Published online Oct 14, 2009. doi: 10.3748/wjg.15.4794
Revised: August 14, 2009
Accepted: August 21, 2009
Published online: October 14, 2009
AIM: To examine the effects of long-term proton pump inhibitor (PPI) therapy on body weight (BW) and body mass index (BMI) in patients with gastroesophageal reflux disease (GERD).
METHODS: The subjects were 52 patients with GERD and 58 sex- and age-matched healthy controls. GERD patients were treated with PPI for a mean of 2.2 years (range, 0.8-5.7 years), and also advised on lifestyle modifications (e.g. selective diet, weight management). BW, BMI and other parameters were measured at baseline and end of study.
RESULTS: Twenty-four GERD patients were treated daily with 10 mg omeprazole, 12 with 20 mg omeprazole, 8 with 10 mg rabeprazole, 5 with 15 mg lansoprazole, and 3 patients with 30 mg lansoprazole. At baseline, there were no differences in BW and BMI between reflux patients and controls. Patients with GERD showed increases in BW (baseline: 56.4 ± 10.4 kg, end: 58.6 ± 10.8 kg, mean ± SD, P < 0.0001) and BMI (baseline: 23.1 ± 3.1 kg/m2, end: 24.0 ± 3.1 kg/m2, P < 0.001), but no such changes were noted in the control group. Mean BW increased by 3.5 kg (6.2% of baseline) in 37 (71%) reflux patients but decreased in only 6 (12%) patients during treatment.
CONCLUSION: Long-term PPI treatment was associated with BW gain in patients with GERD. Reflux patients receiving PPI should be encouraged to manage BW through lifestyle modifications.
- Citation: Yoshikawa I, Nagato M, Yamasaki M, Kume K, Otsuki M. Long-term treatment with proton pump inhibitor is associated with undesired weight gain. World J Gastroenterol 2009; 15(38): 4794-4798
- URL: https://www.wjgnet.com/1007-9327/full/v15/i38/4794.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4794
| Patients | Control | P | |
| Number of subjects | 52 | 58 | - |
| Gender (male/female) | 36/16 | 38/20 | NS |
| Age (yr) | 68.1 ± 10.4 | 68.8 ± 1.5 | NS |
| Duration of observation (yr) | NS | ||
| PPI therapy | 2.1 ± 1.1 | - | - |
| Medical checkup | - | 2.0 ± 0.4 | - |
| Body weight (kg) | 56.4 ± 10.4 | 58.6 ± 8.4 | NS |
| Height (cm) | 156.0 ± 9.8 | 156.0 ± 8.9 | NS |
| Body mass index (kg/m2) | 23.1 ± 3.1 | 24.1 ± 2.7 | NS |
| Blood pressure (mmHg) | - | ||
| Systolic | 133 ± 17 | 132 ± 16 | NS |
| Diastolic | 75 ± 11 | 75 ± 9 | NS |
| Serum total protein (g/dL) | 7.2 ± 0.3 | 7.2 ± 0.3 | NS |
| Serum total cholesterol (mg/dL) | 210 ± 39 | 210 ± 25 | NS |
| Serum triglyceride (mg/dL) | 121 ± 52 | 107 ± 43 | NS |
| Patients (n = 52) | |
| Helicobacter pylori status | |
| Negative | 9 |
| Positive | 11 |
| ND | 32 |
| Endoscopic findings | |
| Normal | 18 |
| LA grade A | 6 |
| LA grade B | 13 |
| LA grade C | 5 |
| LA grade D | 3 |
| ND | 7 |
| Number of patients according to PPI regimens | |
| Omeprazole 10 mg once daily | 24 |
| Omeprazole 20 mg once daily | 12 |
| Rabeprazole 10 mg once daily | 8 |
| Lansoprazole 15 mg once daily | 5 |
| Lansoprazole 30 mg once daily | 3 |
Gastroesophageal reflux disease (GERD) is the most common esophageal disorder, and frequently encountered in the primary care setting. It has been estimated that 15%-25% of persons experience reflux symptoms at least weekly, and 5%-12% suffer on a daily basis[1].
The risk of reflux symptoms, erosive esophagitis, or esophageal adenocarcinoma increases with excessive weight and obesity[2]. Accumulating evidence has confirmed the excellent efficacy and safety of proton pump inhibitor (PPI) therapy in patients with all grades of GERD, making these agents the mainstay of treatment. Consequently, PPIs comprise the largest outpatient pharmacy expenditure in the United States. Body weight loss is commonly recommended as part of a first-line therapeutic measure for GERD, although lifestyle modifications have been relegated to a minor role in the therapeutic regime due to the effectiveness and availability of PPIs as an acid-suppressive therapy[3].
GERD is a chronic condition, necessitating continuous therapy for many patients to control symptoms and prevent complications. Long-term therapeutic options include PPI therapy and surgical or endoscopic procedures[4]. Recently, body weight loss after laparoscopic Nissen fundoplication was reported[5]. There is an extensive literature database that addresses the efficacy and safety of long-term PPI therapy. However, the possible impact of changes in body weight or body mass index (BMI) in reflux patients while on long-term PPI therapy has not been examined. We present herein the first report elucidating the effect on nutritional parameters such as body weight and BMI in patients receiving long-term PPI therapy.
We evaluated 52 adult patients with GERD and 58 healthy controls. We selected patients undergoing daily maintenance therapy of PPI for at least 10 mo at the University Hospital of Occupational and Environmental Health and four Gastroenterology Clinics between June and November 2005. Patients who had prior fundoplication or poor compliance with medication were excluded. Patients with GERD had received advice on lifestyle modifications such as selective diet and weight management to accompany the PPI treatment.
The controls were sex- and age-matched subjects who visited the clinic for a yearly medical examination; they were free of reflux symptoms, and did not take PPIs or histamine receptor antagonists. They did not receive advice on lifestyle modifications. Informed consent was obtained from all subjects and the study was performed in accordance with the Declaration of Helsinki as revised in 1989.
The diagnosis of GERD was made based only on the typical symptoms of troublesome heartburn and/or acid regurgitation. Endoscopy at presentation was performed in patients with alarm symptoms such as dysphagia, odynophagia, bleeding, weight loss, and anemia that together suggested a complicated disease.
Initial therapy was a standard dose of PPI (omeprazole 20 mg, rabeprazole 20 mg, or lansoprazole 30 mg) once daily for 8 wk followed by a daily maintenance half-dose therapy. The patients were followed-up at 4-wk intervals in the clinics to assess symptom recurrence. Patients found to have recurring symptoms of heartburn or acid regurgitation were placed back on their initial PPI dose. The patients were educated on lifestyle modifications by their physicians in addition to the PPI treatment. These instructions included avoidance of overeating, decreased fat intake, elevation of the head of the bed, cessation of smoking, avoiding recumbency for postprandial 3 h, and body weight control.
Body weight, height, and blood pressure, as well as fasting serum levels of total protein, total cholesterol, and triglycerides were determined at baseline and at the last visit. The BMI was calculated as body weight (kg)/[height (m)]2. These parameters obtained within four weeks before the commencement of PPI therapy were defined as baseline data.
All results were expressed as mean ± SD. Categorical outcome variables were analyzed with Fisher’s exact test. For continuous variables, the Mann-Whitney U-test and Student’s t-test were used where appropriate. A P value less than 0.05 denoted the presence of a statistically significant difference between the groups.
Table 1 details the characteristics of the 52 reflux patients and 58 healthy controls. There were no significant differences between the patient and control groups with regard to age, sex, duration of observation, body weight, body height, BMI, blood pressure, and serum values of total protein, total cholesterol, and triglycerides. Helicobacter pylori status, endoscopic findings, and PPI regimens of daily maintenance therapy in reflux patients are listed in Table 2.
No significant differences were found between patient and control groups with respect to changes in blood pressure or serum values of total protein, total cholesterol, and triglycerides. In contrast, patients treated with PPI experienced significantly greater increases from the baseline to the last visit in body weight (P < 0.0001) and BMI (P < 0.0005) than controls (Table 3).
The differences in body weight and BMI between the baseline and the last visit were analyzed separately for both groups (Table 3). Body weight (P < 0.0001) and BMI (P < 0.0001) significantly increased at the last visit in reflux patients. In contrast, there was no significant difference in body weight or BMI at the last visit in controls.
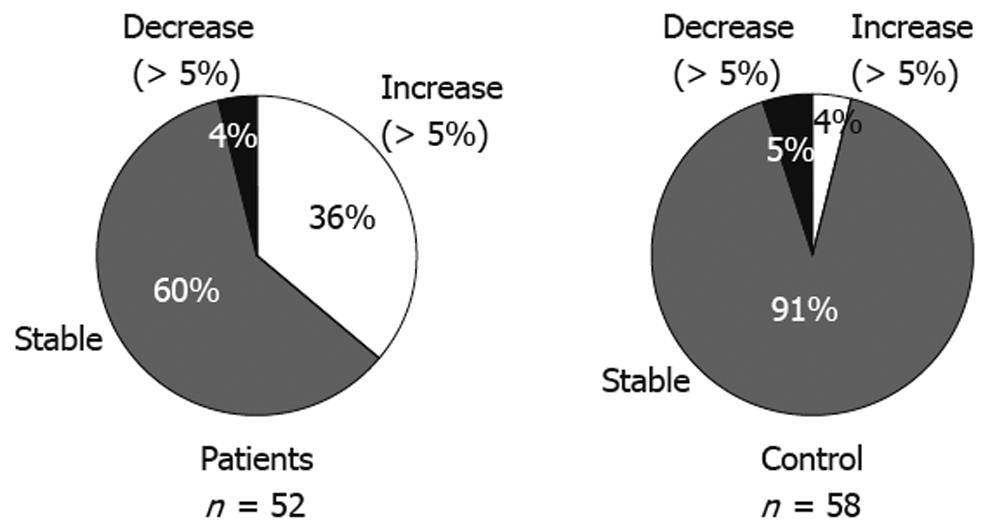
Categorical changes in body weight at the last visit compared to the baseline values are shown in Figure 1. Most of the control group (91%) remained stable, defined by a change of no more than 5% compared to baseline weight; however, only 60% of the PPI group remained stable. In addition, 36% of these patients had an increase in body weight above baseline of more than 5%, compared with 4% of the control group (P < 0.0001).
This study demonstrated for the first time that long-term PPI treatment is associated with undesirable body weight gain in patients with GERD, despite lifestyle modification recommendations by their physicians. Heartburn is the classical symptom of GERD, with patients generally reporting a burning feeling, rising from the stomach and radiating toward the neck and throat. It usually occurs postprandially, particularly after large meals or the consumption of fats. Untreated patients suffering from reflux symptoms find it difficult to have large meals, because this generally aggravates their symptoms. Untreated patients may therefore reduce their meal sizes and intake of fats intentionally or unintentionally. It is conceivable, therefore, that the resolution of reflux symptoms by PPI treatment leads to a higher food intake resulting in body weight gain.
Laparoscopic Nissen fundoplication has evolved as a gold standard in antireflux surgery. This surgical therapy induces a significant and persistent reduction in body weight, possibly due to postoperative dysphagia or delayed gastric emptying[5]. In contrast, the option of long-term PPI therapy was associated with a significant body weight gain in the present study. Omeprazole and other PPIs delay gastric emptying[6-9], which induces postprandial fullness, dyspeptic symptoms, gastrointestinal bacterial overgrowth, and subsequent weight loss[10,11]. Our results have clearly demonstrated that long-term PPI therapy contributed significantly to body weight changes in patients with GERD by relieving the adverse symptoms rather than altering the state of gastric emptying.
Numerous circulating peptides influence appetite. Ghrelin is produced in the stomach and acts as a meal initiator. A recent report revealed that long-term PPI therapy did not change the serum ghrelin level[12]. Another peptide, leptin, is produced in the stomach and acts as an enteric signal involved in energy homeostasis. Change of this peptide associated with PPI therapy has not been reported.
A practice guideline for patients with GERD recommends the use of lifestyle modifications such as elevation of the bed head, a decreased intake of fat, chocolate, alcohol, peppermint, coffee, onions and garlic, cessation of smoking, and avoiding recumbency for three hours postprandially, in addition to taking antireflux medications[13]. However, the positive advantage of such lifestyle modifications on the patient’s condition is not well substantiated. Among these lifestyle interventions, elevation of the bed head, left lateral decubitus positioning, and weight loss are associated with improvement in reflux symptoms in case-control studies[14,15]. These modifications alone, however, are unlikely to control symptoms in the majority of patients[13]. Our results support the finding that lifestyle modifications are an essential component of the treatment for GERD and the prevention of weight gain during PPI treatment.
There is a growing body of literature regarding the association between BMI and GERD[2,16-24]. A recent large meta-analysis of previous studies demonstrated a strong positive relationship between BMI and reflux symptoms[2]. In addition, moderate weight gains, even among normal-weight persons, resulted in the development or exacerbation of symptoms in GERD patients[16]. In the present study, the patients significantly increased their body weight during PPI therapy. Appropriate management of body weight during PPI treatment should reduce the duration of PPI use or PPI dosage.
Excessive weight is associated with an increased risk of coronary heart disease, hypertension, angina, stroke, and diabetes, and constitutes an important cardiovascular health burden[25]. Body weight gain associated with lifetime GERD treatment may induce further medical costs in addition to the PPI therapy. Unfortunately, potentially effective diet modifications are often underestimated in the presence of various PPI regimens. Healthcare providers still recommend lifestyle changes in a moderate percentage of GERD patients[26], and while PPIs have become of pivotal importance for the initial and maintenance treatment of GERD, repeated lifestyle modification recommendations are required.
In conclusion, we elucidated in the present study the impact of long-term PPI therapy on body weight. Undesired body weight gain was observed in GERD patients on long-term PPI treatment. Reflux patients treated with a daily maintenance therapy of PPI should be strongly encouraged to manage their body weight through lifestyle modifications such as proper diet and avoidance of overeating. This measure may reduce the overall medical costs associated with obesity-related illness as well as GERD. Lifestyle modification must therefore remain the backbone of treatment for all patients with GERD, even in the PPI era.
Gastroesophageal reflux disease (GERD) is the most common esophageal disorder, and frequently encountered in the primary care setting. The risk of reflux symptoms and erosive esophagitis increases with excessive weight and obesity.
Many studies have confirmed the efficacy and safety of proton pump inhibitor (PPI) therapy in patients with GERD, making these agents the mainstay of treatment.
This study demonstrated for the first time that long-term PPI treatment is associated with undesirable body weight gain in reflux patients, despite lifestyle modification recommendations by their physicians.
Reflux patients treated with a daily maintenance therapy of PPI should be strongly encouraged to manage their body weight through lifestyle modifications such as proper diet and avoidance of overeating.
GERD is a common esophageal disorder which is becoming increasingly prevalent in the population in parallel with similar rises in the frequency of metabolic disorders.
This study showed that long-term PPI treatment is associated with body weight gain and that lifestyle modification must therefore remain the backbone of treatment for patients with GERD. This report would impact on the ongoing treatment of GERD.
Peer reviewers: Dr. Katsunori lijima, Division of Gastroenterology, Tohoku University Graduate School of Medicine, 1-1 Seiryo-machi, Aobaku., Sendai 980-8574, Japan; Andrew Ukleja, MD, Assistant Professor, Clinical Assistant Professor of Medicine, Director of Nutrition Support Team, Director of Esophageal Motility Laboratory, Cleveland Clinic Florida, Department of Gastroenterology, 2950 Cleveland Clinic Blvd., Weston, FL 33331, United States
S- Editor Tian L L- Editor Logan S E- Editor Zheng XM
| 1. | Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448-1456. |
| 2. | Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199-211. |
| 3. | Kitchin LI, Castell DO. Rationale and efficacy of conservative therapy for gastroesophageal reflux disease. Arch Intern Med. 1991;151:448-454. |
| 4. | Metz DC. Managing gastroesophageal reflux disease for the lifetime of the patient: evaluating the long-term options. Am J Med. 2004;117 Suppl 5A:49S-55S. |
| 5. | Neumayer C, Ciovica R, Gadenstätter M, Erd G, Leidl S, Lehr S, Schwab G. Significant weight loss after laparoscopic Nissen fundoplication. Surg Endosc. 2005;19:15-20. |
| 6. | Anjiki H, Sanaka M, Kuyama Y. Dual effects of rabeprazole on solid-phase gastric emptying assessed by the 13C-octanoate breath test. Digestion. 2005;72:189-194. |
| 7. | Benini L, Castellani G, Bardelli E, Sembenini C, Brentegani MT, Caliari S, Vantini I. Omeprazole causes delay in gastric emptying of digestible meals. Dig Dis Sci. 1996;41:469-474. |
| 8. | Rasmussen L, Oster-Jørgensen E, Qvist N, Pedersen SA. The effects of omeprazole on intragastric pH, intestinal motility, and gastric emptying rate. Scand J Gastroenterol. 1999;34:671-675. |
| 9. | Tougas G, Earnest DL, Chen Y, Vanderkoy C, Rojavin M. Omeprazole delays gastric emptying in healthy volunteers: an effect prevented by tegaserod. Aliment Pharmacol Ther. 2005;22:59-65. |
| 10. | Lewis SJ, Franco S, Young G, O'Keefe SJ. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10:557-561. |
| 12. | Kim BW, Lee BI, Kim HK, Cho YS, Chae HS, Lee HK, Kim HJ, Han SW. [Influence of long-term gastric acid suppression therapy on the expression of serum gastrin, chromogranin A, and ghrelin]. Korean J Gastroenterol. 2009;53:84-89. |
| 13. | DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190-200. |
| 14. | Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med. 2006;166:965-971. |
| 15. | Fraser-Moodie CA, Norton B, Gornall C, Magnago S, Weale AR, Holmes GK. Weight loss has an independent beneficial effect on symptoms of gastro-oesophageal reflux in patients who are overweight. Scand J Gastroenterol. 1999;34:337-340. |
| 16. | Jacobson BC, Somers SC, Fuchs CS, Kelly CP, Camargo CA Jr. Body-mass index and symptoms of gastroesophageal reflux in women. N Engl J Med. 2006;354:2340-2348. |
| 17. | El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol. 2005;100:1243-1250. |
| 18. | Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA. 2003;290:66-72. |
| 19. | Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Risk factors associated with symptoms of gastroesophageal reflux. Am J Med. 1999;106:642-649. |
| 20. | Kulig M, Nocon M, Vieth M, Leodolter A, Jaspersen D, Labenz J, Meyer-Sabellek W, Stolte M, Lind T, Malfertheiner P. Risk factors of gastroesophageal reflux disease: methodology and first epidemiological results of the ProGERD study. J Clin Epidemiol. 2004;57:580-589. |
| 21. | Murray L, Johnston B, Lane A, Harvey I, Donovan J, Nair P, Harvey R. Relationship between body mass and gastro-oesophageal reflux symptoms: The Bristol Helicobacter Project. Int J Epidemiol. 2003;32:645-650. |
| 22. | Stanghellini V. Three-month prevalence rates of gastrointestinal symptoms and the influence of demographic factors: results from the Domestic/International Gastroenterology Surveillance Study (DIGEST). Scand J Gastroenterol Suppl. 1999;231:20-28. |
| 23. | Wu AH, Tseng CC, Bernstein L. Hiatal hernia, reflux symptoms, body size, and risk of esophageal and gastric adenocarcinoma. Cancer. 2003;98:940-948. |
| 24. | Diaz-Rubio M, Moreno-Elola-Olaso C, Rey E, Locke GR 3rd, Rodriguez-Artalejo F. Symptoms of gastro-oesophageal reflux: prevalence, severity, duration and associated factors in a Spanish population. Aliment Pharmacol Ther. 2004;19:95-105. |
| 25. | Overweight, obesity, and health risk. National Task Force on the Prevention and Treatment of Obesity. Arch Intern Med. 2000;160:898-904. |
| 26. | Blair DI, Kaplan B, Spiegler J. Patient characteristics and lifestyle recommendations in the treatment of gastroesophageal reflux disease. J Fam Pract. 1997;44:266-272. |