Published online Oct 14, 2009. doi: 10.3748/wjg.15.4838
Revised: August 12, 2009
Accepted: August 19, 2009
Published online: October 14, 2009
AIM: To investigate and evaluate the pathological features and diagnostic value of focal nodular hyperplasia (FNH) with multi-section spiral computed tomography (MSCT) and postprocessing.
METHODS: A total of 25 patients with FNH who had undergone MSCT and postprocessing were included in the investigation. All patients had been pathologically or clinically confirmed with FNH. A number of 75 cases of hepatic carcinomas, hemangiomas and adenomas were randomly selected at a same period for a comparative study.
RESULTS: There was a single focus in 22 cases and multiple foci in 3 cases. On the plain scan, 17 lesions showed hypodensity, 7 isodensity and 4 hyperdensity (the case with fatty liver). With contrast, 28 lesions were enhanced evenly or in the nodules in the arterial phase; 13 lesions still showed hyperdensity, 11 lesions isodensity and 4 lesions hypodensity in the parenchymatous phase; in the delayed phase only 5 lesions showed hyperdensity but 9 lesions showed isodensity or slight hypodensity and 14 lesions showed hypodensity. Twelve lesions of 28 had central asteroid scars. Thickened feeding arteries in postprocessing were seen in 24 lesions, and were integrated into the parenchymatous lesions with a gradual and smooth course. On the contrary, there were no artery penetrated into the lesion found in any of comparative hepatic tumors.
CONCLUSION: Doctors could make a correct diagnosis and differentiation of FNH on evaluation of the characteristic appearance on MSCT with postprocessing.
- Citation: Liu YJ, Fan WJ, Yuan ZD, Liu PC, Wang CR, Yan WQ, Wang SM, Chen JH, Liu Z. Research on focal nodular hyperplasia with MSCT and postprocessing. World J Gastroenterol 2009; 15(38): 4838-4843
- URL: https://www.wjgnet.com/1007-9327/full/v15/i38/4838.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4838
Hepatic focal nodular hyperplasia (FNH) was first described and proposed by Edmondson[1] in 1958, then verified a decade later by the World Health Organization in 1975. At that time cases were too few to be investigated, and were considered as “local sclerotization”, “hepatic hamartoma”, “hepatic adenoma” and so on[2]. With the improvement in imaging techniques, the number of cases diagnosed with FNH have been gradually increasing. At present, the incidence of FNH is just less than that of hemangiomas and has become the second most common benign hepatic disease[3]. Although there have been a number of papers on FNH with different modalities in recent years[4-7], multi-section spiral computed tomography (MSCT) was considered as one of the common facilities and could demonstrate some radiological characteristics to diagnose with FNH[8]. This article discusses more critical imaging findings on relating to the histological features in FNH through analyzing a total of 25 patients with 28 lesions and 75 cases of other hepatic tumors by MSCT and postprocessing.
Authors collected and analyzed data of 25 FNH patients with 28 lesions within 2 institutions, the Cancer Center of Sun Yat-sen University and Peking University Shenzhen Hospital, from January 2005 to December 2007. The patients underwent CT examination and were confirmed with FNH pathologically or clinically. None of the patients had hepatitis, and all alpha-fetoprotein tests were normal. There were 14 men and 11 women, age range 14-59 years, and mean age 35.5 years. Of the 25 patients, 11 patients were confirmed with FNH by pathology after surgery resection, 14 patients were ascertained to have FNH by relative examinations and clinical follow-up for at least 18 mo with a stable shape and no marked change or diminution of the lesion. Meanwhile 30 hepatic carcinomas, 30 hemangiomas and 15 adenomas were randomly selected at a same period with MSCT and postprocessing for a control group.
All 25 patients underwent 16-section spiral CT scanning (Aquilion 16, Toshiba Medical Corporation). CT projection radiographs were scanned firstly for range confirmation, from the apex of the diaphragm to the base of the liver. After the plain scan, a bolus of about 100 mL (average 1.5 mL/kg body weight) contrast media of iodixanol (Iohexol, 300 mgI/mL) was injected into the antecubital vein at a flow rate of 3-5 m/s for the enhanced scan. Bolus tracking was performed with the region of interest on the descending aorta at the level of diaphragm apex, and the arterial phase scan was automatically started 6 s after attenuation in the region of interest reached a predefined threshold of 120 HU. Three-phrase scans were performed after contrast media injection: arterial phase scan (about 20-25 s), parenchymatous or vein phase scan (about 65-70 s), delayed scan (about 150-180 s). Scan parameters: tube voltage 120 kVp, tube current 300 mA, gantry rotation time 0.5 s per rotation, matrix 512 × 512, beam pitch 0.9375, detector collimation thickness 16 × 1 mm, abdomen standard reconstruction algorithm, and effective thickness of reconstruction images 1 mm.
To study the multi-phase images and analyze the dynamic changes in the lesions, the images from the arterial phase and parenchymatous phase were transferred to workstation (Vitrea2, Vital, USA) for postprocessing, with the use of volume rendering technique (VRT) and maximum intensity projection (MIP) to display the arteries supplying the lesions. Three radiologists evaluated the images in a double-blind manner, recorded the main parameters and analyzed the results.
A total of 25 patients with 28 lesions were studied, among them 22 patients with single lesions, the other 3 with multiple lesions. The sizes of the lesions ranged from 1.0 to 8.3 cm. All lesions assumed a circular or circular-type shape with a clear boundary except 2, which showed irregular lobulated shapes.
There were 6 lesions at the right anterior lobe, 13 at the right posterior lobe, 6 at the left lobe and 2 near the diaphragm in the right lobe, one projecting outside the hepatic boundary.
Specimens showed some pale yellow tubercular structures with many separations, part of which were found with asteroid scars, but no real fibrillar involucrum. Under microscopy fibroplasia, permeated with acute or chronic inflammation, could be seen. The center or periphery of the scars were rich in fibrous tissue and small vessels. No malignant tumor cytology was observed.
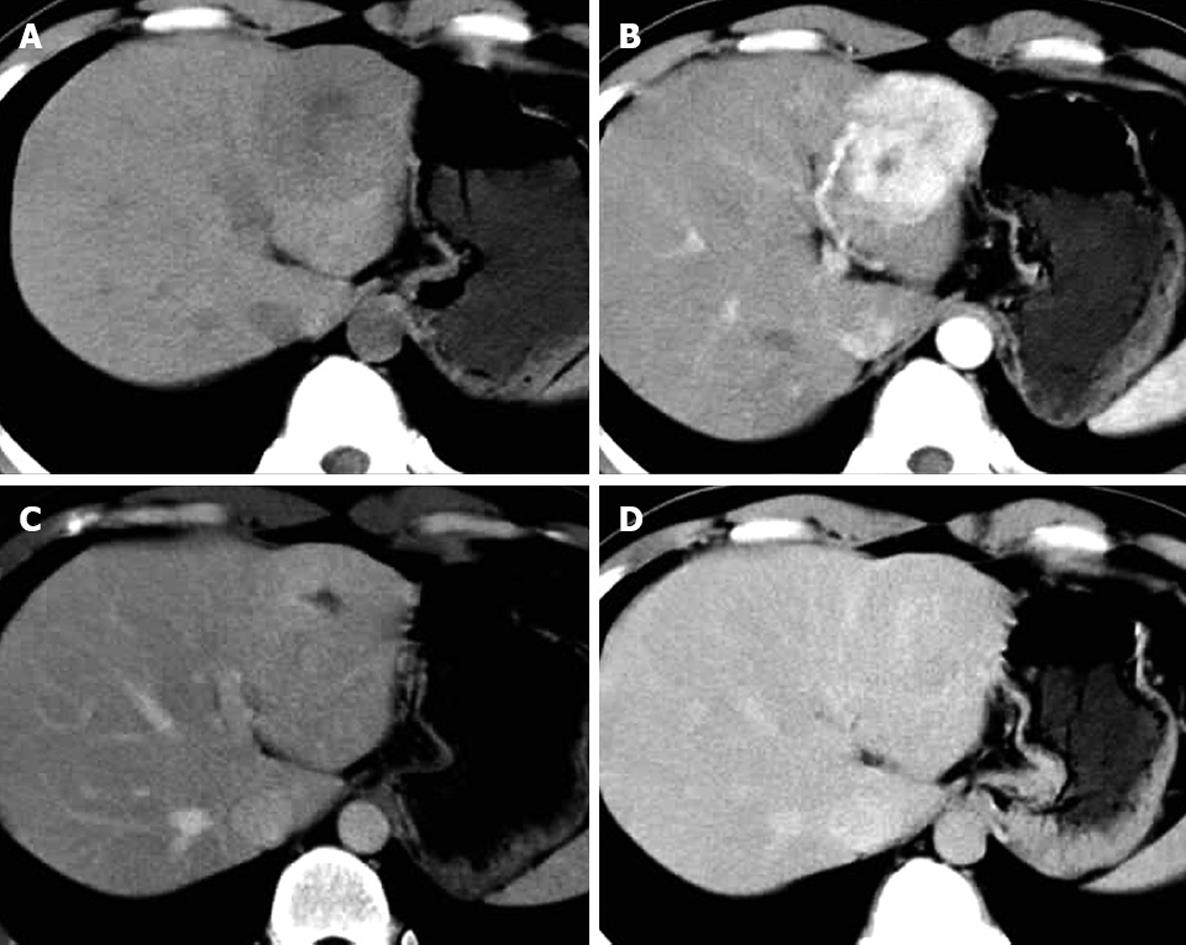
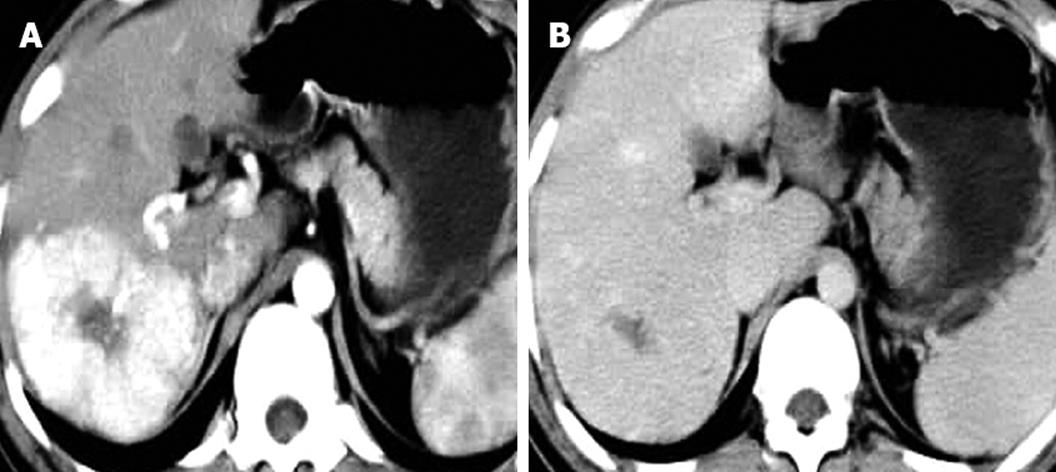
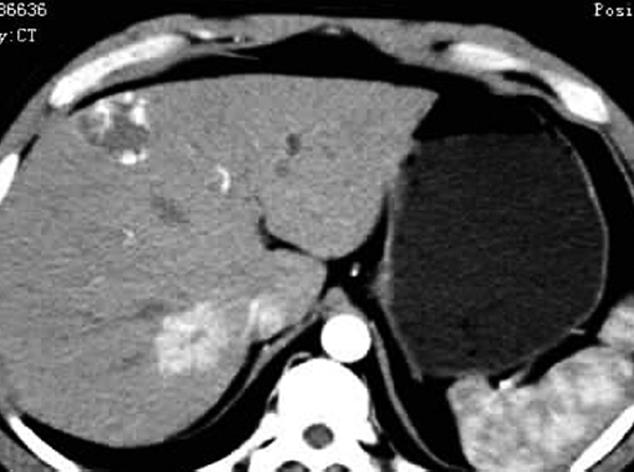
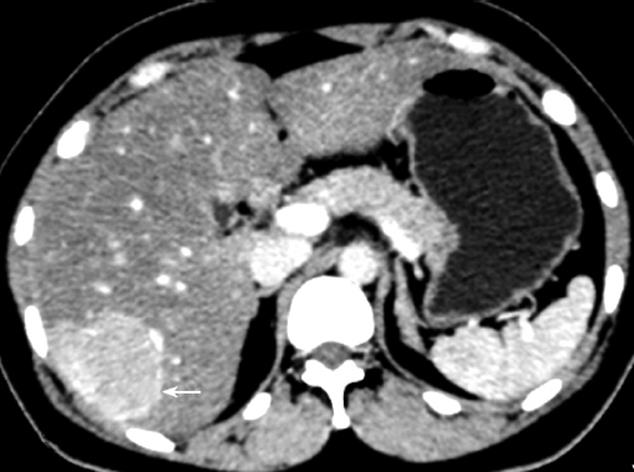
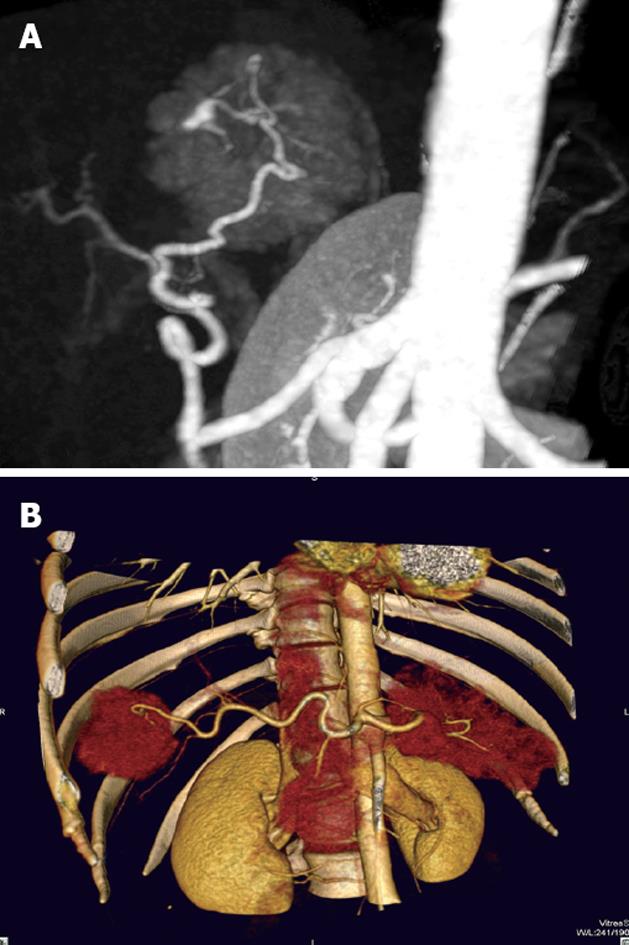
Of the 25 patients with 28 FNH lesions, on plain CT 17 lesions showed hypodensity, 7 isodensity and 4 hyperdensity (in the 4 patients with fatty liver, one of whom had multiple lesions). With contrast, 28 lesions showed even or nodular enhancement in the arterial phase; 13 showed hyperdensity, 11 isodensity and 4 hypodensity in the parenchymatous phase; 5 showed hyperdensity, 9 isodensity or slight hypodensity and 14 hypodensity in the delayed phase. Of the 28 lesions, 12 were found to have a central asteroid scar (Figure 1A-D), with radiating form, and among these, 4 scars were reduced in size in the parenchymatous and delayed phases (Figure 2), 5 scars vanished in the parenchymatous and delayed phases with the lesions showing isodensity, and the other 3 scars showed high density (Figure 1D) in the late delayed phase; also, blood vessels could be seen in some central asteroid scars. The authors also found one example of FNH simultaneously accompanied by hemangiomas (Figure 3). Exceptionally, 6 lesions assumed a ring-like enhancement (Figure 4) in the parenchymatous and delayed phases which were likely mixed with a false involucrum as seen in some hepatic tumors. Thickened blood-supplying arteries were demonstrated in 24 lesions with VRT or MIP in postprocessing (Figure 5A and B), and the arteries followed a gentle line with gradual and direct entry into the body of the lesion. On comparative study, there were no such an artery penetrated into the lesion found in any of other hepatic carcinomas, hemangiomas or adenomas.
FNH pathogenesis was once thought to occur most frequently in young women[9], whereas in this study it occurred in more males than females, but the shape of the lesion was larger in females than in males. A relationship between FNH and oral contraceptives remains inconclusive. The use of contraceptives does not increase FNH incidence, but may possibly promote FNH growth[10]. A recent report indicates that FNH is possibly linked with hereditary factors in an unusual manifestation of hepatic organization[11].
This FNH group primarily appeared with a single lesion (22/25). Most of these cases had no marked clinical symptoms, and only a minority of this group presented with pain and feeling unwell, or a suspicious lump in the right upper abdomen, one of which was clinically thought to be in danger of rupture or hemorrhage. The majority of FNH cases were discovered on routine physical examination and then investigated further.
The FNH pathology has 3 major characteristics: (1) The pathological change is not a real tumor, but mainly normally functioning hepatic cells, with proliferation of the connectivum, the abnormal bile duct and macrophages; (2) The existence of abnormal blood vessels and fiber structure, namely a scar in the center of the pathological change; (3) The blood supply pattern radiated from the inside to the periphery[12-14]. Under the microscope may be seen fibrous divisions and an area of proliferating hepatic cells in FNH, which lacks a normal vein and portal vein, and sometimes has acute or chronic inflammatory cells[15]. In addition there is a special type of FNH called multiple FNH syndrome[16,17], which comprises more than 2 FNH lesions, simultaneously merged at least with one of the following pathological changes: hepatic angiocavernoma, artery structure damage, blood vessel abnormality in the central nervous system, meningioma or neurogliocytoma. This group included an example of multiple FNH combined with hepatic angiocavernoma (Figure 3).
It should be noted that on the border of the FNH lesions, there were expanded blood vessels or blood sinuses, which assumed an incomplete enhanced link round the tumor (Figure 4), and could easily be taken for a malignant tumor with involucrum enhancement.
The majority of FNH showed as normal or slightly low density tumors in CT scans, demonstrating a clear boundary, even density, and no calcification[18,19]. In enhanced scanning, the FNH characteristics alternated as follows: in the arterial phase the lesion quickly and obviously increased evenly or in the nodules from the central area; in the parenchymatous and delayed phases the contrast agent flowed out evenly and the low density lesion appeared. These enhanced characteristics were the result of the rich arterial blood supply and major vein drainage and generous blood sinuses within FNH. The center asteroid low density scar in 12 of 28 (42.8%) lesions has a distinctive feature to differentiate FNH from other tumors with a rich blood supply. MIP or direct volume rendering (DVR) could demonstrate the supplying artery to the central scar (Figure 5). In the parenchymatous and delayed phase the scars were enhanced gradually to equilibrium or to slightly high density, and only 2 remained at low density. The authors believe that it might be possible that scar proliferation could lead to obliteration of the vessel lumen, which could easily be confused with necrosis or a collagen scar within fibrous lamina-like cancer, hepatocellular carcinoma or cholangiocellular carcinoma[20].
The technology of DVR or MIP may directly demonstrate the pattern of blood supply in FNH with just the enhanced scanning raw data, the results of which provide new significant evidence for FNH diagnosis. The arteries supplying blood in FNH often appeared thickened, and had a smooth, gradual course, entering directly into the central area of the lesion. The pathology demonstrated that FNH has one or several blood-supplying arteries, which radiate from the center to the circumference[21]. The major blood supply and drainage vessels have been demonstrated by color Doppler[22,23]. In the scar center were vasa vasorum which proliferated and radiated throughout. All of these characteristics showed marked features different from hemangiomas or other cancers of the liver.
The central scar may either appear in FNH or fibrous lamina-like hepatocellular carcinoma, and their imaging appears extremely similar. However, the FNH may occur as a single or multiple lesion, and in the arterial phase the lesions are markedly enhanced, their density approaching that of the aorta, whereas the fibrous lamina-like hepatocellular carcinoma usually appears as a single lesion with a diameter sometimes greater than 10 cm, with the central scar seldom or slightly enhanced. FNH may present false involucrum signs in certain case which could be confused with malignancy[24], but, by postprocessing, arterial vessels of hepatic carcinoma were revealed to be unevenly thickened, damaged, and discontinuous[25].
Both FNH and hemangiomas of the liver could be markedly enhanced but in different ways. The typical enhancement of hemangiomas started from the periphery, with nodular or ring-like enhancement, advancing gradually to the center, with delayed phase backfill to equal density or high density. In contrast, enhancement of FNH started at the center of the lesion, rapidly and evenly spreading throughout, except for the central scar, which consistently strengthened then abated on the delayed phase. The process in hemangiomas enhanced slowly (representing slow bloodstream) and some atypical small hemangiomas may backfill completely in the arterial phase, but in the parenchymatous and delayed phases still assumed a high or equal density, which was a crucial difference from FNH[26].
A patient with hepatic adenoma commonly has a history of taking contraceptives and/or has had celialgia for a long time. Sometimes a ring-like bright band may be seen at the edge of an adenoma. As they bleed easily, some hematoma within the lesion result in a very different outcome of imaging compared with FNH[27]. The peripheral enhancement in adenoma possibly indicated a wealth of blood-supplying vessels under the big involucrum. The enhancement in adenoma was mostly centripetal, in contrast to the FNH centrifugal type[28].
There are a variety of imaging techniques for visualizing FNH in different aspects[29]: single photon emission CT (SPECT) can make a differentiation according to whether or not there are Kupffer cells in the lesion[30]; color ultrasound may allow a rudimentary diagnosis based on finding radiating blood flow; magnetic resonance imaging emphasizes the special signal intensity in the FNH and its central scar and even its spectrum[31]. However, MSCT with postprocessing technology provides a common mean of entirely recognizing the pathological characteristics of FNH, not only demonstrating the central asteroid scar and shape of the lesion, but also the tissue enhancing process, the blood-supplying model and hemodynamics[32]. The following points should be used clinically to consider as FNH: (1) The MSCT plain scan presents a low or slightly low density lesion with an even, clear boundary and lower density central asteroid scar, which shows delayed enhancement; (2) In the arterial phase, the lesion is rapidly and evenly enhanced except for the central scar, in the parenchymatous phase, the lesion has normal or slightly high density, and in the delayed phase it has low density; (3) The lesion has a thickened but symmetrical blood-supplying artery[33] entering gradually into the lesion. These are FNH pathological characteristics, on which clinicians could make a definite FNH diagnosis, no longer requiring further auxiliary investigations, biopsy or surgery.
There are several kinds of lesions which provoke a conflict in diagnosis of diseases of the liver. As a benign disease, correct diagnosis of focal nodular hyperplasia (FNH) is vital for choosing the appropriate treatment protocol.
Imaging techniques have been used to investigate the variants of FNH, e.g. single photon emission CT (SPECT) examined whether or not there were Kupffer cells in the lesion, color ultrasound investigated radiating blood flow, magnetic resonance imaging measured particular signal intensity in the central scar, whereas MSCT, by intravenous injection of contrast medium, can delineate the lesion shape, size, supplying arteries and hemodynamics, which could reflect the pathological manifestations of FNH.
On detailed FNH case observation, this paper has introduced different enhanced modes in the lesion and the central scar. By postprocessing in a workstation, the authors found a thickened but symmetrical artery entering into the lesion with a radiating blood supply, which is crucial in revealing the pathological characteristics of FNH and in making a conclusive diagnosis.
Based on the results of this study, a contrast method with postprocessing is recommended in diagnosis of hepatic lesions with MSCT, in order to reveal the features of enhancement, the central scar and supplying artery in FNH.
FNH was one of the more recently recognized diseases, but its pathogenesis was still unknown. It is not a real tumor, but a mass of normal functional hepatic cells, with proliferation of the connectivum. FNH was often confused clinically with some other malignant and benign tumors such as hepatocellular carcinoma, hepatic hemangioma, etc.
An important imaging technique has been addressed in this paper. Using a contrast method and postprocessing in a workstation, multi-section spiral computed tomography offers significant features which may reveal the characteristics of FNH pathology, and therefore the technique could make a reliable FNH diagnosis before biopsy or surgery.
Peer reviewers: Boris Guiu, MD, Department of Radiology, CHU le Bocage, Bd Maréchal de Lattre de Tassigny, 21000 Dijon, France; Dr. Giuseppe Currò, University of Messina, Via Panoramica, 30/A, 98168 Messina, Italy
S- Editor Tian L L- Editor Cant MR E- Editor Ma WH
| 1. | Wilson TS, Macgregor JW. Focal nodular hyperplasia of the liver: the solitary cirrhotic liver nodule. Can Med Assoc J. 1969;100:567-572. |
| 2. | Ohmoto K, Honda T, Hirokawa M, Mitsui Y, Iguchi Y, Kuboki M, Yamamoto S. Spontaneous regression of focal nodular hyperplasia of the liver. J Gastroenterol. 2002;37:849-853. |
| 3. | Wasif N, Sasu S, Conway WC, Bilchik A. Focal nodular hyperplasia: report of an unusual case and review of the literature. Am Surg. 2008;74:1100-1103. |
| 4. | Leconte I, Van Beers BE, Lacrosse M, Sempoux C, Jamart J, Materne R, Baudrez V, Horsmans Y. Focal nodular hyperplasia: natural course observed with CT and MRI. J Comput Assist Tomogr. 2000;24:61-66. |
| 5. | Bonney GK, Gomez D, Al-Mukhtar A, Toogood GJ, Lodge JP, Prasad R. Indication for treatment and long-term outcome of focal nodular hyperplasia. HPB (Oxford). 2007;9:368-372. |
| 6. | Faccioli N, D’Onofrio M, Comai A, Cugini C. Contrast-enhanced ultrasonography in the characterization of benign focal liver lesions: activity-based cost analysis. Radiol Med. 2007;112:810-820. |
| 7. | Koffron A, Geller D, Gamblin TC, Abecassis M. Laparoscopic liver surgery: Shifting the management of liver tumors. Hepatology. 2006;44:1694-1700. |
| 8. | Chen RC, Lii JM, Chou CT, Chang TA, Chen WT, Li CS, Tu HY. T2-weighted and T1-weighted dynamic superparamagnetic iron oxide (ferucarbotran) enhanced MRI of hepatocellular carcinoma and hyperplastic nodules. J Formos Med Assoc. 2008;107:798-805. |
| 9. | Reddy KR, Kligerman S, Levi J, Livingstone A, Molina E, Franceschi D, Badalamenti S, Jeffers L, Tzakis A, Schiff ER. Benign and solid tumors of the liver: relationship to sex, age, size of tumors, and outcome. Am Surg. 2001;67:173-178. |
| 10. | Di Carlo I, Urrico GS, Ursino V, Russello D, Puleo S, Latteri F. Simultaneous occurrence of adenoma, focal nodular hyperplasia, and hemangioma of the liver: are they derived from a common origin? J Gastroenterol Hepatol. 2003;18:227-230. |
| 11. | Mindikoglu AL, Regev A, Levi JU, Casillas J, Schiff ER. Focal nodular hyperplasia in identical twins. Am J Gastroenterol. 2005;100:1616-1619. |
| 12. | Fabre A, Audet P, Vilgrain V, Nguyen BN, Valla D, Belghiti J, Degott C. Histologic scoring of liver biopsy in focal nodular hyperplasia with atypical presentation. Hepatology. 2002;35:414-420. |
| 13. | Elsayes KM, Peterson CM, Menias CO. The central scar: pathophysiology and imaging features. Curr Probl Diagn Radiol. 2007;36:247-257. |
| 14. | Fukukura Y, Nakashima O, Kusaba A, Kage M, Kojiro M. Angioarchitecture and blood circulation in focal nodular hyperplasia of the liver. J Hepatol. 1998;29:470-475. |
| 15. | Fan MJ, Wang L, Zeng YJ, Li HG, Shen XM, Lin ML. Focal nodular hyperplasia of the liver:a clinicopathological analysis in 20 patients. Zhonguo Redai Yixue. 2008;8:2139-2140, 2077. |
| 16. | Petsas T, Tsamandas A, Tsota I, Karavias D, Karatza C, Vassiliou V, Kardamakis D. A case of hepatocellular carcinoma arising within large focal nodular hyperplasia with review of the literature. World J Gastroenterol. 2006;12:6567-6571. |
| 17. | Finley AC, Hosey JR, Noone TC, Shackelford DM, Varadarajulu S. Multiple focal nodular hyperplasia syndrome: diagnosis with dynamic, gadolinium-enhanced MRI. Magn Reson Imaging. 2005;23:511-513. |
| 18. | Turowski C, Feist H, Alzen G, Gluer S, Petersen C. Conversion of a neonatal hepatic hemangioma to focal nodular hyperplasia. Pathol Int. 2009;59:251-254. |
| 19. | Carlson SK, Johnson CD, Bender CE, Welch TJ. CT of focal nodular hyperplasia of the liver. AJR Am J Roentgenol. 2000;174:705-712. |
| 20. | Sanada Y, Yoshida K, Itoh H. Comparison of CT enhancement patterns and histologic features in hepatocellular carcinoma up to 2 cm: assessment of malignant potential with claudin-10 immunohistochemistry. Oncol Rep. 2007;17:1177-1182. |
| 21. | Ungermann L, Elias P, Zizka J, Ryska P, Klzo L. Focal nodular hyperplasia: spoke-wheel arterial pattern and other signs on dynamic contrast-enhanced ultrasonography. Eur J Radiol. 2007;63:290-294. |
| 22. | Bartolotta TV, Taibbi A, Midiri M, Lagalla R. Focal liver lesions: contrast-enhanced ultrasound. Abdom Imaging. 2009;34:193-209. |
| 23. | Yen YH, Wang JH, Lu SN, Chen TY, Changchien CS, Chen CH, Hung CH, Lee CM. Contrast-enhanced ultrasonographic spoke-wheel sign in hepatic focal nodular hyperplasia. Eur J Radiol. 2006;60:439-444. |
| 24. | Zheng L, Wu PH, Shen JX, Mo YX, Xie CM, Ruan CM, Li L. [Typical and atypical features of focal nodular hyperplasia of the liver on helical CT images]. Ai Zheng. 2006;25:861-865. |
| 25. | Zheng XH, Guan YS, Zhou XP, Huang J, Sun L, Li X, Liu Y. Detection of hypervascular hepatocellular carcinoma: Comparison of multi-detector CT with digital subtraction angiography and Lipiodol CT. World J Gastroenterol. 2005;11:200-203. |
| 26. | Miyayama S, Matsui O, Ueda K, Kifune K, Yamashiro M, Yamamoto T, Komatsu T, Kumano T. Hemodynamics of small hepatic focal nodular hyperplasia: evaluation with single-level dynamic CT during hepatic arteriography. AJR Am J Roentgenol. 2000;174:1567-1569. |
| 27. | Ibrahim S, Chen CL, Wang SH, Lin CC, Yang CH, Yong CC, Jawan B, Cheng YF. Liver resection for benign liver tumors: indications and outcome. Am J Surg. 2007;193:5-9. |
| 28. | Brancatelli G, Federle MP, Vullierme MP, Lagalla R, Midiri M, Vilgrain V. CT and MR imaging evaluation of hepatic adenoma. J Comput Assist Tomogr. 2006;30:745-750. |
| 29. | Attal P, Vilgrain V, Brancatelli G, Paradis V, Terris B, Belghiti J, Taouli B, Menu Y. Telangiectatic focal nodular hyperplasia: US, CT, and MR imaging findings with histopathologic correlation in 13 cases. Radiology. 2003;228:465-472. |
| 30. | Schmidt E, Udvaros E, Szabo Z, Zambo K. Varying appearance of focal nodular hyperplasia in nuclear medicine imaging. Clin Nucl Med. 2008;33:71-73. |
| 31. | Hong HS, Kim HS, Kim MJ, De Becker J, Mitchell DG, Kanematsu M. Single breath-hold multiarterial dynamic MRI of the liver at 3T using a 3D fat-suppressed keyhole technique. J Magn Reson Imaging. 2008;28:396-402. |
| 32. | Winterer JT, Kotter E, Ghanem N, Langer M. Detection and characterization of benign focal liver lesions with multislice CT. Eur Radiol. 2006;16:2427-2443. |
| 33. | Kamel IR, Liapi E, Fishman EK. Focal nodular hyperplasia: lesion evaluation using 16-MDCT and 3D CT angiography. AJR Am J Roentgenol. 2006;186:1587-1596. |