Published online Jan 28, 2009. doi: 10.3748/wjg.15.478
Revised: November 7, 2008
Published online: January 28, 2009
AIM: To compare the histologic features of the liver in intrahepatic neonatal cholestasis (IHNC) with infectious, genetic-endocrine-metabolic, and idiopathic etiologies.
METHODS: Liver biopsies from 86 infants with IHNC were evaluated. The inclusion criteria consisted of jaundice beginning at 3 mo of age and a hepatic biopsy during the 1st year of life. The following histologic features were evaluated: cholestasis, eosinophilia, giant cells, erythropoiesis, siderosis, portal fibrosis, and the presence of a septum.
RESULTS: Based on the diagnosis, patients were classified into three groups: group 1 (infectious; n = 18), group 2 (genetic-endocrine-metabolic; n = 18), and group 3 (idiopathic; n = 50). There were no significant differences with respect to the following variables: cholestasis, eosinophilia, giant cells, siderosis, portal fibrosis, and presence of a septum. A significant difference was observed with respect to erythropoiesis, which was more severe in group 1 (Fisher’s exact test, P = 0.016).
CONCLUSION: A significant difference was observed in IHNC of infectious etiology, in which erythropoiesis was more severe than that in genetic-endocrine-metabolic and idiopathic etiologies, whereas there were no significant differences among cholestasis, eosinophilia, giant cells, siderosis, portal fibrosis, and the presence of a septum.
- Citation: Bellomo-Brandao MA, Escanhoela CA, Meirelles LR, Porta G, Hessel G. Analysis of the histologic features in the differential diagnosis of intrahepatic neonatal cholestasis. World J Gastroenterol 2009; 15(4): 478-483
- URL: https://www.wjgnet.com/1007-9327/full/v15/i4/478.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.478
The frequency of cholestatic jaundice is difficult to evaluate with certainty, but varies between 1:2500 and 1:5000 newborns[123]. The initial approach in the diagnosis of cholestasis is to distinguish between intrahepatic and extrahepatic causes, as the latter etiology requires early surgical intervention[4]. In general, intrahepatic neonatal cholestasis (IHNC) represents 2/3 of the cases of neonatal cholestasis[56789]. The most common causes of IHNC are of infectious origin[101112]. In septicemia, manifestations of hepatic origin represent only one component of the involvement of multiple organs, of which adequate treatment offers the best chance of recovery[13]. Any serious bacterial infections during the neonatal period can result in jaundice[14]; however, there seems to be a more frequent association with urinary tract infections, especially when the pathogen is E. coli[15]. In addition, other infections have been observed, such as syphilis, toxoplasmosis, rubella, and cytomegalovirus (CMV)[1617181920].
Despite the many possible etiologies for IHNC[5], 13%-78% of the cases have been reported to be idiopathic[21222324]. Idiopathic IHNC implies that the liver suffers inflammatory alterations of unknown cause, with no evidence of blockage of the biliary tree, and infectious agents or metabolic errors have been ruled out[12526]. There are cases of idiopathic IHNC which are considered spontaneous in which there is familial recurrence, therefore sporadic cases could possibly consist of a viral injury or another environmental factor that affect the transitory form of the immature liver of the newborn; however, the characteristics are similar in both cases[1327].
Liver biopsy is currently used to confirm the clinical diagnosis and to assess the degree of necroinflammatory injury or fibrosis. Most studies of percutaneous liver biopsy are retrospective analyses and the aim is usually to differentiate biliary atresia from neonatal hepatitis[12212829303132].
There are no data available in the literature pertaining to the histologic features present in neonatal hepatitis to aid in the differential diagnosis of IHNC. The objectives of the present study were to analyze and compare the histologic features of the liver in IHNC of infectious, genetic-endocrine-metabolic, and idiopathic etiologies, in the search for features which can facilitate the diagnostic process.
Eighty-six patients submitted to liver biopsy during IHNC investigation between March 1982 and December 2005, 72 from the State University of Campinas Teaching Hospital (UNICAMP) and 14 from the Children’s Institute of the University of São Paulo (USP). Among the 86 hepatic biopsies, 5 were surgical and 81 were percutaneous. The inclusion criteria consisted of jaundice beginning at 3 mo of age and hepatic biopsy during the first year of life.
In order to establish the etiology of IHNC, the following were reviewed: serum alpha-1-antitrypsin, sweat sodium and chloride test, innate metabolic errors in urine, polymerase chain reaction for CMV antigenemia, and serology for CMV, human immunodeficiency virus, hepatitis B virus (HBV), hepatitis C virus (HCV), Epstein-Barr virus, rubella virus, Toxoplasma gondii, and T. pallidum.
Seven histologic variables were evaluated by means of an investigation protocol: cholestasis, eosinophilia in the inflammatory infiltrate, presence of giant cells, erythropoiesis, siderosis, portal fibrosis and the presence of a septum. Siderosis and cholestasis were evaluated by Perls’ staining and portal fibrosis and the presence of a septum by Masson staining.
The histologic variables (cholestasis, eosinophilia in the inflammatory infiltrate, presence of giant cells, erythropoiesis, siderosis and portal fibrosis) were classified according to the degree of intensity using a grading system (Table 1). The histologic variable, septum was classified by: presence or absence.
| Mild | Moderate | Severe | |
| Cholestasis | Biliary pigment deposits in few hepatocytes of zone 3 acini | Hepatocytes with biliary pigment in two zone 3 acini associated with the presence of rare canalicular bilirubinostasis | The majority of the hepatocytes with biliary pigment biliary associated with several canalicular bilirubinostasis |
| Eosinophilia in the inflammatory infiltrate | Rare eosinophils in few space-porta | Some eosinophils in many space-porta and rare in the parenchyma | Many eosinophils in all the space-porta and several in the parenchyma |
| Presence of giant cells | Occurring in a maximum of 30% of the hepatocytes | Between 30% and 60% of the hepatocytes | > 60% of the hepatocytes |
| Erythropoiesis | Rare groupings of erythroblasts | Some groupings of erythroblasts | Groupings of erythroblasts and megakaryocytes |
| Siderosis | Deposits of ferric pigment in only a few Kupffer cells | Deposits of ferric pigment in Kupffer cells and a few hepatocytes | Deposits of ferric pigment in the majority of Kupffer cells and many hepatocytes |
| Portal fibrosis | Discrete widening of some space-porta | Widening of some space-porta | Widening of all space-porta |
The present research study was approved by the medical research ethics committees of both institutions. Informed consent was not required because liver biopsies were performed during the course of clinical evaluation.
In order to verify associations between categorical variables, the χ2 test was used. When the expected values were < 5, the Fisher’s exact test was used[33]. Significance was established as P ≤ 0.05 in all tests. The computer software used was SAS for Windows, version 8.02 (SAS Institute Inc., Cary, NC, USA).
Based on the etiology of IHNC, the patients were classified into three groups: group 1 (infectious; n = 18), group 2 (genetic-endocrine-metabolic; n = 18), and group 3 (idiopathic; n = 50). The etiologies of IHNC are presented in Table 2.
| Groups | Etiology | Number of cases |
| 1 | Neonatal sepsis | 6 |
| Cytomegalovirus | 6 | |
| Urinary tract infection | 3 | |
| Syphilis | 1 | |
| Toxoplasmosis | 2 | |
| 2 | Alpha1-antitripsyn deficiency | 2 |
| Other metabolic diseases | 6 | |
| Galactosemia | 2 | |
| Alagille syndrome | 2 | |
| Byler’s disease | 1 | |
| Cystic fibrosis | 1 | |
| Secondary to use of parenteral nutrition | 1 | |
| Down’s sindrome | 1 | |
| Panhypopituitarism | 2 | |
| 3 | Idiopathic | 50 |
| Total | 86 |
Twenty-seven patients were females and 59 were males. Patients were predominantly boys in all 3 groups (P = 0.407).
The mean age at the time of liver biopsy was as follows: group 1, 2 mo and 15 d (range, 1-6 mo and 9 d); group 2, 2 mo and 15 d (range, 1-6 mo and 8 d); and group 3, 2 mo and 24 d (range, 13 d-9 mo and 5 d). There were no statistical differences among the groups (P = 0.428).
Table 3 shows values for birth weight, weight during the first medical visit and stature at birth for the groups. There were no significant differences among the groups according to the variables: weight during the first medical visit and stature at birth. However, a significant difference was observed for birth weight, which was lower in group 1 in relation to groups 2 and 3 (P = 0.014).
| Group 1 | Group 2 | Group 3 | P | |
| Birth weight (g) | 2160 | 2780 | 2750 | 0.014 |
| (SD = 650) | (SD = 594) | (SD = 767) | ||
| Weight during the first medical visit (g) | 3040 | 3567 | 3970 | 0.105 |
| (SD = 1014) | (SD = 1170) | (SD = 1074) | ||
| Stature at birth (cm) | 44.5 | 47 | 48 | 0.373 |
| (SD = 4.26) | (SD = 3.51) | (SD = 5.22) |
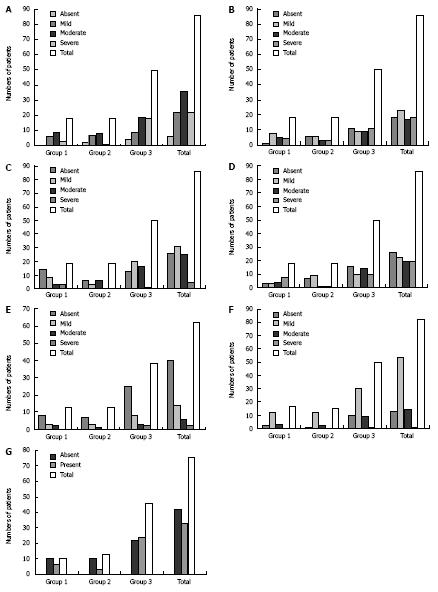
The degree of cholestasis was not significantly different between the 3 groups studied (P = 0.078; Figure 1A). The presence of giant cells, graded as absent, mild, moderate, and severe in groups 1, 2, and 3 did not show any significant differences (P = 0.144; Figure 1B).
The presence of eosinophils in the inflammatory infiltrate did not differ when the genetic-endocrine-metabolic and/or idiopathic groups were compared (P = 0.056; Figure 1C). A significant difference was observed for to the variable, erythropoiesis in group 1 (P < 0.05; Figure 1D).
With respect to siderosis, there was no correlation with the etiology of IHNC (P = 0.973; Figure 1E).
With respect to progression of IHNC to chronic stages, portal fibrosis (Fisher’s exact test P = 0.86) and the presence of a septum (χ2 = 3.83; P = 0.147) were not related to the etiology of IHNC (Figure 1F and G).
Liver biopsy is recommended for the diagnosis of cholestasis of unknown etiology. The interpretation of a single liver biopsy in a child with neonatal cholestasis is limited by the dynamics of the disease. Interpretation of the biopsy findings is pathologist-dependent and requires experience[433].
Most studies involving percutaneous liver biopsies are retrospective analyses, using the clinical course, surgical or autopsy results as a gold standard. However, the goal is usually to differentiate biliary atresia from neonatal hepatitis[212829303132].
Many cholestatic conditions are expressed differently with time. Liver biopsy can also provide disease-specific findings. Examples include PAS-positive granules in alpha-1 antitrypsin deficiency, paucity of ducts in Alagille syndrome, necroinflammatory duct lesions in sclerosing cholangitis, and other findings that are relatively specific for metabolic and storage diseases[43435].
Hepatic erythropoiesis was more evident in INHC of infectious etiology. This was not clear in the idiopathic group, which was likely due to the participation of an undetected infectious agent. On the other hand, hepatic erythropoiesis strongly suggests an infection.
Fetal hematopoiesis has an onset in the beginning of gestation. Initially, hematopoiesis is restricted to the vitelline bag up to the gestational age of 6-8 wk, when the liver starts to be the predominant organ of production. At 20 wk, the bone marrow becomes the main organ of hematopoiesis and remains as the primary reservoir for the circulating population of immune cells. However, when there are infectious processes, hepatic production persists[34].
The presence of eosinophilia in the inflammatory infiltrate, an important marker of neonatal hepatitis[35], did not show significant differences when compared to the genetic-endocrine-metabolic and/or idiopathic groups. The degree of cholestasis, a morphologic variable, which is extremely important for the differential diagnosis of extra- and intra-hepatic cholestasis, did not show a significant difference between the 3 patient groups. Similar findings were observed in relation to progression to chronic stages, demonstrating that portal fibrosis or a septum is not related to the etiology of the process. In relation to siderosis and the presence of giant cells, our findings did not demonstrate any correlation with the etiology of IHNC.
In conclusion, there are no data available in the literature analyzing the histologic features usually present in neonatal hepatitis in the differential diagnosis of IHNC. There were no significant differences among different etiologies of IHNC in relation to the following histologic features: cholestasis, eosinophilia, giant cells, portal fibrosis, the presence or absence of a septum, and siderosis. A significant difference was observed in IHNC of infectious etiology, which presented with more severe erythropoiesis than the genetic-endocrine-metabolic and idiopathic etiologies.
In the present study, erythropoiesis was more severe in cases of infectious etiology than in genetic-endocrine-metabolic and idiopathic etiologies and should prompt an investigation for infection.
Seven histologic variables were evaluated in 86 patients submitted to liver biopsy during intrahepatic neonatal cholestasis (IHNC) investigation: cholestasis, eosinophilia in the inflammatory infiltrate, presence of giant cells, erythropoiesis, siderosis, portal fibrosis and the presence of a septum. There were no significant differences among the different etiologies of IHNC in relation to: cholestasis, eosinophilia, giant cells, portal fibrosis, the presence or absence of a septum, and siderosis. A significant difference was observed in IHNC of infectious etiology, which presented with more severe erythropoiesis than the genetic-endocrine-metabolic and idiopathic etiologies and should prompt an investigation for infection.
Because of the several possible diagnoses with similar clinical presentation we need to look for features that can help in the diagnostic process of IHNC and a scoring system for histologic features could be useful.
The initial approach of liver biopsy is usually used to distinguish between intrahepatic and extrahepatic neonatal cholestasis. This is the first study to analyze standardized histologic features usually present in IHNC (cholestasis, eosinophilia in the inflammatory infiltrate, presence of giant cells, erythropoiesis, siderosis, portal fibrosis and the presence of a septum) in the differential diagnosis of IHNC.
Liver biopsy should be interpreted by a pathologist with expertise in pediatric liver disease.
Cholestasis, eosinophilia in the inflammatory infiltrate, presence of giant cells, erythropoiesis, siderosis, portal fibrosis and the presence of a septum are histologic features usually present in neonatal hepatitis. A scoring system for these histologic features was used in the differential diagnosis of IHNC.
This is a well written analysis of the histologic features in the differential diagnosis of IHNC. Although it is not a landmark finding the report is of value in that it points out that severe erythropoiesis would be a criterion indicating IHNC of infectious etiology.
| 2. | Danks DM. Management of neonatal obstructive jaundice. Aust Paediatr J. 1977;13:71-76. |
| 3. | Dick MC, Mowat AP. Hepatitis syndrome in infancy--an epidemiological survey with 10 year follow up. Arch Dis Child. 1985;60:512-516. |
| 4. | Moyer V, Freese DK, Whitington PF, Olson AD, Brewer F, Colletti RB, Heyman MB. Guideline for the evaluation of cholestatic jaundice in infants: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39:115-128. |
| 5. | Dellert SF, Balistreri WF. Neonatal cholestasis. Pediatric Gastrointestinal Disease. 3rd ed. B.C. Decker: Canada 2000; 880-894. |
| 6. | Fischler B, Papadogiannakis N, Nemeth A. Aetiological factors in neonatal cholestasis. Acta Paediatr. 2001;90:88-92. |
| 7. | Henriksen NT, Drablos PA, Aagenaes O. Cholestatic jaundice in infancy. The importance of familial and genetic factors in aetiology and prognosis. Arch Dis Child. 1981;56:622-627. |
| 8. | Mowat AP. Hepatite e colestase em lactentes: Afecções intra-hepáticas. Doenças Hepáticas em Pediatria. 2nd ed. Revinter: Rio de Janeiro 1991; 41-80. |
| 9. | Yachha SK, Sharma A. Neonatal cholestasis in India. Indian Pediatr. 2005;42:491-492. |
| 10. | Silveira TR, Pires ALG. Icterícia colestática neonatal. 2nd ed. Edição. Guanabara: Rio de Janeiro 1991; 465-687. |
| 11. | Prado ET, Araujo Mde F, Campos JV. [Prolonged neonatal cholestasis: prospective study]. Arq Gastroenterol. 1999;36:185-194. |
| 12. | Zerbini MC, Gallucci SD, Maezono R, Ueno CM, Porta G, Maksoud JG, Gayotto LC. Liver biopsy in neonatal cholestasis: a review on statistical grounds. Mod Pathol. 1997;10:793-799. |
| 13. | Bezerra JA. Colestase neonatal. Gastroenterologia e hepatologia em pediatria. Medsi: Rio de Janeiro São Paulo Belo Horizonte 2003; 582-597. |
| 14. | Tiker F, Tarcan A, Kilicdag H, Gurakan B. Early onset conjugated hyperbilirubinemia in newborn infants. Indian J Pediatr. 2006;73:409-412. |
| 15. | Garcia FJ, Nager AL. Jaundice as an early diagnostic sign of urinary tract infection in infancy. Pediatrics. 2002;109:846-851. |
| 16. | American Academy of Pediatrics. Cytomegalovirus infection. Pickering LK. Redbook: In 2003; report of the Committee on Infectious Diseases. 26th ed. Elk Grove Village (IL): American Academy of Pediatrics, 2003: 259-262. |
| 17. | Felber S, Sinatra F. Systemic disorders associated with neonatal cholestasis. Semin Liver Dis. 1987;7:108-118. |
| 18. | Munro SC, Hall B, Whybin LR, Leader L, Robertson P, Maine GT, Rawlinson WD. Diagnosis of and screening for cytomegalovirus infection in pregnant women. J Clin Microbiol. 2005;43:4713-4718. |
| 19. | Roberts EA. A criança ictérica. Doenças hepáticas e do sistema biliar em crianças. Livraria Santos Editora: São Paulo 2001; 11-45. |
| 20. | Shibata Y, Kitajima N, Kawada J, Sugaya N, Nishikawa K, Morishima T, Kimura H. Association of cytomegalovirus with infantile hepatitis. Microbiol Immunol. 2005;49:771-777. |
| 21. | Dehghani SM, Haghighat M, Imanieh MH, Geramizadeh B. Comparison of different diagnostic methods in infants with Cholestasis. World J Gastroenterol. 2006;12:5893-5896. |
| 22. | Eliot N, Odievre M, Hadchouel M, Hill C, Flamant R. [Statistical analysis of clinical, biological and histologic data in 288 cases of neonatal cholestasis]. Arch Fr Pediatr. 1977;34:CCXIII-CCXX. |
| 23. | Hessel G, Yamada RM, Escanhoela CA, Bustorff-Silva JM, Toledo RJ. [The role of the abdominal ultrasonography and percutaneous liver biopsy in the differential diagnosis of neonatal cholestasis]. Arq Gastroenterol. 1994;31:75-82. |
| 24. | Mieli-Vergani G, Howard ER, Mowat AP. Liver disease in infancy: a 20 year perspective. Gut. 1991;Suppl:S123-S128. |
| 25. | Wang JS, Tan N, Dhawan A. Significance of low or normal serum gamma glutamyl transferase level in infants with idiopathic neonatal hepatitis. Eur J Pediatr. 2006;165:795-801. |
| 26. | Balistreri WF, Heubi JE, Suchy FJ. Immaturity of the enterohepatic circulation in early life: factors predisposing to “physiologic” maldigestion and cholestasis. J Pediatr Gastroenterol Nutr. 1983;2:346-354. |
| 27. | Balistreri WF, Bezerra JA. Whatever happened to “neonatal hepatitis”? Clin Liver Dis. 2006;10:27-53, v. |
| 28. | Brough AJ, Bernstein J. Conjugated hyperbilirubinemia in early infancy. A reassessment of liver biopsy. Hum Pathol. 1974;5:507-516. |
| 29. | Landing BH, Wells TR, Ramicone E. Time course of the intrahepatic lesion of extrahepatic biliary atresia: a morphometric study. Pediatr Pathol. 1985;4:309-319. |
| 30. | Fox VL, Cohen MB, Whitington PF, Colletti RB. Outpatient liver biopsy in children: a medical position statement of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 1996;23:213-216. |
| 31. | Santos JL, Almeida H, Cerski CT, Silveira TR. Histo-pathological diagnosis of intra- and extrahepatic neonatal cholestasis. Braz J Med Biol Res. 1998;31:911-919. |
| 32. | Okcu-Heper A, Erden E, Doganci T, Kuloglu Z, Kansu A, Genc Y. Nonobstructive neonatal cholestasis: clinical outcome and scoring of the histopathological changes in liver biopsies. Pediatr Dev Pathol. 2006;9:44-51. |
| 33. | Russo P. Liver including tumors, gallbladder, and biliary tree. Potter’s Pathology of the fetus, infant and Child. Mosby Elsevier: Salt Lake City 2007; 1207-1268. |
| 34. | Bazlul Karim A, Kamal M. Cholestatic jaundice during infancy: experience at a tertiary-care center in Bangladesh. Indian J Gastroenterol. 2005;24:52-54. |
| 35. | Matthai J, Paul S. Evaluation of cholestatic jaundice in young infants. Indian Pediatr. 2001;38:893-898. |