Published online Jan 28, 2009. doi: 10.3748/wjg.15.473
Revised: October 20, 2008
Published online: January 28, 2009
AIM: To evaluate the prevalence and clinical characteristics of Nonalcoholic fatty liver disease (NAFLD) among asymptomatic Brazilian adolescents.
METHODS: Transversal observational study included asymptomatic adolescents with central obesity from private and public schools in Salvador-Bahia, northeastern Brazil. The children answered a questionnaire that included age, gender, race, and medical history, and were submitted to a complete physical exam and abdominal ultrasound. Biochemical exams included: ALT, AST, GGT, C reactive protein (CRP), fasting glucose, insulin, cholesterol and triglycerides. Criteria for NAFLD included: the presence of steatosis in ultrasound and/or high level of ALT, negative or occasional historic of intake of alcohol (≤ 140 g/wk), negative investigation for hepatitis A, B, C, auto-immune hepatitis, Wilson disease and hemochromatosis.
RESULTS: From October, 2005 to October, 2006, the study included 1801 subjects between 11 and 18 years of age and a mean age of 13.7 ± 2.0 years. One hundred ninety-nine had central obesity. The prevalence of NAFLD was 2.3%, most of whom were male and white. Insulin resistance (IR) was observed in 22.9% of them and had positive correlations with ALT and GGT (P < 0.05). Elevated CRP was observed in 6.9% of the cases; however, it was not associated with WC, IR or liver enzymes.
CONCLUSION: The prevalence of NAFLD in Brazilian adolescents was low. The ethnicity may have influence this frequency in the population studied, which had a large proportion of African descendents.
- Citation: Rocha R, Cotrim HP, Bitencourt AGV, Barbosa DBV, Santos AS, Almeida AM, Cunha B, Guimarães I. Nonalcoholic fatty liver disease in asymptomatic Brazilian adolescents. World J Gastroenterol 2009; 15(4): 473-477
- URL: https://www.wjgnet.com/1007-9327/full/v15/i4/473.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.473
Nonalcoholic fatty liver disease (NAFLD) is a clinical-pathological condition of wide spectrum, which includes steatosis and steatohepatitis, that has a potential to advance to cirrhosis and hepatocellular carcinoma[1].
Obesity is the most relevant risk factor for NAFLD in children and several studies have described the prevalence and risk factors of NAFLD in these populations[23].
Reports of NAFLD in the child population was described in boys and girls between 10 and 13 years of age who showed elevated aminotransferase levels and variable degrees of steatosis, inflammation and fibrosis in hepatic biopsies[4]. NAFLD occurrence varies with race/ethnicity and gender[5]. A recent study shows body mass index (BMI) as an independent factor for hepatic fibrosis in children and adolescents with NAFLD[6]. In adults and adolescents, visceral obesity has been considered the main risk factor for the development of hepatic steatosis[78]. However, most studies have been done with convenient samples of obese adolescent groups treated in clinics for obesity, which may influence the prevalence of NAFLD in these individuals[910].
In Brazil, previous studies estimated that 15% of students are overweight or obese[1112]; however, the prevalence of NAFLD in this population is not known. The present study aimed to evaluate the prevalence and the clinical characteristics of NAFLD among asymptomatic Brazilian adolescents.
Transversal observational study included asymptomatic adolescents from seven high schools in Salvador, Bahia, Brazil who presented central obesity. Salvador is a city in the northeast of Brazil, where it is relevant to account for the proportion of African descendents.
The study was approved by the Ethics Committee for Research from MCO-Programa de Pós Graduação em Medicina e Saúde-Universidade Federal da Bahia, Brazil.
Inclusion criteria: Age between 11 and 18 years old; abdominal circumference > 75th percentile, according to age and sex[13]; free and pre-explained consent term, signed by the adolescents and their caregivers.
Exclusion criteria: Previous historic or serologic markers for liver diseases (hepatitis A, B, C), auto-immune disease, metabolic diseases such as Wilsons Disease and hemochromatosis and intake of alcohol above 140 g/wk.
Criteria for diagnosis of NAFLD: Presence of steatosis in ultrasound scan and/or high levels of ALT and/or AST; negative or occasional historic alcohol intake (≤ 140 g/wk); negative diagnosis of other liver diseases (A, B and virus, autoimmune hepatitis, metabolic liver disease).
For anthropometric evaluation, a sample of blood was collected and a superior abdomen ultrasound scan was performed during the same session at the nursing of the schools.
Anthropometric measures: waist circumference (WC) was measured at the end of normal expiration in the middle portion between the last rib and the iliac ridge. For evaluation, reference values were adopted that consider normal abdominal circumference to be ≤ 75th percentile and increased abdominal circumference to be > 75th percentile according to age and sex[13].
Participants were weighed while not wearing coats or shoes or carrying any objects. A Filizola balance was used, with a scale resolution of 0.1 kg. Height was measured by a stadiometer with no shoes and hair accessories; the resolution was 0.5 cm. BMI was calculated by dividing weight by height squared. Subjects were considered to be overweight at a BMI between the 85th and 95th percentile and obese at a BMI above the 95th percentile for a given age and sex, based on Cole[14].
All adolescents underwent superior abdomen ultrasound scan (AUS). The AUS was performed by only one medical doctor. The scanner used was an Aloka, model DynaView II, with colored Doppler and a 3.5 MHz drill. The discrepancy of echogenicity between the liver and kidney was considered as a criterion. Hepatic steatosis was graded as mild, moderate or severe, according to the Saverumuttu et al[15] classification.
Blood samples were collected by the technical staff from adolescents after a 12 h fast. The samples were analyzed in a referential laboratory for AST, ALT, GGT, C reactive protein (CRP), fasting glucose and insulin. Aminotransferase alterations were considered when AST was > 36 U/L for girls and > 59 U/L for boys, ALT > 52 U/L for girls and >72 U/L for boys, and GGT > 43 for girls and > 73 U/L for boys. High-sensitivity CRP assay was conducted using latex-enhanced nephelometry.
Individuals who presented elevated ALT, AST and GGT levels underwent the following exams: HBsAg, anti-HCV, anti-HAV-IgM, antibodies (anti-nuclear, anti-muscle, anti-LKM, anti-mitochondrial) α1-antitrypsin, ceruloplasmin and serum copper.
Serum glucose and insulin were used for the calculation of homeostasis model assessment (HOMA) = [fast insulin (&mgr;IU/mL) × fast glucose (mg/dL)/22.5] (mg/dL = mmol/L × 18.182), considering ≥ 3.16 as a cutoff point to define insulin resistance[16].
The adolescents diagnosed with NAFLD were provided assistance at the NASH Group.
The data were analyzed using Statistical Package for Social Science (SPSS) 13.0. Mistake typeIwas estimated to be 5%. Spearman was used for the correlation between variables and t-test to compare the mean. The data are expressed as percentage, medium and standard deviation.
Between October, 2005 and October, 2006, seven public and private schools participated in the study. This corresponded to 3500 students. Among these students, 1801 (51.5%) adolescents agreed to participate in the study. The present study included 199 individuals who had central obesity (WC > 75 percentile). Twenty-four (12.1%) of them were excluded: one had a history of alcohol consumption above 140 g/wk and 23 had incomplete data.
Among 175 adolescents evaluated, the mean age was 13.7 ± 2.0 years, 54.3% were female and 71.4% (125) were nonwhite. AST level was statistically higher in white adolescents as compared to nonwhite adolescents (P < 0.05), as shown in Table 1.
| Variable | Nonwhite (n = 125) | White (n = 50) |
| Sex F/M (%) | 58.4/41.6 | 44.0/5.0 |
| Age (yr) | 13.74 ± 2.02 | 13.70 ± 2.00 |
| BMI (kg/m2 ) | 27.41 ± 3.27 | 26.64 ± 3.26 |
| WC (cm) | 88.09 ± 7.76 | 88.65 ± 9.26 |
| AST (U/L) | 24.11 ± 4.83a | 26.53 ± 6.77 |
| ALT (U/L) | 31.41 ± 7.32 | 32.96 ± 13.70 |
| GGT (U/L) | 22.03 ± 6.68 | 21.04 ± 11.69 |
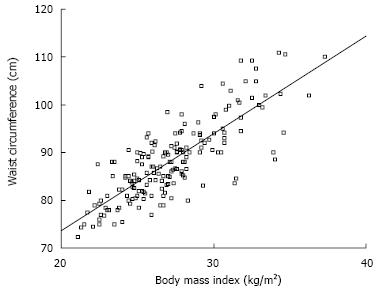
Overweight was observed in 64.0% of studied adolescents with central obesity. There was a positive correlation between BMI and WC values, as shown in Figure 1.
Hepatic steatosis to AUS was observed in 1.7% (3) individuals. All of them were males and white, two were obese and one was overweight. One of the obese adolescents showed moderate steatosis on ultrasound and elevated ALT, AST and GGT. Two of them had mild steatosis on AUS and normal ALT, AST and GGT.
Among the 175 adolescents studied, 2.8% (5) showed elevated aminotransferases. One girl had isolated elevated ALT. One boy had elevated AST, ALT and GGT. All had BMI > 95th percentile. Elevated GGT was observed in 3 cases, two girls and one boy.
According NAFLD criteria (presence of steatosis in ultrasound scan and/or high levels of ALT and/or AST) four (2.3%) adolescents had NAFLD diagnosis (Table 2).
| Adolescents | Age(yr) | Sex | Elevated enzyme | Steatosis | Class BMI | Ethnicity |
| 1 | 17 | Boy | AST, ALT, GGT | Moderate steatosis | Obese | White |
| 2 | 15 | Boy | No | Mild steatosis | Obese | White |
| 3 | 12 | Boy | No | Mild steatosis | Overweight | White |
| 4 | 15 | Girl | ALT | No | Obese | Nonwhite |
WC had a statistically significant correlation with ALT and GGT (P < 0.05). This finding remained after exclusion of a few outliers. IR was observed in 22.9% (40) of adolescents. Only one (1) adolescent with isolated elevated GGT had IR. Insulin resistance also had a statistically significant correlation with ALT and GGT (P < 0.05) after exclusion of a few outliers.
Elevated CRP was observed in 6.8% (12) of adole-scents. However, adolescents with hepatic steatosis and/or elevated aminotransferases did not have elevated CRP.
The present study shows the prevalence and clinical characteristics of NAFLD among Brazilian asymptomatic adolescents.
The prevalence of 2.3% is consistent with some studies based on the frequency of elevated aminotransferases or fatty liver in obese populations[817]. Tominaga et al[18] found a prevalence of 2.5% fatty liver in a Japanese study of 810 obese children between 4 and 12 years old, and Alavian et al[19] found among 966 obese children in Iran aged 7-18 years 1.8% with elevated ALT.
However, the majority of the cases presented central obesity, identified through isolated measure of WC. It also has been demonstrated as an independent risk factor for development of NAFLD in adults and children, and it is considered more important than obesity[20]. Central obesity has been related to IR, one of the factors related to the pathogenesis of NAFLD[2122].
Positive correlation between BMI and WC in adolescents, as observed in this study, is also a common finding related to cardiovascular disease in this population[2324].
This investigation also observed that the majority of adolescents with fatty liver on AUS were obese (BMI ≥ 95th percentile), which may be related to the degree of association between steatosis and BMI[925].
Higher frequency of NAFLD in adolescents has been shown[59]; however, other factors may explain this difference between the present investigation and prior ones. The majority of them have included adolescents or children from obesity clinics[2627]. Another factor was that only half of the eligible children agreed to participate of the study. The discrimination that may occur among obese young people may influence the decision of these people to not consent to the study.
Another influence factor may be ethnicity. It has been considered as an important factor for the different frequencies of NAFLD in children populations. It is more common in Hispanic and less so in black adolescents[5]. In Salvador-Bahia, a city in the northeast of Brazil, the majority of the population are of African descendents. Although this study did not aim to evaluate race, these simple of the population came from an area in Brazil where a relevant factor is the proportion of African descendents, and it may explain the low prevalence of NAFLD in this study.
The characteristics of disease appear to be similar to those in other studies. Gender has not been considered to be a risk factor for NAFLD in children[28]; however, a higher degree of hepatic steatosis has been found in boys compared to girls, at a ratio of 2:1[518]. In this sample, the adolescents with NAFLD were males, corroborating previous reports.
Elevated ALT levels in asymptomatic individuals have been considered useful as a marker to screen patients with NAFLD; they have a correlation with abdominal fat[29], and higher levels of ALT correlated to grades of fat in hepatocytes[325]. In obese children, ALT has been considered as a predictor of NAFLD[27]. Elevated levels of ALT were observed only in one adolescent, who had moderate degree of liver steatosis, and in one adolescent with normal AUS, thus showing a correlation between WC and IR.
The frequency of IR was relevant to this study. And despite the positive correlation with ALT and GGT, elevated hepatic enzymes or steatosis were not common. The literature shows that IR is a risk factor for and is associated with NAFLD, mainly with elevated ALT[230].
The elevation of CRP in obese adults has been considered a risk factor for progression of NASH[31], and the low prevalence of NAFLD in this study may justify the low occurrence of elevated CRP.
Two female adolescents had isolated elevated GGT. However, GGT is not considered a predictor of NAFLD in obese children. In studies with adults, GGT is associated with NAFLD and the metabolic syndrome, especially in women[323334]. Fraser et al[35], when studying hepatic enzymes in women who had and had not diabetes, observed an association of serum GGT with IR in both groups. We observe that one adolescent with elevated GGT also had IR.
In conclusion, NAFLD in asymptomatic Brazilian adolescents was most frequent among white males with central obesity and the mean age of 13.7 ± 2.0 years. These adolescents came from an area in Brazil where it is relevant to consider the proportion of African descendents, and the influence of ethnicity on the prevalence the NAFLD may be an important factor in this population. However, this hypothesis deserves future study.
The authors thank FAPESB (Fundação de Amparo a Pesquisa do Estado da Bahia) for their financial support, as well as the schools and individuals who agreed take part in this project.
Non-alcoholic fatty liver disease (NAFLD) is a common condition among in obese children patients. However, the prevalence of NAFLD has varied in different populations. The influence of gender, risk factors and ethnicity have been discussed. However, more studies are considered necessary to elucidate the relevance of this liver disease in adolescents.
This study reported the findings of NAFLD prevalence of 2.3% in 175 adolescents with central obesity. The prevalence of NAFLD in these adolescents was lower than others studies and we speculate that ethnicity may influence the prevalence in this population. They come from an area in Brazil where a relevant factor is the proportion of African descendents. However, this hypothesis deserves future study.
This is one of the first Brazilian studies which evaluated the prevalence of NAFLD in adolescents with central obesity. This investigation showed a low prevalence of this liver disease and we hypothesized the influence of ethnicity in the lower frequency NAFLD in this population.
The prevalence of NAFLD and associated factors in different populations is important for understanding its development, and to implement preventive strategies to control of the disease.
NAFLD means Non-alcoholic Fatty Liver Disease; HOMA-IR is homeostasis model assessment index-insulin resistance
The work is interesting. Rocha et al describe the epidemiology of NAFLD in a cohort of children from Brazil. The study is strengthened by a solid research design and large numbers. While the findings are not particularly novel, it is helpful to have this sort of basic epidemiology data in the literature.
| 1. | Cotrim HP, Parana R, Braga E, Lyra L. Nonalcoholic steatohepatitis and hepatocellular carcinoma: natural history? Am J Gastroenterol. 2000;95:3018-3019. |
| 2. | Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500-505. |
| 3. | Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999-2004. Gastroenterology. 2007;133:1814-1820. |
| 4. | Moran JR, Ghishan FK, Halter SA, Greene HL. Steatohepatitis in obese children: a cause of chronic liver dysfunction. Am J Gastroenterol. 1983;78:374-377. |
| 5. | Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561-e565. |
| 6. | Iacobellis A, Marcellini M, Andriulli A, Perri F, Leandro G, Devito R, Nobili V. Non invasive evaluation of liver fibrosis in paediatric patients with nonalcoholic steatohepatitis. World J Gastroenterol. 2006;12:7821-7825. |
| 7. | Kral JG, Schaffner F, Pierson RN Jr, Wang J. Body fat topography as an independent predictor of fatty liver. Metabolism. 1993;42:548-551. |
| 8. | Fishbein MH, Mogren C, Gleason T, Stevens WR. Relationship of hepatic steatosis to adipose tissue distribution in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2006;42:83-88. |
| 9. | Franzese A, Vajro P, Argenziano A, Puzziello A, Iannucci MP, Saviano MC, Brunetti F, Rubino A. Liver involvement in obese children. Ultrasonography and liver enzyme levels at diagnosis and during follow-up in an Italian population. Dig Dis Sci. 1997;42:1428-1432. |
| 10. | Sathya P, Martin S, Alvarez F. Nonalcoholic fatty liver disease (NAFLD) in children. Curr Opin Pediatr. 2002;14:593-600. |
| 11. | Leão LCLS, Araújo LMB, Moraes LTLP, Assis AM. Prevalence of obesity in school children from Salvador, Bahia. Arq Bras Endocrinol Metab. 2003;47:151-157. |
| 12. | Guimaraes IC, Guimaraes AC. Prevalence of cardiovascular risk factors in selected samples of schoolchildren--socioeconomic influence. Prev Cardiol. 2005;8:23-28. |
| 13. | Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439-444. |
| 14. | Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240-1243. |
| 15. | Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed). 1986;292:13-15. |
| 16. | Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115:e500-e503. |
| 17. | Burgert TS, Taksali SE, Dziura J, Goodman TR, Yeckel CW, Papademetris X, Constable RT, Weiss R, Tamborlane WV, Savoye M. Alanine aminotransferase levels and fatty liver in childhood obesity: associations with insulin resistance, adiponectin, and visceral fat. J Clin Endocrinol Metab. 2006;91:4287-4294. |
| 18. | Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, Kusano Y. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995;40:2002-2009. |
| 19. | Alavian SM, Mohammad-Alizadeh AH, Esna-Ashari F, Ardalan G, Hajarizadeh B. Non-alcoholic fatty liver disease prevalence among school-aged children and adolescents in Iran and its association with biochemical and anthropometric measures. Liver Int. 2008;40:Epub ahead of print. |
| 20. | Hsieh SD, Yoshinaga H, Muto T, Sakurai Y, Kosaka K. Health risks among Japanese men with moderate body mass index. Int J Obes Relat Metab Disord. 2000;24:358-362. |
| 21. | Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450-455. |
| 22. | Perseghin G, Bonfanti R, Magni S, Lattuada G, De Cobelli F, Canu T, Esposito A, Scifo P, Ntali G, Costantino F. Insulin resistance and whole body energy homeostasis in obese adolescents with fatty liver disease. Am J Physiol Endocrinol Metab. 2006;291:E697-E703. |
| 23. | Rosa ML, Mesquita ET, da Rocha ER, Fonseca Vde M. Body mass index and waist circumference as markers of arterial hypertension in adolescents. Arq Bras Cardiol. 2007;88:573-578. |
| 24. | Kelishadi R, Gheiratmand R, Ardalan G, Adeli K, Mehdi Gouya M, Mohammad Razaghi E, Majdzadeh R, Delavari A, Shariatinejad K, Motaghian M. Association of anthropometric indices with cardiovascular disease risk factors among children and adolescents: CASPIAN Study. Int J Cardiol. 2007;117:340-348. |
| 25. | Chan DF, Li AM, Chu WC, Chan MH, Wong EM, Liu EK, Chan IH, Yin J, Lam CW, Fok TF. Hepatic steatosis in obese Chinese children. Int J Obes Relat Metab Disord. 2004;28:1257-1263. |
| 26. | Sagi R, Reif S, Neuman G, Webb M, Phillip M, Shalitin S. Nonalcoholic fatty liver disease in overweight children and adolescents. Acta Paediatr. 2007;96:1209-1213. |
| 27. | Sartorio A, Del Col A, Agosti F, Mazzilli G, Bellentani S, Tiribelli C, Bedogni G. Predictors of non-alcoholic fatty liver disease in obese children. Eur J Clin Nutr. 2007;61:877-883. |
| 28. | Quiros-Tejeira RE, Rivera CA, Ziba TT, Mehta N, Smith CW, Butte NF. Risk for nonalcoholic fatty liver disease in Hispanic youth with BMI > or =95th percentile. J Pediatr Gastroenterol Nutr. 2007;44:228-236. |
| 29. | Stranges S, Dorn JM, Muti P, Freudenheim JL, Farinaro E, Russell M, Nochajski TH, Trevisan M. Body fat distribution, relative weight, and liver enzyme levels: a population-based study. Hepatology. 2004;39:754-763. |
| 30. | Dubern B, Girardet JP, Tounian P. Insulin resistance and ferritin as major determinants of abnormal serum aminotransferase in severely obese children. Int J Pediatr Obes. 2006;1:77-82. |
| 31. | Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol. 2007;42:573-582. |
| 32. | Araújo LMB, Lima DS, Daltro C. Association of Gamma-Glutamyl Transferase and the Metabolic Syndrome in Obese Women. Arq Bras Endocrinol Metab. 2005;49:557-562. |
| 33. | Kim SG, Kim HY, Seo JA, Lee KW, Oh JH, Kim NH, Choi KM, Baik SH, Choi DS. Relationship between serum adiponectin concentration, pulse wave velocity and nonalcoholic fatty liver disease. Eur J Endocrinol. 2005;152:225-231. |
| 34. | Marchesini G, Avagnina S, Barantani EG, Ciccarone AM, Corica F, Dall’Aglio E, Dalle Grave R, Morpurgo PS, Tomasi F, Vitacolonna E. Aminotransferase and gamma-glutamyltranspeptidase levels in obesity are associated with insulin resistance and the metabolic syndrome. J Endocrinol Invest. 2005;28:333-339. |
| 35. | Fraser A, Ebrahim S, Smith GD, Lawlor DA. A comparison of associations of alanine aminotransferase and gamma-glutamyltransferase with fasting glucose, fasting insulin, and glycated hemoglobin in women with and without diabetes. Hepatology. 2007;46:158-165. |