INTRODUCTION
Midkine (MK), also known as MDK, FLJ27379, and NEGF2, is a 13-kDa heparin-binding growth factor with a high affinity for heparin, which shares a 50% homology in amino acid sequence with pleiotrophin (PTN), another member of MK family. Previous findings indicate that MK could be internalized depending on lipoprotein receptor-related protein (LRP), a low-density lipoprotein (LDL) receptor-related protein, and is further transported to nuclei depending on nucleolin. In our study, MK could be translocated to and accumulated in nuclei and nucleoli of different kinds of tumor cell[1]. Furthermore, K79R81, K86K87, and C-terminal tail of MK constitute the nuclear localization determinants of MK. The C-terminal tail is the key element controlling MK nucleoli accumulation though the N-terminal tail. K79R81 and K86K87 also contribute to this process. Immunogold-labeling electron microscopy can investigate the exact location of MK mainly localized in granular component (GC), dense fibrillar component (DFC) and the border between DFC and FC[1]. The phenomenon of nuclear targeting of MK is related to the promotion of cell survival and anti-apoptotic activity[2].
MK is detectable in various carcinomas such as breast, prostate, grastric, colon, hepatocellular and urinary bladder carcinomas, neuroblastoma, glioma and Wilms’ tumor, exhibiting a proto-oncogene function[3456]. However, its expression is low or undetectable in adult normal tissues. MK is deeply involved in cancer progression, onset of inflammatory diseases and repair of injured tissues. MK suppresses tumor growth both in vitro and in vivo and it is believed that MK is a new therapeutic target for curing tumors.
MK TRANSLOCATED TO AND ACCUMULATED IN NUCLEI
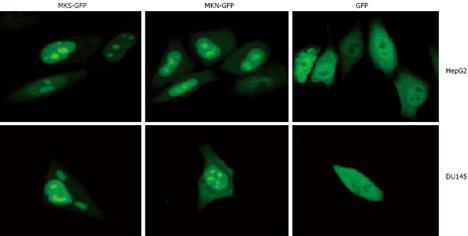
It has been reported that MK localizes in the nuclei[27], nucleoli[8], or cytosol[9], which is inconsistent with the intracellular localization of MK. Human MK exclusively localizes in nuclei and nucleoli in HepG2, DU145, and MCF7 cells by using GFP as a tracking molecule. GFP protein is stable in vivo and fused to the C or N terminus of many cellular and extracellular proteins without loss of activity, permitting tagging of proteins for gene regulation analysis, protein localization, or specific organelle labeling, and thus can be used as a perfect positive control to detect the information of MK. We cloned two MK gene fragments with or without signal peptide and separately inserted them into pEGFP-N2 plasmids (enhanced green fluorescent protein N-terminal protein fusion vector). Then, two recombinant plasmids pEGFP-MKS (EGFP fused with MK with signal peptide) and pEGFP-MKN (EGFP fused with MK without signal peptide) were obtained. The results showed that 24 h after transfection, both fusion proteins of MKS-GFP and MKN-GFP in the two cell lines, HepG2 and DU145, were localized exclusively in the nuclei and accumulated in the nucleoli, while GFP control was detected diffusely over the entire cell body except in nucleoli, indicating that signal peptide in pEGFP-MKS does not participate in nuclear and nucleolar localization (Figure 1). Similar results were obtained in MCF7 cell line (data not shown).
Figure 1 Subcellular localization analysis of MKS-GFP and MKN-GFP fusion proteins in carcinoma cell lines.
MKS-GFP and MKN-GFP fusion proteins were localized to nuclei and accumulated in nucleoli, but rarely localized to cytoplasm. The high light “spots” in the center of nuclei are nucleoli. HepG2 cells (upper panel) and DU145 cells (bottom panel) are transfected with pEGFP-N2, and diffuse freely in the whole cell except in nucleoli.
MK IS INTERNALIZED IN A LRP-DEPENDENT MANNER AND PROTEASOMAL DEGRADATION
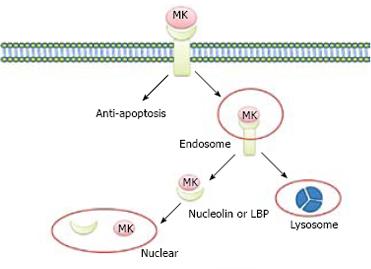
Lipoprotein receptor-related protein (LRP), also known as a low-density lipoprotein (LDL), is a midkine-binding protein[10]. LRP belongs to the LDL receptor family, which includes five prototype members: LDL receptor, ApoE receptor2, very low-density lipoprotein (VLDL) receptor, LRP, and LRP2/megalin. The major functions of these receptors are to endocytose and deliver their ligands to lysosomes for degradation or catabolism[1112]. MK is not internalized in LRP-deficient cells, whereas transfection of a LRP expression vector can restore MK internalization and subsequent nuclear translocation, suggesting that LRP binds to heparin-binding growth factor, MK, and mediates nuclear targeting by MK. Internalized MK in cytoplasm binds to nucleolin, a nucleocytoplasmic shuttle protein and tanslocates to nuclei. When the nuclear localization signal located next to the acidic stretches is deleted, the mutant nucleolin not only accumulates in cytoplasm but also suppresses the nuclear translocation of MK. With respect to nuclear targeting by MK, 37-kDa laminin-binding protein precursor (LBP) binds to MK and is cotranslocated with MK into nuclei[7]. It is possible that MK uses both nucleolin and LBP precursor as shuttle proteins, revealing a novel role of LRP in intracellular signaling by its ligand, and the importance of nucleolin and LBP in the process of nuclear target of MK (Figure 2).
Figure 2 Nuclear targeting by growth factor MK.
MK was endocytosed into cell cytosol through binding to the receptor of LRP, and then tanslocated to nuclei in the presence of nucleulin or LBP. At the same time, the internalized MK is degraded by proteasome to “off” the signals from the cell surface receptors.
It is widely held that growth factor signaling is terminated by lysosomal degradation of its activated receptor and endocytosed growth factor is transported to lysosome. Nuclear targeting is another important pathway through which signals of growth factors are mediated. The nuclear targeting pathway is down-regulated by the proteasome system. Degradation of endocytosed MK is suppressed by both proteasome and lysosome inhibitors. By contrast, proteasome inhibitor rather than lysosome inhibitor accelerates the nuclear accumulation of MK. Expression vector of less signal sequence MK in cytosol, can be constructed because endocytosed MK may be translocated to cytosol from cellular compartments before entering nuclei. Cytosol-produced MK undergoes proteasomal degradation, accumulates in nuclei as endocytosed MK, and is polyubiquitinated with its nuclear accumulation increased by proteasome inhibitors. MK molecule can be further dissected by cytosol-produced MK to investigate its role in degradation and trafficking. The N terminal half-domain of MK is more significantly susceptible to proteasomal degradation, whereas the C terminal half-domain is sufficient to locate nuclei. These data highlight the desensitization of nuclear targeting by growth factor MK and indicate that the proteasome system plays a critical role in desensitization of nuclear targeting. MK is prone to proteasomal degradation[13]. Because “off” signaling is essential for life, it is reasonable that nuclear targeting growth factor MK is prone to degradation by proteasome (Figure 2).
CONFORMATIONAL DETERMINANTS OF INTRACELLULAR LOCALIZATION OF MK
Nuclei orchestrate the running of cells and is a highly dynamic organelle containing dynamic compartments created by relatively immobile binding or assembly sites[14]. In order to function properly, nuclei should rely on a constant flow of molecules between cytosol and nuclei. Many proteins can be translocated into nuclei by the presence of import signals such as nuclear localization signal or sequence (NLS) which can be specifically recognized by receptors on nuclear pore complex[15], or certain shuttling proteins such as nucleolin[16]. After transported into nuclei, proteins can be docked at different subnuclear compartments. In subnuclear structures such as nucleoli, proteins may accumulate in a steady-state compartment mediated by nucleolar localization sequence (NoLS) or domain which might interact with local high-affinity binding sites[17]. We have identified the conformational determinants for MK nuclear and nucleolar localization[18], showing that K79R81, K86K87, and C-terminal tail of MK constitute the nuclear localization determinant, and play an important role in nuclear localization.
DISTRIBUTION OF MK IN NUCLEOLI
At ultra-structure level, nucleoli include three components: fibrillar center (FC), dense fibrillar component (DFC), and granular component (GC)[1920]. Although MK tanslocated to nucleoli and accumulated in nucleoli, its exact location in nucleoli is still unclear. Immunogold-labeling electron microscopy shows that MK is mainly localized in GC, DFC and the border between DFC and FC (data unpublished). Because each component is corresponding to a special biological function, the nascent transcripts appear in the junction region between FC and DFC, accumulate in DFC and continue intranucleolar migration of rRNA towards GC[2122], suggesting that the role of MK in nucleoli may be related to the control of rRNA gene transcription, pre-rRNA processing, and nascent ribosome subunit assembly, the downstream elements to control cell proliferation.
INVOLVEMENT OF MK IN CARCINOGENESIS
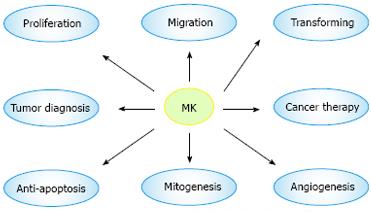
MK exhibits various cancer-related activities and is involved in carcinogenesis and cancer advancement. MK exerts its fibrinolytic activity by enhancing the level of plasminogen activator in endothelial cells[23], and transforms NIH3T3 cells[24]. MK promotes migration of neutrophils, osteoblastic, osteosarcoma cells, neural cells, and macrophages, which is always dependent on the receptors: N-syndecan and PTPζ[25]. MK mediates cell survival and growth mainly through P13-kinase and extracellular signal-regulated kinase (ERK) in intracellular signaling[26]. MK exerts its anti-apoptotic activity to rescue Wilms’ tumor cells from cisplatin[27]. MK also induces a strong angiogenic response in rabbit cornea, when its cDNA is transfected[28]. Resistance to cytotoxic agents is a major limitation for its use in treatment of human cancers. MK has been recently identified as a mediator of intercellular transfer of drug resistance[29] (Figure 3).
Figure 3 Biological function and medical application of MK.
The most attractive feature of MK is its involvement in carcinogenesis, which plays a great role in proliferation, migration, anti-apoptosis, mitogenesis, transformation, and angiogenesis. Furthermore, MK is expected to be a target molecule of diagnosis of and therapy for tumor.
MEDICAL APPLICATION OFMKIN CANCER THERAPY
MK is frequently over-expressed in most carcinomas[30]. Serum and urinary MK levels are becoming prognostic and diagnostic markers of various tumors. Furthermore, MK demonstrates many activities that are significantly correlated to carcinogenesis, indicating that MK is a candidate target of therapy for carcinomas.
MOLECULAR TARGET
Anti-sense ODNs have a huge potential as agents to turn off the expression of specific proteins in vitro and in vivo. Indeed, anti-sense MK ODNs show anti-tumor activities in various carcinoma cells. Moreover, they also suppress the growth of pregrown tumors in nude mice via atelocollagen-mediated gene transfer, and exert their inhibitory effect on mitosis of cancer cells[313233]. Thus, abolition of MK production or disruption of its signaling pathway is a good treatment modality for human carcinomas.
GENE TARGET
Currently, studies on gene therapy for cancer are carried out. The most difficult aspect of developing an in vivo approach to cancer is correctly targeting cancer cells. Many vectors target tumors for gene delivery, including viral and synthetic vectors (liposome and emulsion), which show promise in targeting cancer cells. It is essential to use a strong and tissue-specific promoter region if a suicide gene is to be expressed selectively in cancer cells. Compared with cytomegalovirus (CMV) promoter, MK promoter exhibits a stronger expression in Wilms’ tumor cells than CMV promoter[34]. Furthermore, adenovirus containing CMV promoter-thymidine kinase gene can cause severe side effect to liver, while the MK promoter-thymidine kinase gene does not have such a side effect. Therefore, a suicide gene under the control of MK promoter is a highly potential strategy for the treatment of carcinomas[35].
CONCLUSION AND PERSPECTIVES
In this review, we have described that MK can be translocated to nucleoli, where it accumulates in different tumor cells. A growing body of evidence indicates that nuclear targeting plays an indispensable role in the biological activities of growth factors. For example, nuclear localization of fibroblast growth factor 1 and Schwannoma-derived growth factor is necessary for their mitogenic activity[3637]. Increased ribosomal gene transcription and cell proliferation are closely correlated to the nuclear translocation of fibroblast growth factor-2[38]. It is, thus, important to make clear that signals from tumor cell surface receptors and ligands translocated to nuclei play a role in the biological activities of MK.
MK is comparatively ubiquitous in many kinds of tumor, whereas its expression is usually low or undetectable in normal adult tissue. Serum MK levels are also elevated in patients with various carcinomas, such as gastric, colon, hepatocellular, and lung carcinomas[27]. These observations indicate that MK should be considered indispensable to the development and enlargement of tumors. Further analyses of MK mechanisms related to carcinogenesis should shed light on therapies for tumors and cancers. To further establish blood MK as a tumor marker, prospective studies on blood levels and prognosis will provide useful information. Further study should focus on the application of MK in gene therapy for cancer.