadelphia VAMC.
Peer reviewer: Dr. Sk Md Fazle Akbar, Assistant Professor, Third Department of Internal Medicine, Ehime University School of Medicine, Shigenobu-Cho, Ehime 791-0295, Japan
S- Editor Li LF L- Editor Logan S E- Editor Zheng XM
| 1. | McHutchison JG, Dev AT. Future trends in managing hepatitis C. Gastroenterol Clin North Am. 2004;33:S51-S61. |
| 2. | Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26:62S-65S. |
| 3. | Dominitz JA, Boyko EJ, Koepsell TD, Heagerty PJ, Maynard C, Sporleder JL, Stenhouse A, Kling MA, Hrushesky W, Zeilman C. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41:88-96. |
| 4. | Sloan KL, Straits-Tröster KA, Dominitz JA, Kivlahan DR. Hepatitis C tested prevalence and comorbidities among veterans in the US Northwest. J Clin Gastroenterol. 2004;38:279-284. |
| 5. | Cheung RC. Epidemiology of hepatitis C virus infection in American veterans. Am J Gastroenterol. 2000;95:740-747. |
| 6. | McHutchison JG, Fried MW. Current therapy for hepatitis C: pegylated interferon and ribavirin. Clin Liver Dis. 2003;7:149-161. |
| 7. | Barth H, Liang TJ, Baumert TF. Hepatitis C virus entry: molecular biology and clinical implications. Hepatology. 2006;44:527-535. |
| 8. | Egger D, Wölk B, Gosert R, Bianchi L, Blum HE, Moradpour D, Bienz K. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J Virol. 2002;76:5974-5984. |
| 9. | Lundin M, Monné M, Widell A, Von Heijne G, Persson MA. Topology of the membrane-associated hepatitis C virus protein NS4B. J Virol. 2003;77:5428-5438. |
| 10. | Ye J, Wang C, Sumpter R Jr, Brown MS, Goldstein JL, Gale M Jr. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc Natl Acad Sci USA. 2003;100:15865-15870. |
| 11. | Ikeda M, Abe K, Yamada M, Dansako H, Naka K, Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117-125. |
| 12. | Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci USA. 2005;102:2561-2566. |
| 13. | O'Leary JG, Chan JL, McMahon CM, Chung RT. Atorvastatin does not exhibit antiviral activity against HCV at conventional doses: a pilot clinical trial. Hepatology. 2007;45:895-898. |
| 14. | Bader T, Fazili J, Madhoun M, Aston C, Hughes D, Rizvi S, Seres K, Hasan M. Fluvastatin inhibits hepatitis C replication in humans. Am J Gastroenterol. 2008;103:1383-1389. |
| 15. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. |
| 16. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. |
| 17. | Backus LI, Boothroyd DB, Phillips BR, Mole LA. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46:37-47. |
| 18. | Hatanaka T. Clinical pharmacokinetics of pravastatin: mechanisms of pharmacokinetic events. Clin Pharmacokinet. 2000;39:397-412. |
| 19. | Chong PH, Seeger JD, Franklin C. Clinically relevant differences between the statins: implications for therapeutic selection. Am J Med. 2001;111:390-400. |
| 20. | Frese M, Pietschmann T, Moradpour D, Haller O, Bartenschlager R. Interferon-alpha inhibits hepatitis C virus subgenomic RNA replication by an MxA-independent pathway. J Gen Virol. 2001;82:723-733. |
| 21. | Lonardo A, Loria P, Bertolotti M, Carulli N. Statins and HCV: a complex issue. Hepatology. 2007;45:257. |
| 22. | Walsh MJ, Jonsson JR, Richardson MM, Lipka GM, Purdie DM, Clouston AD, Powell EE. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529-535. |
| 23. | Nielsen SU, Bassendine MF, Burt AD, Martin C, Pumeechockchai W, Toms GL. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J Virol. 2006;80:2418-2428. |
| 24. | Nahmias Y, Goldwasser J, Casali M, van Poll D, Wakita T, Chung RT, Yarmush ML. Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology. 2008;47:1437-1445. |