Published online Jan 28, 2009. doi: 10.3748/wjg.15.431
Revised: June 29, 2008
Published online: January 28, 2009
AIM: To explore the possibility of reversing multi-drug resistance (MDR) to HepG2/mdr1 in vitro and in vivo with RNA interference (RNAi).
METHODS: HepG2/mdr1 was obtained by cloning the whole gene mdr1 into HepG2 cells. shRNA targeting sequence was designed to be homologous to the P-gp encoding MDR1 mRNA consensus sequence. pSUPER-shRNA/mdr1 was constructed using the enzyme-digested technique. HepG2/mdr1 cells were transfected with vectors of pSUPER-shRNA/mdr1 to measure their efficacy by real-time PCR for mdr1 mRNA, flow cytometry (FCM) for P-gp expression, and Rhodamine efflux, MTT method for HepG2/mdr1 function, respectively. In vivo, mice tumors were treated by injecting pSUPER-shRNA/mdr1 in situ and into intra-abdominal cavity. Tumors were collected to create cell suspension and cryosections after chemothearpy with adiramycin and mytomycin. The cell suspension was incubated in RPMI-1640 supplemented with G418 to screen stable cells for appreciating the reversal of MDR. Cryosections were treated with immunohistochemistry technique to show the effectiveness of transfection and the expression of P-gp.
RESULTS: pSUPER-shRNA/mdr1 was successfully constructed, which was confirmed by sequencing. The MDR phenotype of HepG2/mdr1 was decreased significantly in vitro transfection. HepG2/mdr1 showing its MDR was reversed notably in P-gp expression (11.0% vs 98.2%, P < 0.01). Real-time PCR showed that mRNA/mdr1 was lower in test groups than in control groups (18.73 ± 1.33 vs 68.03 ± 2.21, P < 0.001). Compared with HepG2, the sensitivity of HepG2/mdr1 and HepG2/mdr1-dsRNA cells to ADM was decreased by 1.64 times and 15.6 times, respectively. The accumulation of DNR in positive groups was decreased evidently. In vivo, the p-gp expression in positive groups was significantly lower than that in control groups (65.1% vs 94.1%, P < 0.05). The tumor suppressing rate in test groups was 57.8%. After chemotherapy, the growth rate in test groups was lower than that in control groups (700.14 ± 35.61 vs 1659.70 ± 152.54, P < 0.05). Similar results were also observed under fluorescence microscope, and confirmed by Image-Pro Plus 4.5 analysis.
CONCLUSION: pSUPER-shRNA/mdr1 vector system allows simple, stable and durable nonviral knockdown of P-gp by RNAi in malignant cells and animals to restore their sensitivity to adriamycin.
-
Citation: Pan GD, Yang JQ, Yan LN, Chu GP, Liu Q, Xiao Y, Yuan L. Reversal of multi-drug resistance by pSUPER-shRNA-mdr1
in vivo andin vitro . World J Gastroenterol 2009; 15(4): 431-440 - URL: https://www.wjgnet.com/1007-9327/full/v15/i4/431.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.431
It has been reported that a relatively new technique using RNA interference (RNAi) is able to inhibit gene expression[1]. RNAi is triggered by the presence of double-stranded RNA (dsRNA) in cells and results in a rapid destruction of mRNA containing an identical or nearly identical sequence[2]. Studies on the RNAi pathway in invertebrates, however, have demonstrated that cleavage of long dsRNAs to a length of 21-25 bp results in dsRNAs that are active in eliciting RNAi[34]. It has recently been shown that RNAi can be achieved in cultured mammalian cells using small interfering RNA (siRNAs) with a length of 21-23 bp[5].
Liu et al[6] have developed a technique to efficiently deliver plasmid DNA to organs of postnatal mice by rapidly injecting a large volume of physiological solution into their tail vein. A further research showed that high-pressure delivery technique can deliver siRNAs for RNAi induction used as a reporter gene, the modified luciferase gene, luc+[7]. In liver, injection of 0.5 &mgr;g siRNA-luc+ can increasingly inhibit luc+. These results indicate that inhibition of target gene expression by siRNA in organs of postnatal mice can efficiently deliver siRNA.
Resistance to cytotoxic drugs represents a serious hindrance in cancer chemotherapy. One form of multi-drug resistance (MDR) is caused by over-expression of P-gp, a mdr1 gene product[8]. P-gp is a transmembrane phosphoglycoprotein capable of transporting a variety of structurally and functionally diverse chemotherapeutic drugs, such as vinblastine, doxorubicin and paclitaxel, leading to reduced intracellular drug concentration and cytotoxicity[9]. In human hepatocellular carcinoma (HCC), the best treatment is radical operation of the tumor. However, only 30%-40% of HCC can undergo a radical operation, while others must receive chemotherapy[10]. To circumvent the MDR of HCC resulting from chemotherapeutic drugs, several ways including inhibitors of P-gp, oligonucleotide,anti-sense RNA, transient siRNA, hammerhead ribozyme techniques and others regulating MDR-related gene measurements have been developed[111213141516]. However, the half-life of P-gp (at least 16 h) makes it difficult to achieve a complete knockdown of P-gp[17]. To solve this problem, siRNA vectors have been used in retro-, adeno-, and lentiviral systems. Virus-associated difficulties, such as insertional mutagenesis and cis-activation of silent genes by their strong promoters, and difficult generation of recombinant viruses, have limited the use of such systems[1819].
Reports showed that RNAi technology is currently used not only as a powerful tool for analyzing gene function, but also for developing highly specific therapeutics[2021]. In the RNAi approach, sequence-specific post-transcriptional gene silencing is achieved by siRNA, short double-stranded RNA molecules in which the anti-sense strand is complementary to the target mRNA of a given gene[2223]. It has not been determined whether suppressing mdr1 expression with siRNA is sufficient to reverse the MDR of HepG2/mdr1 in vitro and .
An alternative new strategy is the plasmid-based expression system. We used the plasmid-based RNAi system for the knockdown of MDR-1, restoring its sensitivity to adriamycin and doxorubicin in P-gp over expression. This vector system-pSUPER is a powerful tool for the attenuation of P-gp-mediated drug resistance in malignant cells.
The effective dsRNA sequence has been screened from 4 siRNA transfecting HepG2/mdr1 cells with an oligofectamine kit (result not shown). In the present study, we used this sequence in design of shRNA template oligonucleotide.
HepG2/mdr1 was obtained by cloning the whole gene mdr1 into HepG2 cells to construct its MDR[24]. HepG2 and HepG2/mdr1 cells were cultured in RPMI-1640 containing 2% glutamine, 100 U/mL penicillin, 100 &mgr;g/mL streptomycin, 10% fetal calf serum, and 200 &mgr;g/mL G418 at a density of 2 × 105 and sub-cultured upon reaching a density of 1 × 106 cells per Ml. Three days before transfection, G418 was omitted from the medium.
ShRNA target sequence was designed to be homologous to the P-gp encoding MDR1 mRNA consensus sequence (GeneBank accession number AF 16 535) tested by transfection assay[24]. Complementary oligonucleotides encoded a hairpin structure with a 19-mer stem derived from the mRNA target site. A 19-bp loop sequence separated the two complementary domains. Near the 3' end of shRNA template is a 5 nucleotide poly (T) tract recognized as a RNA pol III termination signal. The 5' end of two oligonucleotides was Bal II and Hind III restriction site overhangs. shRNA insert template oligonucleotides, MDR1 DNA oligos, control insert sequence DNA oligos encoding the GFP shRNA (sense: 5'-GATCCGGTTATGTACAGGAACGCATTCAAGAGATGCGTTCCTGTACATAACCT-TTTTGGGAAA-3' and anti-sense: 5'-AGCTTTTCCAAAAAGGTTATGTAC-AGGAACGCATCTCTTGAATGCGTTCCTGTACATA-ACCG-3') were synthesized, annealed and ligated into the Bal II and Hind III sites of linearized pSuper RNA system shRNA expression vector for each target site.
Ligation products were transformed into E. coli, clones were selected, and plasmid DNA was isolated. Plasmid was digested, clones with the shRNA insert were selected, and purification of pSUPER-neo shRNA plasmid for transfection was performed using the Wizard plus SV Minipreps DNA purification system (Promega, USA).
HepG2/mdr1 was kindly provided by Dr. Yong-Bing Chen (Department of Biliary-hepatology Surgery, Beijing Youan Hospital, Beijing 100 000, China). All cells were cultured in RPMI-1640 medium (Sigma, USA) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 &mgr;g/mL streptomycin. The cells were plated in a fully humidified atmosphere containing 5% CO2, 95% air at 37°C. HepG2/mdr1 cells were established by gene clone technique. In brief, the mdr1 gene was cloned into the vector and then transfected into HepG2 cells to construct its MDR[20]. In order to maintain the MDR phenotype, cell culture medium for P-gp-expressing HepG2/mdr1 cells was supplemented with 0.05 &mgr;g/mL of doxorubicin, 400 &mgr;g/mL of G418. HepG2/mdr1 cells were incubated in a doxorubicin-free medium with 400 &mgr;g/mL of G418 for over 2 wk and then used in study. All the cells were discarded after 3 mo and new cells were obtained from frozen stocks.
HepG2/mdr1 cells in the exponential phase of growth were plated in 6-well plates at 2 × 105 cells per well, grown for 24 h, then transfected with siRNA expression plasmid pSUPER-shRNA (OligoEngine Co, USA) according to the manufacturer’s protocol. Negative control cells were treated with pSUPER negative control plasmid consisting of a circle plasmid encoding a siRNA whose sequence was not found in mouse, human or rat genome databases. Seventy-two hours after transient transfection, silencing was examined, then the cells were trypsinized for 72 h and seeded onto selective media. HepG2/mdr1 cells stably expressing siRNA were established by selection with a medium containing 400 &mgr;g/mL of G418 (Sigma, USA). The medium was renewed every 3 d. After 2 wk, resistant colonies were trypsinized, combined in pools and cultured in a selective medium. Stable expression was examined 8 wk after transfection.
Sequences of the primers and probes were selected from the mdr1 gene for MDR and P-glycoprotein. The primers and TaqMan probes were designed using the Primer Express Software (Applied Biosystems, Foster City, CA). Molecular beacons were designed according to the guidelines available at internet. A DNA folding program was used to estimate the stability of stem and loop structures of the molecular beacons. The forward and reversed primer sequences for mdr1 gene and GAPDH are 5'-GACATGACCAGGTATGCCTA-3' and 5'-CTTGGAGACATCATCTGTAAGTC-3', and 5'-TGGGTGTGAACCACGAGAA-3' and 5'-GGCATGGACTGTGGTCATGA-3', respectively. The primer sequences of TaqMan probes for mdr1 and GAPDH are 5'-CTGCACCACCAACTGCTTAGC-3' and 5'-CTGACTCACCACACCAATGAC-3', respectively. First, total RNA was extracted from all groups with a RNA extraction kit (Omega, USA) and verified by 1% gel electropherogram and A260/A280. Reverse transcription PCR was carried out to produce cDNA. PCR was performed in a 50 &mgr;L volume containing 10 × buffer, 25 mmol/L MgCl2, 25 mmol/L dNTP mix, 10 &mgr;mol/L primer F, 10 &mgr;mol/L primer R, Taq DNA polymerase and DEPC-H2O. The reactions were initially heated to 94°C for 4 min, and then subjected to 35 cycles at 94°C for 30 s, at 55°C for 30 s, at 72°C for 1 min, at 72°C for 1 min in an iCycle iQ PCR detection system (Bio-Rad, CA). Finally, real-time PCR was performed in a 30 &mgr;L volume containing 10 × buffer, 10 mmol/L dNTPs, 250 mmol/L MgCl2, 10 Pmol/&mgr;L primer F, 10 Pmol/&mgr;L primer R, 10 Pmol/&mgr;L Taq Man probe, Taq enzyme, cDNA template and ddH2O, for 35 cycles at 94°C for 2 min, at 94°C for 20 s, at 50°C for 30 s, and at 60°C for 40 s for the measurement of mdr1 mRNA level. Data were collected during reactions. PCR threshold cycle (Ct) defined as the fractional cycle number at which the fluorescence reaches 10 times the standard deviation (SD) of the baseline, was determined. Average Ct for duplicate standards and test samples was calculated. Standard curve equations were calculated by regression analysis of average Ct versus log10 of the standard copy number. ΔCt and ΔΔCt of all samples were calculated to produce 2-ΔΔCt. The results were linearized and analyzed by ANVON.
Nucleofected cells of HepG2/mdr1, including test groups-HepG2/mdr1-si and control groups-HepG2/mdr1 were collected, analyzed for Pgp expression with staining 50 &mgr;L of Pgp monoclonal antibody for 60 min at 37°C, and washed two times in phosphate-buffered saline (PBS). The cells were incubated fro another 60 min with 50 &mgr;L of PE-labled antibody against Pgp antibody, washed two times with PBS, and finally, analyzed with a flow cytometer (FACSCalibur, BD, Vienna, Austria). The data were then subjected to CellQuest software (BD).
All cells in the exponential growth phase were plated in a 96-well plate at 2 × 104 cells/well, and grown for 24 h. The cells were incubated for 48 h after different concentrations of doxorubicin were added. The cells were incubated for 4 h after 20 &mgr;L of 5 mg/mL MTT (Sigma, USA) was added. The absorbance at 490 nm was read and the IC50 values were determined (at least three times) in multiple independent experiments. Relative reversal rate = (IC50A-IC50B)/(IC50A-IC50C), where IC50A is IC50 values for non-transfected multi-drug resistant cells, IC50B is IC50 values for sensitive cells. Survival data of cells transfected with shRNA vectors were evaluated by ANOVA for statistical significance.
To assess the steady accumulation of daunorubicin, all cells transfected with shRNA expression plasmids or control plasmids and transfected reagents were incubated with 1.0 &mgr;g/mL daunorubicin for 1 h at 37°C, washed three times with ice-cold PBS. The fluorescence intensity of intracellular daunorubicin was determined by FCM.
Animal care and sacrifice were approved by Sichuan University Medical School Animal Studies Committee. Prior to injection, HepG2/mdr1 and HepG2 cells were resuspended in a serum-free medium at 2 × 107 cells/mL. Nude mice (BALBC/C-nu/nu, Animal Breeding Center, Sichuan University, Chengdu, China) were divided into experimental group and control group. Mice in each group were subcutaneously injected with 200 &mgr;L cell suspension into the right flank. The process of tumorigenesis was monitored and newly formed tumor volume (volume = m12× m2× 0.5236, m1 represents the short axis, and the m2 long axis) was measured every 2-3 d.
When the newly formed subcutaneous tumor reached 0.5-0.6 cm in diameter, mice in the experimental group underwent 2 variant tests (5 mice per test): pSUPER-shRNA-MDR1 at the ratio of 1 mg/kg and 1.5 mg/kg was injected into the tumor in situ and peritoneal cavity, respectively. Mice in the negative control group were injected with blank pSUPER vectors and mice in the blank control group of HepG2 were not treated. The vectors were mixed with liposome at the ratio of 1:1 for transfection. Seventy-two hours after injection, mice in all groups underwent chemotherapy with adriamycin (ADM) at a dose of 1.5 mg/kg, twice a week, for two weeks. Then, tumorigenic tissue was carefully separated from its surrounding fibrous capsule for measurement of tumor volume. The relative growth rate of tumorigenic tissue [ΔTV = (V2 - V1)/V1, V1: tumor volume before chemotherapy; V2: tumor volume at mouse sacrifice] demonstrated the sensitivity of variant cells to ADM in vivo. Tumorigenic tissue was further ground, suspended as single cells, and cultured in RPMI-1640 medium supplemented with 200 &mgr;g/mL of G418. Cells with stable expression of shRNA were selected for flow cytometry or incubated with different concentrations of ADM (0.032-5 &mgr;g/mL). Their IC50 values were determined by MTT assay.
Seventy-two hours after treatment with pSUPER-shRNA-mdr1 injection, mice in all groups underwent chemotherapy with adiramycin at a dose of 1.5 mg/kg, two times a week, for two weeks, and executed to harvest the tumor for monoplast suspension. The exact volume and relative growth rate of tumor [ΔTV = (V2 - V1)/V1, where V1 is the tumor volume before chemotherapy and V2 is the tumor volume after mouse sacrifice] were measured to evaluate drug sensitivity of cell lines to ADM. Cell suspension was cultured in a RPMI-1640 medium supplemented with 200 &mgr;g/mL of G418 to screen stably expressing cells. The screened cells were incubated at 1 × 106 cells/mL to study the sensitivity of HepG2/mdr1 to ADM. Cells were treated at different concentrations of ADM (0.032-5 &mgr;g/mL) to determine their IC50 by MTT assay.
Variant cells were incubated with 50 &mgr;L of monoclonal antibody to P-gp at 37°C for 60 min, washed twice in phosphate-buffered saline (PBS), incubated for another 60 min with 50 &mgr;L of PE-labeled secondary antibody, sorted with a flow cytometer (FACSCalibur, BD, Vienna, Austria) and analyzed using CellQuest software (BD).
Sections of tumorigenic tissue were fixed in cold acetone at 4°C for 10 min. The sections were incubated for 10 min at room temperature with an endogenous peroxidase blocking solution, and then with 1:100 anti-P-gp mouse monoclonal antibodies at 37°C for 1 h. After washed with PBS, the cells were incubated with 1:150 PE-labeled rabbit anti-mouse IgG at 37°C for 1 h, stained with glycerine and photographed under inverted light microscope (Olympus, Japan) and confocal fluorescence microscope (Olympus, Japan). The images were analyzed with Image-Pro Plus 4.5 system (Media Cybernetics, Inc. USA). The total OD value and area of intracellular fluorescence for each section were measured.
Average values were expressed as mean ± SD. The statistical significance of differences in mean values was assessed by Student’s t test and ANVON using SPSS 10.0 statistical software. P < 0.05 was considered statistically significant.
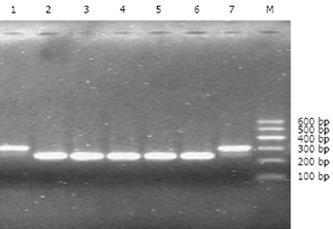
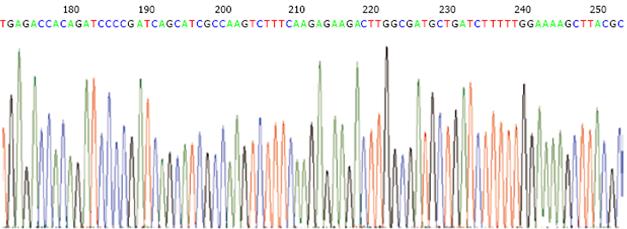
The shRNA/mdr1 expression plasmids were digested by restriction enzyme Bag II and Hind III, and 1.5% agarose gel electrophoresis produced a fragment of 64 bp (Figure 1). DNA sequencing confirmed that ligation reaction between the shRNA insert and pSUPER-shRNA/mdr1 expression vector was correct. No.1 and No.7 indicated the positive clone size of 310 bp (insert element 64 bp) (Figure 2).
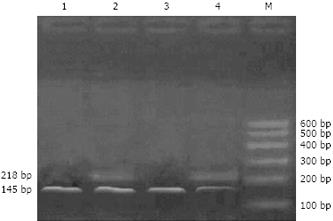
Seventy-two hours after transient transfection, mdr1 mRNA expression was significantly reduced in cells treated with pSUPER-shRNA/mdr1 vector, compared with the control (56.3-fold vs 1.0-fold, P < 0.01; Figure 3 and Table 1).
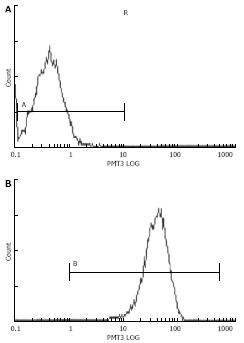
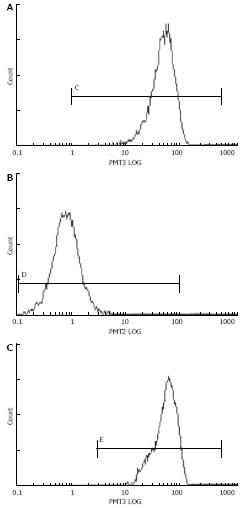
The effect of transfection with pSUPER-shRNA/mdr1 vectors on cellular P-gp expression in HepG2/mdr1 cells was assessed by FCM. Seventy-two hours after transient transfection with pSUPER-shRNA/mdr1 vectors, the protein expression of P-gp was decreased from 98.6% to 11.0% (P < 0.01, Table 1 and Figure 4), indicating that transfection with pSUPER-shRNA/mdr1 vectors could significantly decrease the cellular P-gp expression levels in HepG2/mdr1 cells after transient and stable transfection.
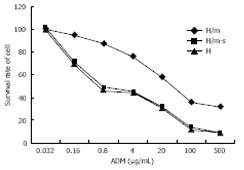
MTT assay was performed to examine the effects of doxorubicin after transient and stable transfection. Seventy-two hours after transient transfection, pSUPER-shRNA/mdr1 vectors decreased the resistance to adiramycin and mytomycin from 44.6-fold to 1.4-fold and from 138.1-fold to 1.14-fold compared to the control groups (Table 1 and Figure 5). The IC50 was lower than that in control groups (0.56 &mgr;g/mL vs 25.0 &mgr;g/mL, 0.08 &mgr;g/mL vs 9.67 &mgr;g/mL, P < 0.01), suggesting that transfection of pSUPER-shRNA/mdr1 vectors could reverse the resistance to adiramycin and mytomycin.
Transfection with hairpin shRNA vectors improved intracellular drug accumulation. Compared with control groups, accumulation of daunorubicin in cells treated with shRNA vectors was significantly increased (79.32% vs 37.96%, P < 0.05). There was no difference in sensitive cells of HepG2 (79.32% vs 87.56%) between test groups (Table 1 and Figure 6).
Two weeks after HepG2/mdr1 and HepG2 cells were injected into the right flank of 4-wk-old nude mice, all mice suffered from tumors measuring 0.5 cm × 0.6 cm × 0.6 cm to 0.6 cm × 0.6 cm × 0.65 cm (Table 1). There was no difference in the mean tumor volume between HepG2/mdr1 and HepG2 groups (64.23 ± 2.15 mm3vs 66.41 ± 1.58 mm3).
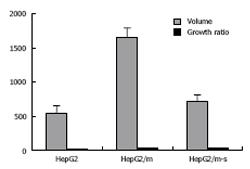
After treatment with ADM, changes of tumor volume were more significant in the transfected groups than in the control groups (1695.70 ± 152.54 mm3vs 700.14 ± 25.61 mm3, P < 0.01; Figure 7, Table 2). In vivo implant of HepG2/m-sh was more sensitive to ADM than that of HepG2/m. However, the difference in ADM sensitivity between in vivo implant of HepG2 and HepG2/m-sh was not significant. The results demonstrate that tumor cells in transfected groups could reverse their sensitivity to ADM.
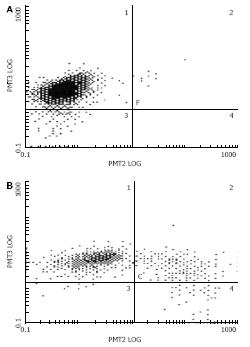
P-gp expression was detected by FCM after the mice were sacrificed. P-gp expression in test groups was lower than that in control groups (65% vs 94.1%, P < 0.05), but there was no significant difference between groups treated with in situ and intra-abdominal injection of pSUPER-mdr1 (65.1% vs 58.7%), showing that effect of pSUPER-shRNA-mdr1 could be achieved both by in situ injection and by intra-abdominal injection of pSUPER-mdr1 (Figure 8).
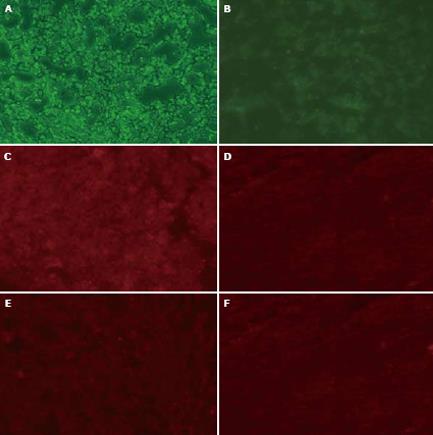
After chemotherapy, nude mice were sacrificed the next day. Tumors were collected by cervical dislocation or by drawing blood from heart, and cryo-sectioned (8 &mgr;m). The sections were treated with immnohistochemistry and analyzed by Image-Pro Plus 4.5 software (Media Cybernetics, Inc USA). The density of green fluorescence represented the tranfected vector pSUPER-shRNA/mdr1 into tumor. The density of red fluorescence was equal to the amount of P-gp antibodies binding to cellular membrane. The transfection ratio of test groups was greater than that of control groups (86.70% vs 35.20%, P < 0.05). The expression of P-gp in test groups was lower than that in control groups (15.75% vs 97.90%, P < 0.05). However, there was no difference in expression of P-gp between in situ and intra-abdominal injection of pSUPER-shRUA/mdr1 (97.90% vs 92.62%, Figure 9).
In cancer, over-expression of mdr1 P-glycoprotein (ABCB1) is a possible cause of chemotherapy-based treatment failure[25]. P-glycoprotein confers cross-resistance to unrelated drugs that differ widely in molecular structure and target specificity, including natural agents and new anticancer agents[26].
Of the novel strategies to circumvent MDR, hammerhead ribozymes against the mdr1 mRNA and anti-sense oligonucleotides have been widely used[12131415]. Both hammerhead ribozymes and anti-sense must be delivered across the plasma membrane, and are susceptible to degradation. Recent studies exploiting the promise of RNAi in mammalian cells have led to the reversal of gene-dependent MDR using short hairpin RNA expression vectors[2728]. In vitro study has not achieved complete reversal of MDR with RNAi[29]. Use of shRNAs, when expressed in stably integrated plasmids, can decrease mdr1 mRNA by 95%-97% in intact cells and restore drug sensitivity to that of parent cell lines.
In the present study, shRNA expression cassettes targeting mdr1 were used not only in cells in culture, but also in animals. Pichler et al[27] reported that gene silencing induced by pSuper and pRetrosuper constructs is specific and potent in cell lines, and stably express shRNAi for several months. It was reported that high-pressure delivery technique of siRNA through tail vein inhibits expression of genes in a specific manner in vivo[7]. However, in this study, siRNA delivered with this method was susceptible to degradation, the effect of RNAi only lasted several days, a time frame insufficient for its application.
The potent and ubiquitous gene-silencing mechanism of RNAi is to generate considerable excitement in the fields of molecular biology and gene therapy[30], but delivery is probably the single biggest obstacle to the development of RNAi-based therapeutic agents[31]. Vector-based delivery of shRNA could improve the efficiency of siRNA delivery. pSUPER RNAi system provides a mammalian expression vector that directs intracellular syntheses of siRNA-like transcripts. The vector uses a polymerase III H1-RNA gene promoter, since it produces a small RNA transcript lacking a polyadenosine tail and has a well-defined start of transcription and a termination signal consisting of five thymidines in a row (T5). Cleavage of the transcript at the termination site is after the second uridine, yielding a transcript resembling the ends of synthetic siRNAs, which also contain two 3’overhanging T or U nucleotides (nt)[3233]. The pSUPER RNAi system has been used to induce efficient and specific down-regulation of gene expression[3435], resulting in functional inactivation of targeted genes. This vector-induced stable expression of siRNAs mediates persistent suppression of gene expression, allowing analysis of loss-of-function phenotypes that develop over a longer period of time. We used this vector system to deliver shRNA/mdr1. Combined with bioluminescence imaging, pSUPER was easier to be followed, especially in vivo. shRNA- mediated down-regulation of P-glycoprotein transport activity both in cultured cells and in tumor implants in living animals could be followed by direct noninvasive bioluminescence imaging using the Renilla luciferase fluorophore. In vitro, the vector of pSUPER-shRNA/mdr1 was constructed using enzyme-digested technique according to the pSUPER protocol. Transfected with the recombinant plasmid, HepG2/mdr1 showed that its MDR was reversed notably in P-gp expression between positive and control groups (11.0% vs 98.2%, P < 0.01). Real-time PCR showed that mRNA/mdr1 in test groups was lower than that in control groups (56.30-fold vs 1.0-fold, P < 0.001). Compared with HepG2/mdr1 cells, the sensitivity of HepG2/mdr1-dsRNA cells to adiramycin and doxorubicin was decreased from 15.6-fold to 1.64-fold and from 138.1-fold to 1.14-fold, respectively. The accumulation of DNR in positive groups was decreased evidently. These results indicate that plasmid vector of pSUPER-shRNA/mdr1 can effectively transfect target cells and suppress gene function. In vivo, after transfected with pSUPER-shRNA/mdr1 through the peritoneal cavity and in situ, carcinoma cells(HepG2 and HepG2/mdr1) measured by flow cytometry and immunohistochemistry analysis showed that dsRNA/mdr1 was successfully and effectively brought into cells, and that p-gp expression in positive groups was significantly down regulated compared to control groups(65.1% vs 94.1%, P < 0.05). The tumor suppressing rate for test groups was 57.8%. After chemotherapy, the growth rate of tumor for test groups was slower than that for control groups (700.14 ± 35.61 vs 1659.70 ± 152.54, P < 0.05). Similar results were also observed under fluorescence microscope, and confirmed by Image-ProPlus 4.5 software analysis.
The results indicate that vector-based delivered RNAi can improve its efficiency in vitro and in vivo. There was no difference in the efficacy of vector pSUPER- shRNA/mdr1 transfected with in situ injection and that intra-abdominal injection (data not shown). Our study showed that transfection of vector pSUPER- shRNA/mdr1 with intra-abdominal injection was effective due to its diffusivity, which can extend from location to system. It was recently reported that administration of RNAi targeted mdr1 gene can effectively reverse MDR both in vitro and in vivo human epidermoid carcinoma KB(v200) cells[21]. This is the first time to investigate the possibility of reversing MDR in HepG2/mdr1 cells and tumor implants with RNAi.
The authors thank Mrs. Xia QJ, Zhang FQ, and Ms. Yan NH, Chen QY, and Cao GQ for their excellent technical support in shRNAi vector construction and real-time PCR. The authors also thank Dr. Gottesman MM (NIH, New York, USA) for proviing human MDR1 gene plasmid of pHaMDR1-1.
Hepatocellular-carcinoma is very resistant to chemotherapy.There is a growing interest in the reversal of multi-drug resistance to tumors with RNA interfer-ence (RNAi). In hepatocellular carcinoma, such research is almost restricted in vitro. In the present study, the authors investigated the effect of RNAi on the reversal of multi-drug resistance to hepatocellular carcinoma in vitro and in vivo.
This study focused on the reversal of multi-drug resistance to hepatocellular carcinoma with RNAi in vivo.
The present study showed that RNAi targeting mdr1 gene could reverse the resistance of hepatocellular carcinoma to chemotherapy. Transfection with in situ injection and intra-abdominal injection showed that RNAi could reverse multi-drug resistance of hepatocellular carcinoma.
These data demonstrate that RNAi targeting mdr1 gene could reverse drug resistance of hepatocellular carcinoma in vivo, thus contributing to the development of drugs used in gene therapy for hepatocellular carcinoma.
This is a carefully performed study with novel findings, which can be used in the diagnosis and treatment of hepatocellular carcinoma. The study showed that RNAi targeting mdr1 gene can reverse drug resistance of hepatocellular carcinoma in vivo, and therefore improves its sensitivity to chemotherapy for HepG2/mdr1 cells and transplant tumors of HepG2/mdr1.
| 1. | Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806-811. |
| 2. | Tuschl T. RNA interference and small interfering RNAs. Chembiochem. 2001;2:239-245. |
| 3. | Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25-33. |
| 4. | Elbashir SM, Lendeckel W, Tuschl T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001;15:188-200. |
| 5. | Caplen NJ, Parrish S, Imani F, Fire A, Morgan RA. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc Natl Acad Sci USA. 2001;98:9742-9747. |
| 6. | Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258-1266. |
| 7. | Lewis DL, Hagstrom JE, Loomis AG, Wolff JA, Herweijer H. Efficient delivery of siRNA for inhibition of gene expression in postnatal mice. Nat Genet. 2002;32:107-108. |
| 8. | Ling V. Multidrug resistance and P-glycoprotein expression. Ann N Y Acad Sci. 1987;507:7-8. |
| 9. | Roninson IB, Chin JE, Choi KG, Gros P, Housman DE, Fojo A, Shen DW, Gottesman MM, Pastan I. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci USA. 1986;83:4538-4542. |
| 10. | Wang HJ, Lee H. [Surgical treatment of hepatocellular carcinoma]. Korean J Gastroenterol. 2005;45:247-257. |
| 11. | Warmann S, Göhring G, Teichmann B, Geerlings H, Pietsch T, Fuchs J. P-glycoprotein modulation improves in vitro chemosensitivity in malignant pediatric liver tumors. Anticancer Res. 2003;23:4607-4611. |
| 12. | Stuart DD, Kao GY, Allen TM. A novel, long-circulating, and functional liposomal formulation of antisense oligodeoxynucleotides targeted against MDR1. Cancer Gene Ther. 2000;7:466-475. |
| 13. | Chan JY, Chu AC, Fung KP. Inhibition of P-glycoprotein expression and reversal of drug resistance of human hepatoma HepG2 cells by multidrug resistance gene (mdr1) antisense RNA. Life Sci. 2000;67:2117-2124. |
| 14. | Huesker M, Folmer Y, Schneider M, Fulda C, Blum HE, Hafkemeyer P. Reversal of drug resistance of hepatocellular carcinoma cells by adenoviral delivery of anti-MDR1 ribozymes. Hepatology. 2002;36:874-884. |
| 15. | Wu H, Hait WN, Yang JM. Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res. 2003;63:1515-1519. |
| 16. | Nieth C, Priebsch A, Stege A, Lage H. Modulation of the classical multidrug resistance (MDR) phenotype by RNA interference (RNAi). FEBS Lett. 2003;545:144-150. |
| 17. | Alemán C, Annereau JP, Liang XJ, Cardarelli CO, Taylor B, Yin JJ, Aszalos A, Gottesman MM. P-glycoprotein, expressed in multidrug resistant cells, is not responsible for alterations in membrane fluidity or membrane potential. Cancer Res. 2003;63:3084-3091. |
| 18. | An DS, Xie Y, Mao SH, Morizono K, Kung SK, Chen IS. Efficient lentiviral vectors for short hairpin RNA delivery into human cells. Hum Gene Ther. 2003;14:1207-1212. |
| 19. | Barton GM, Medzhitov R. Retroviral delivery of small interfering RNA into primary cells. Proc Natl Acad Sci USA. 2002;99:14943-14945. |
| 20. | Holm PS, Lage H, Bergmann S, Jürchott K, Glockzin G, Bernshausen A, Mantwill K, Ladhoff A, Wichert A, Mymryk JS. Multidrug-resistant cancer cells facilitate E1-independent adenoviral replication: impact for cancer gene therapy. Cancer Res. 2004;64:322-328. |
| 21. | Shi Z, Liang YJ, Chen ZS, Wang XW, Wang XH, Ding Y, Chen LM, Yang XP, Fu LW. Reversal of MDR1/P-glycoprotein-mediated multidrug resistance by vector-based RNA interference in vitro and in vivo. Cancer Biol Ther. 2006;5:39-47. |
| 22. | Fuchs B, Zhang K, Schabel A, Bolander ME, Sarkar G. Identification of twenty-two candidate markers for human osteogenic sarcoma. Gene. 2001;278:245-252. |
| 23. | Rubin Grandis J, Zeng Q, Drenning SD. Epidermal growth factor receptor--mediated stat3 signaling blocks apoptosis in head and neck cancer. Laryngoscope. 2000;110:868-874. |
| 24. | Chen YB, Yu SH, Yan LN, Gou XH, Li DH, Zhao LY, Zhang SB. Construction of mdr1 Expression Vector and Detection of Its Expression in HepG2 Cells. Zhongguo Puwaijichu Yu Linchuang Zazhi. 2005;12:254-257. |
| 25. | Borst P, Elferink RO. Mammalian ABC transporters in health and disease. Annu Rev Biochem. 2002;71:537-592. |
| 26. | Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48-58. |
| 27. | Pichler A, Zelcer N, Prior JL, Kuil AJ, Piwnica-Worms D. In vivo RNA interference-mediated ablation of MDR1 P-glycoprotein. Clin Cancer Res. 2005;11:4487-4494. |
| 28. | Gan HZ, Zhang GZ, Zhao JS, Zhang FC, Bu LS, Yang SJ, Piao SL, Du ZW, Gao S, Zheng DM. Reversal of MDR1 gene-dependent multidrug resistance using short hairpin RNA expression vectors. Chin Med J (Engl). 2005;118:893-902. |
| 29. | Yagüe E, Higgins CF, Raguz S. Complete reversal of multidrug resistance by stable expression of small interfering RNAs targeting MDR1. Gene Ther. 2004;11:1170-1174. |
| 30. | Shankar P, Manjunath N, Lieberman J. The prospect of silencing disease using RNA interference. JAMA. 2005;293:1367-1373. |
| 31. | Stevenson M. Therapeutic potential of RNA interference. N Engl J Med. 2004;351:1772-1777. |
| 32. | Koper-Emde D, Herrmann L, Sandrock B, Benecke BJ. RNA interference by small hairpin RNAs synthesised under control of the human 7S K RNA promoter. Biol Chem. 2004;385:791-794. |
| 33. | Karkare S, Daniel S, Bhatnagar D. RNA interference silencing the transcriptional message: aspects and applications. Appl Biochem Biotechnol. 2004;119:1-12. |
| 34. | Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550-553. |
| 35. | Brummelkamp TR, Bernards R, Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243-247. |