Published online Oct 14, 2009. doi: 10.3748/wjg.15.4810
Revised: July 22, 2009
Accepted: July 29, 2009
Published online: October 14, 2009
AIM: To evaluate the effectiveness of three-dimensional endoanal ultrasound (3D-EAUS) in the assessment of anal fistulae with and without H2O2 enhancement.
METHODS: Sixty-one patients (37 males, aged 17-74 years) with anal fistulae, which were not simple low types, were evaluated by physical examination and 3D-EAUS with and without enhancement. Fistula classification was determined with each modality and compared to operative findings as the reference standard.
RESULTS: The accuracy of 3D-EAUS was significantly higher than that of physical examination in detecting the primary tract (84.4% vs 68.7%, P = 0.037) and secondary extension (81.8% vs 62.1%, P = 0.01) and localizing the internal opening (84.2% vs 59.7%, P = 0.004). A contrast study with H2O2 detected several more fistula components including two primary suprasphincteric fistula tracks and one supralevator secondary extension, which were not detected on non-contrast study. However, there was no significant difference in accuracy between 3D-EAUS and H2O2-enhanced 3D-EAUS with respect to classification of the primary tract (84.4% vs 89.1%, P = 0.435) or secondary extension (81.8% vs 86.4%, P = 0.435) or localization of the internal opening (84.2% vs 89.5%, P = 0.406).
CONCLUSION: 3D-EAUS was highly reliable in the diagnosis of an anal fistula. H2O2 enhancement was helpful at times and selective use in difficult cases may be economical and reliable.
- Citation: Kim Y, Park YJ. Three-dimensional endoanal ultrasonographic assessment of an anal fistula with and without H2O2 enhancement. World J Gastroenterol 2009; 15(38): 4810-4815
- URL: https://www.wjgnet.com/1007-9327/full/v15/i38/4810.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4810
| Classification based on surgical findings | Overall accuracy | |||
| Intersphincteric (n = 26) | Transsphincteric (n = 34) | Suprasphincteric (n = 4) | ||
| Physical examination | ||||
| Intersphincteric | 201 | 9 | 0 | |
| Transsphincteric | 7 | 231 | 2 | 68.7% (44/64)a |
| Suprasphincteric | 0 | 2 | 11 | |
| 3D-EAUS | ||||
| Intersphincteric | 241 | 4 | 0 | |
| Transsphincteric | 4 | 291 | 1 | 84.4% (54/64)b |
| Suprasphincteric | 0 | 1 | 11 | |
| H2O2 3D-EAUS | ||||
| Intersphincteric | 241 | 3 | 0 | |
| Transsphincteric | 2 | 301 | 1 | 89.1% (57/64)c |
| Suprasphincteric | 0 | 1 | 31 | |
| Classification based on surgical findings (No. of secondary extensions detected) | Overall accuracy | ||||
| Absent (37) | Intersphincteric (7) | Ischiorectal (19) | Supralevator (3) | ||
| Physical examination | |||||
| Absent | 241 | 1 | 3 | ||
| Intersphincteric | 5 | 41 | 3 | 2 | |
| Ischiorectal | 8 | 2 | 121 | 62.1% (41/66)a | |
| Supralevator | 0 | 0 | 1 | 11 | |
| 3D-EAUS | |||||
| Absent | 321 | 0 | 2 | 1 | |
| Intersphincteric | 2 | 51 | 1 | 2 | |
| Ischiorectal | 3 | 2 | 161 | 0 | 81.8% (54/66)b |
| Supralevator | 0 | 0 | 0 | 11 | |
| H2O2 3D-EAUS | |||||
| Absent | 331 | 0 | 1 | 1 | |
| Intersphincteric | 1 | 51 | 1 | 0 | |
| Ischiorectal | 3 | 2 | 171 | 0 | 86.4% (57/66)c |
| Supralevator | 0 | 0 | 0 | 21 | |
| Classification based on surgical findings (No. of internal openings detected) | Overall accuracy | ||||
| Anterior (12) | Left lateral (8) | Posterior (31) | Right lateral (6) | ||
| Physical examination | |||||
| Anterior | 61 | 0 | 0 | 0 | |
| Left lateral | 2 | 51 | 1 | 0 | |
| Posterior | 0 | 1 | 201 | 1 | 59.7% (34/57)a |
| Right lateral | 0 | 0 | 2 | 31 | |
| 3D-EAUS | |||||
| Anterior | 101 | 0 | 1 | 0 | |
| Left lateral | 0 | 61 | 0 | 0 | |
| Posterior | 0 | 1 | 261 | 0 | 84.2% (48/57)b |
| Right lateral | 0 | 0 | 1 | 61 | |
| H2O2 3D-EAUS | |||||
| Anterior | 101 | 0 | 1 | 0 | |
| Left lateral | 0 | 71 | 0 | 0 | |
| Posterior | 0 | 0 | 281 | 0 | 89.5% (51/57)c |
| Right lateral | 0 | 0 | 1 | 61 | |
Despite the fact that anal fistulae are very common and have been studied extensively, some complex forms still continue to pose a difficult surgical problem. The aim of treatment for an anal fistula is to permanently eliminate abscess formation and achieve healing while preserving anal function and continence. Overly aggressive fistulotomy can lead to postoperative fecal incontinence, whereas inappropriate conservative treatment could lead to fistula recurrence. Therefore, accurate preoperative assessment of a fistula is necessary for optimal surgical results.
Inspection and digital examination with or without anesthesia are basic diagnostic methods. However, digital examination may fail to detect complex fistulae or to localize the internal opening. It is now well established that preoperative imaging modalities can alert the surgeon to fistula components that might otherwise be missed[1,2].
Endoanal ultrasonography (EAUS) has been increasingly used in the preoperative evaluation of anal fistulae. Initial EAUS evaluation was not satisfactory[3], but the diagnostic accuracy of EAUS has improved with technical advancements in ultrasonography, including the use of H2O2 as a contrast agent and 3D image reconstruction[4,5]. The image is no longer limited to the axial plane in 3D-EAUS. Instead, it is possible to cut across any part of the data set in the coronal, sagittal, or oblique plane. This property is expected to be helpful in tracing the tract and internal opening.
The H2O2-enhanced 3D-EAUS is the latest development in EAUS and is expected to diagnose anal fistulae with high accuracy. However, there is limited data on the accuracy and clinical usefulness of 3D-EAUS and H2O2-enhanced 3D-EAUS. The purpose of this study was to evaluate the accuracy of 3D-EAUS with or without H2O2 enhancement in identifying the primary tract, secondary extension, and internal opening in an anal fistula, compared to surgical findings as the standard reference.
Between January 2007 and February 2009, 61 patients (37 men and 24 women; mean age 39 years, range 17-74 years) with anal fistulae were preoperatively evaluated with physical examination, 3D-EAUS with and without enhancement, and subsequently underwent surgery. EAUS was performed when the surgeon thought preoperative imaging was clinically necessary. EAUS was not performed in patients with obvious simple fistulae with low, straight configuration and external openings close to the anus. Patients without visible external openings were also excluded.
Three patients had known Crohn’s disease, and two patients had a history of anal trauma leading to anal fistula formation. All other patients were believed to have fistulae of cryptoglandular origin. Twelve patients, including the three patients with Crohn’s disease, had previously undergone 15 fistula operations.
Physical examination was performed during the first visit to the outpatient clinic. Patients were examined in the left lateral position without any anesthesia. By inspection and palpation of the perianal area and by digital rectoanal examination, the fistula anatomy was determined by a single surgeon. Insertion of a probe was attempted on each occasion, provided it did not cause significant discomfort. The relationship between the components of the anal fistula and the sphincter complex was categorized (whether diagnosed with full confidence or by suspicion only) and recorded.
Physical examination aimed to determine the following fistula characteristics: (1) the primary tract, categorized according to the criteria of Parks et al[6] as intersphincteric, transsphincteric, extrasphincteric, or suprasphincteric; (2) secondary extension, including horseshoe tract and abscess formation; and (3) the internal opening, localized with respect to a clock face and categorized as anterior, left lateral, posterior, or right lateral. The anatomic location of any secondary extension arising from the primary fistula track was recorded as intersphincteric, ischiorectal, or supralevator. A horseshoe extension was defined as any extension from the primary track that appeared to extend to both sides of the internal opening, and such an extension was classified as intersphincteric or ischiorectal.
Patients underwent surgical treatment after these examinations, and each component of the anal fistula was categorized and recorded using the same criteria.
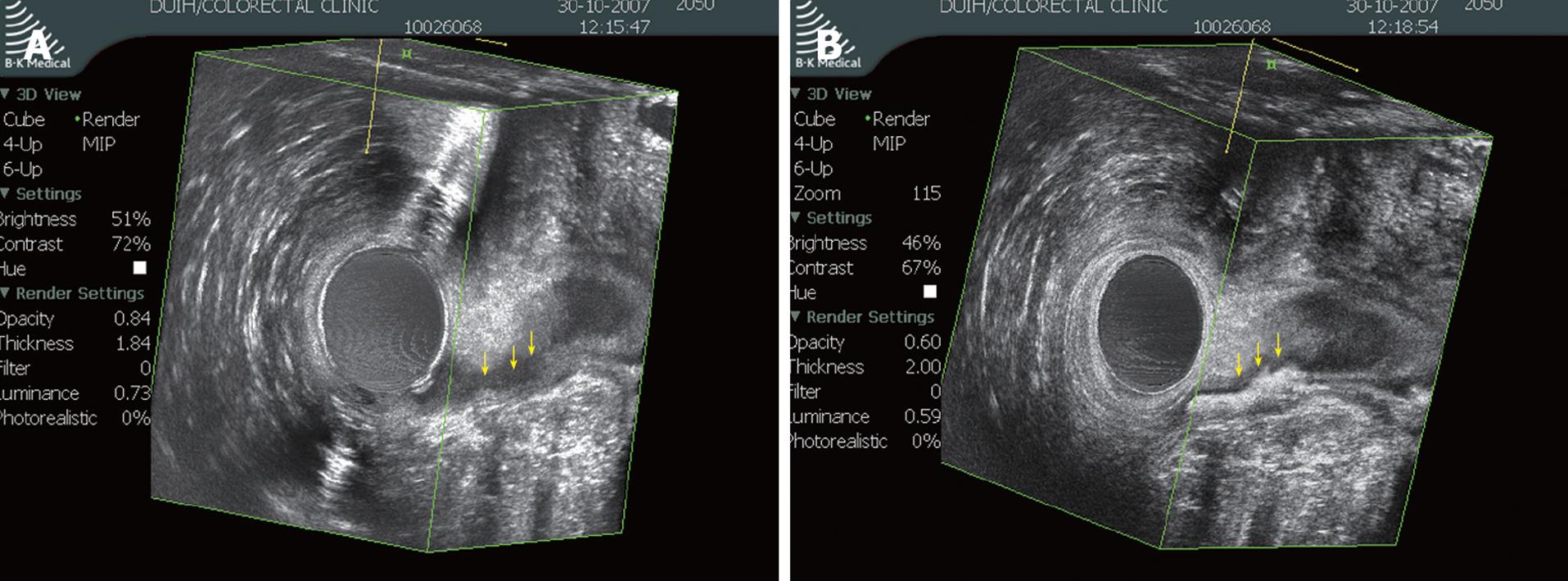
Images were acquired with a 10-MHz, 360°, rotating endoprobe (type 2050, BK Medical, Herlev, Denmark). The data from a series of closely spaced EAUS images (0.25 mm) were combined to create a 3D volume displayed as a cube. The endoprobe was introduced into the anal canal with the patient in the left lateral position. Serial radial images were made of the distal part of the rectum and the anal canal using the automatic probe withdrawal system. Three-dimensional ultrasound images were obtained with a software program used for 3D reconstruction (Life Imaging System 2000, L3D1 version 3.5.5; B-K Medical). Fistula tracks were visualized as tube-like, hypoechoic lesions. The internal fistula opening was identified as a hypoechoic area in the intersphincteric plane, as a defect in the internal anal sphincter, or as a subepithelial breach that connected to the fistulous tract through an internal sphincter defect[7].
After the pre-enhanced data set was saved, H2O2-enhanced sonography was performed as previously described, with some modifications[8]. Briefly, a flexible intravenous cannula (16-21 G Angiocath) was inserted into the fistula tract through the external opening and fixed with adhesive tape. This cannula was connected to a 10 mL syringe containing 3% H2O2. Ultrasonic scanning was started approximately 20 s after instillation of H2O2 to allow time for bubble release. The contrast study was usually performed with infusion of a small amount (0.5-3.0 mL) of H2O2. Further injection of H2O2 at a little higher pressure was performed when contrast was not sufficient. The low echogenic tract seen on the non-enhanced study became bright due to reflection from the gas, with acoustic shadowing deep to the track after H2O2 installation.
After the EAUS procedures, the characteristics of the fistula were classified according to the same criteria used in the clinical evaluation.
The accuracy of the preoperative diagnosis established through physical examination, 3D-EAUS, and H2O2-enhanced 3D-EAUS was compared against the surgical findings. Primary fistula tracts were considered correctly classified when placed in the correct anatomic group of the Parks classification[6]. Secondary extensions (abscess and/or horseshoe extension) were considered to be correctly classified when placed in the correct anatomical compartment and quadrant, and internal openings were considered to be correctly classified when placed in the correct quadrant and level. The accuracy of 3D-EAUS and H2O2-enhanced 3D-EAUS in detecting and classifying components of a given fistula was compared with the surgical findings as a reference standard. Fisher’s exact test was used to detect significant differences in diagnostic accuracy. Statistical significance was defined as P < 0.05.
Preoperative clinical assessment and 3D-EAUS were conducted in all 61 patients. One patient complained of severe local pain, and another patient noted lower abdominal pain during the instillation of H2O2. The instillation was stopped instantly, and the patient with abdominal pain was closely monitored. The symptoms spontaneously subsided the next day without any problems. There were no adverse effects from the instillation of H2O2 except in these cases. The mean duration between ultrasound and surgery was 6 d (range, 0-18 d).
After the surgical procedures, we identified a total of 64 primary fistula tracts in 61 patients: 26 intersphincteric fistulae, 34 transsphincteric fistulae, and 4 suprasphincteric fistulae. The accuracy of physical examination in detecting the primary fistula tract was 68.7%, with surgical findings serving as the standard reference.
The concordance rates of 3D-EAUS and H2O2-enhanced 3D-EAUS with surgical findings with respect to identification of the primary tract were 84.4% and 89.1%, respectively (Table 1). These figures indicate that both 3D-EAUS and H2O2-enhanced 3D-EAUS were superior to physical examination in detecting the primary tract (P = 0.037 and P = 0.005, respectively). The H2O2 enhancement enabled detection of one more transsphincteric fistula and two more suprasphincteric fistulae. Apart from the effect on accuracy, H2O2 enhancement made the tract more conspicuous in ambiguous cases (Figure 1). However, there was no statistically significant difference between 3D-EAUS and H2O2-enhanced 3D-EAUS with respect to accurate detection of the primary tract (P = 0.435).
Twenty-nine secondary extensions, including abscesses and horseshoe tracts, were detected in 26 patients during surgical exploration: 7 intersphincteric, 19 ischiorectal, and 3 supralevator. The overall accuracy of clinical evaluation in identifying secondary extension was 62.1%, as shown in Table 2. The overall accuracy of 3D-EAUS (81.8%) was significantly higher than that of physical examination (P = 0.01). The use of H2O2 did not further improve the diagnostic accuracy (P = 0.482), but one ischiorectal extension and one supralevator type extension that were not clear on non-enhanced study were identified in enhanced study.
A total of 57 internal openings were detected in 54 patients at the time of surgery. No internal openings were found in seven patients, while two openings were found in three patients. The internal opening was most frequently found in the posterior position (36 patients), followed by anterior, right, and left positions. The concordance between physical examination and surgery with respect to the location of the internal opening was 59.7%. The concordance between surgical finding and 3D-EAUS was 84.2%, and that between surgical finding and H2O2-enhanced 3D-EAUS was 89.5%. Statistical analysis indicated that both 3D-EAUS and H2O2-enhanced 3D-EAUS were more accurate than physical examination (Table 3). No difference in diagnostic accuracy was noted between 3D-EAUS and H2O2-enhanced 3D-EAUS, with respect to identifying the internal opening (P = 0.406).
There has been much controversy regarding the accuracy and usefulness of EAUS in anal fistula. According to our literature review, the accuracy of EAUS compared with surgery in the detection of the internal opening has varied between 28% and 94%, and the accuracy in the detection of the primary tract and secondary extension have been in the range of 36%-100% and 23%-92%, respectively[9-12]. Such a wide range of accuracy rates could be related to differences in the criteria used to identify the internal opening, differences in operator experience, and differences in the complexities of fistulae recruited. In addition, the use of H2O2 and variable ultrasound equipment in individual studies may also be responsible for such differences.
The most noticeable improvement in the accuracy of EAUS may derive from the use of H2O2 as a contrast material. Many studies have shown significantly superior results in H2O2-enhanced EAUS, compared to unenhanced study[4,13,14]. Another technical development is the use of high frequency transducers and the introduction of 3D technology in EAUS[15].
In the present study, the agreement of 3D-EAUS with surgery was excellent: 84% in the detection of primary tracts, 84% in internal openings, and 82% in secondary extensions. These outcomes are consistent with recent data concerning 3D-EAUS[16-18].
Most reports using conventional 2D-EAUS have shown superior results with H2O2 enhancement. However, only a few reports have addressed the effect of H2O2 in 3D-EAUS. Ratto et al[16] obtained improved diagnostic accuracy by using H2O2, and West et al[5] reported high diagnostic accuracy with H2O2 enhanced 3D-EAUS, comparable to MRI. However, the improved accuracy was not apparent in the study of Buchanan et al[17]. We identified a few more primary tracts and secondary extensions by using H2O2. However, there was no statistically significant difference between 3D-EAUS and H2O2 enhanced 3D-EAUS with respect to classifying primary tracks, internal openings, and secondary tracks.
Several explanations may be offered concerning the lack of favorable results with H2O2. First, 3D-EAUS was already highly accurate prior to the use of H2O2 in this study. The already elevated baseline may have left little room for improvement on the contrast study.
One of theoretical limitations of non-contrast EAUS is difficulty discriminating between an active tract and scar tissue since both tissues appear hypoechoic on noncontrast EAUS[19]. The gas generated after H2O2 instillation makes the active tract hyperechoic. In this regard, contrasting with H2O2 could be more useful in patients with recurrent fistulae, which usually accompany previous operative scars. Several authors have suggested that H2O2 might be more helpful in recurrent cases[8,17]. The number of recurrent or Crohn’s disease cases in this study was relatively low. The paucity of recurrent and complicated cases might have limited the advantages of H2O2 enhancement.
Instillation of H2O2 is generally accepted to be very safe, but several complications have been reported, including air embolism[20,21]. Such complications have been associated with large infusion volume or forceful instillation. Of note, one patient in this study complained of abdominal pain in the early period of this study, although symptoms subsided spontaneously. The cause for the abdominal pain is not clear, but it might be associated with the relatively large volume (10 mL) of H2O2 and the high injection pressure. We were very careful not to inject forcefully after that event, and no patients experienced complications thereafter. Such gentle instillation might negatively influence the usefulness of enhancement.
Another point to consider is that although H2O2 enhancement had little influence on diagnostic accuracy improvement, H2O2 enhancement often improved the quality of images in cases where the unenhanced study barely delineated the tract as shown in Figure 1 of this study and in another report[17]. The presence of gas in the internal opening or fistula track made the tract more obvious. It is notable that this beneficial effect was observed often in difficult cases including suprasphincteric fistula tract and recurrent fistulae, in which accurate anatomical assessment is far more important.
Considering these results, selective use of H2O2 in difficult cases rather than routine use may be economical and helpful.
EAUS was inferior to MRI in most earlier studies[11,22], and it was inferior[2,23] or equivalent[24,25] in subsequent studies. A conventional 2D-EAUS was used in most of these comparative studies. In the only prospective comparative study evaluating 3D-EAUS and MRI, both modalities were shown to be equally accurate[18]. The high accuracy of 3D-EAUS, which is comparable with MRI, indicates that 3D-EAUS may be the first choice in the assessment of anal fistulae, since it has advantages over MRI including easier use and lower cost.
In addition to the high accuracy and low cost, EAUS provides additional usefulness in clinical practice. Yee et al[26] recognized a high incidence (92%) of coexisting occult sphincter defects in the evaluation of rectovaginal fistulae. Sphincter defects were noted more frequently based on EAUS than based on manometry or a history of fecal incontinence. In fact, the ability to accurately display the anal sphincter muscle anatomy is an inherent advantage of EAUS. This method clearly shows the overall volume of the sphincter muscle, as well as sphincter injury. Knowledge of sphincter status and associated injuries recognized on EAUS facilitates surgical decisions on whether to proceed with a sphincter-saving procedure or lay open. Surgical treatment in this study was chosen largely based on preoperative EAUS findings. Seton or mucosal advancement flap was favored over lay open when there was associated sphincter damage or a high type fistula.
Most previous reports have regarded surgical results as the reference standard. However, surgery as a gold standard has been questioned, as studies have shown that EAUS and MRI are able to detect fistula tracts that are not seen on surgical exploration[13,18]. Buchanan et al[2] suggested using clinical outcomes rather than surgical findings as a reference standard because missed occult infection is possible during surgical exploration. In view of the high accuracy of 3D-EAUS, the lack of data on clinical outcomes and the use of surgical findings as the gold standard in this study might in part have biased our results. This study was also limited by the retrospective study design and the low prevalence of high type fistulae. The latter made it difficult to draw clear conclusions as to how adequate 3D-EAUS was in detecting high type fistulae.
In conclusion, 3D-EAUS is highly reliable in the preoperative evaluation of anal fistulae. The use of H2O2 for enhancement offers some benefits, although it did not significantly improve the diagnostic accuracy in this study. The selective use of H2O2 may be economical and reliable in difficult cases.
Accurate preoperative assessment of anal fistula is very important for optimal surgical results. Physical examination is a basic diagnostic method, but it often fails to accurately diagnose complex fistula. Endoanal ultrasonography (EAUS) is a useful tool in this situation, and recently introduced 3D-EAUS is expected to further increase diagnostic accuracy.
In this study, the authors demonstrated that 3D-EAUS is highly accurate in the evaluation of anal fistula without or without H2O2 enhancement. They also showed that the selective application of H2O2 enhancement in difficult cases is an effective and economic strategy.
Recently, EAUS has been widely applied in the diagnosis of anal fistula. However, only a few reports have been published regarding the diagnostic accuracy of 3D-EAUS. This report would be helpful in accumulating data in this field.
This study would be a useful guideline to the surgeon dealing with anal fistula.
The author evaluated the effectiveness of 3D-EAUS in the assessment of the anal fistula with and without H2O2 enhancement. This paper is written well and is an important paper for further studies.
Peer reviewer: Subbaramiah Sridhar, MB, BS, MPH, FRCP (Edin), FRCP (Glasg), FRCP (Lond), FRSS (Lond), FRCPC (Medicine & Gastroenterology), FACP, FACG, FASGE, AGAF, Section of Gastroenterology, BBR 2544, Medical College of Georgia, 15th Street, Augusta, GA 30912, United States
S- Editor Li LF L- Editor Webster JR E- Editor Ma WH
| 1. | Lindsey I, Humphreys MM, George BD, Mortensen NJ. The role of anal ultrasound in the management of anal fistulas. Colorectal Dis. 2002;4:118-122. |
| 2. | Buchanan GN, Halligan S, Bartram CI, Williams AB, Tarroni D, Cohen CR. Clinical examination, endosonography, and MR imaging in preoperative assessment of fistula in ano: comparison with outcome-based reference standard. Radiology. 2004;233:674-681. |
| 3. | Choen S, Burnett S, Bartram CI, Nicholls RJ. Comparison between anal endosonography and digital examination in the evaluation of anal fistulae. Br J Surg. 1991;78:445-447. |
| 4. | Cheong DM, Nogueras JJ, Wexner SD, Jagelman DG. Anal endosonography for recurrent anal fistulas: image enhancement with hydrogen peroxide. Dis Colon Rectum. 1993;36:1158-1160. |
| 5. | West RL, Dwarkasing S, Felt-Bersma RJ, Schouten WR, Hop WC, Hussain SM, Kuipers EJ. Hydrogen peroxide-enhanced three-dimensional endoanal ultrasonography and endoanal magnetic resonance imaging in evaluating perianal fistulas: agreement and patient preference. Eur J Gastroenterol Hepatol. 2004;16:1319-1324. |
| 7. | Cho DY. Endosonographic criteria for an internal opening of fistula-in-ano. Dis Colon Rectum. 1999;42:515-518. |
| 8. | Kruskal JB, Kane RA, Morrin MM. Peroxide-enhanced anal endosonography: technique, image interpretation, and clinical applications. Radiographics. 2001;21 Spec No:S173-S189. |
| 9. | Cataldo PA, Senagore A, Luchtefeld MA. Intrarectal ultrasound in the evaluation of perirectal abscesses. Dis Colon Rectum. 1993;36:554-558. |
| 10. | Deen KI, Williams JG, Hutchinson R, Keighley MR, Kumar D. Fistulas in ano: endoanal ultrasonographic assessment assists decision making for surgery. Gut. 1994;35:391-394. |
| 11. | Hussain SM, Stoker J, Schouten WR, Hop WC, Lameris JS. Fistula in ano: endoanal sonography versus endoanal MR imaging in classification. Radiology. 1996;200:475-481. |
| 12. | Navarro-Luna A, Garcia-Domingo MI, Rius-Macias J, Marco-Molina C. Ultrasound study of anal fistulas with hydrogen peroxide enhancement. Dis Colon Rectum. 2004;47:108-114. |
| 13. | Poen AC, Felt-Bersma RJ, Eijsbouts QA, Cuesta MA, Meuwissen SG. Hydrogen peroxide-enhanced transanal ultrasound in the assessment of fistula-in-ano. Dis Colon Rectum. 1998;41:1147-1152. |
| 14. | Ratto C, Gentile E, Merico M, Spinazzola C, Mangini G, Sofo L, Doglietto G. How can the assessment of fistula-inano be improved? Dis Colon Rectum. 2000;43:1375-1382. |
| 15. | Gravante G, Giordano P. The role of three-dimensional endoluminal ultrasound imaging in the evaluation of anorectal diseases: a review. Surg Endosc. 2008;22:1570-1578. |
| 16. | Ratto C, Grillo E, Parello A, Costamagna G, Doglietto GB. Endoanal ultrasound-guided surgery for anal fistula. Endoscopy. 2005;37:722-728. |
| 17. | Buchanan GN, Bartram CI, Williams AB, Halligan S, Cohen CR. Value of hydrogen peroxide enhancement of three-dimensional endoanal ultrasound in fistula-in-ano. Dis Colon Rectum. 2005;48:141-147. |
| 18. | West RL, Zimmerman DD, Dwarkasing S, Hussain SM, Hop WC, Schouten WR, Kuipers EJ, Felt-Bersma RJ. Prospective comparison of hydrogen peroxide-enhanced three-dimensional endoanal ultrasonography and endoanal magnetic resonance imaging of perianal fistulas. Dis Colon Rectum. 2003;46:1407-1415. |
| 19. | Law PJ, Talbot RW, Bartram CI, Northover JM. Anal endosonography in the evaluation of perianal sepsis and fistula in ano. Br J Surg. 1989;76:752-755. |
| 20. | Tsai SK, Lee TY, Mok MS. Gas embolism produced by hydrogen peroxide irrigation of an anal fistula during anesthesia. Anesthesiology. 1985;63:316-317. |
| 21. | Schwab C, Dilworth K. Gas embolism produced by hydrogen peroxide abscess irrigation in an infant. Anaesth Intensive Care. 1999;27:418-420. |
| 22. | Lunniss PJ, Barker PG, Sultan AH, Armstrong P, Reznek RH, Bartram CI, Cottam KS, Phillips RK. Magnetic resonance imaging of fistula-in-ano. Dis Colon Rectum. 1994;37:708-718. |
| 23. | Maier AG, Funovics MA, Kreuzer SH, Herbst F, Wunderlich M, Teleky BK, Mittlbock M, Schima W, Lechner GL. Evaluation of perianal sepsis: comparison of anal endosonography and magnetic resonance imaging. J Magn Reson Imaging. 2001;14:254-260. |
| 24. | Gustafsson UM, Kahvecioglu B, Astrom G, Ahlstrom H, Graf W. Endoanal ultrasound or magnetic resonance imaging for preoperative assessment of anal fistula: a comparative study. Colorectal Dis. 2001;3:189-197. |
| 25. | Schwartz DA, Wiersema MJ, Dudiak KM, Fletcher JG, Clain JE, Tremaine WJ, Zinsmeister AR, Norton ID, Boardman LA, Devine RM. A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn's perianal fistulas. Gastroenterology. 2001;121:1064-1072. |
| 26. | Yee LF, Birnbaum EH, Read TE, Kodner IJ, Fleshman JW. Use of endoanal ultrasound in patients with rectovaginal fistulas. Dis Colon Rectum. 1999;42:1057-1064. |