Published online Oct 14, 2009. doi: 10.3748/wjg.15.4775
Revised: August 4, 2009
Accepted: August 11, 2009
Published online: October 14, 2009
AIM: To evaluate the predictive value of tissue transglutaminase (tTG) antibodies for villous atrophy in adult and pediatric populations to determine if duodenal biopsy can be avoided.
METHODS: A total of 324 patients with celiac disease (CD; 97 children and 227 adults) were recruited prospectively at two tertiary centers. Human IgA class anti-tTG antibody measurement and upper gastrointestinal endoscopy were performed at diagnosis. A second biopsy was performed in 40 asymptomatic adults on a gluten-free diet (GFD) and with normal tTG levels.
RESULTS: Adults showed less severe histopathology (26% vs 63%, P < 0.0001) and lower tTG antibody titers than children. Levels of tTG antibody correlated with Marsh type in both populations (r = 0.661, P < 0.0001). Multiple logistic regression revealed that only tTG antibody was an independent predictor for Marsh type 3 lesions, but clinical presentation type and age were not. A cut-off point of 30 U tTG antibody yielded the highest area under the receiver operating characteristic curve (0.854). Based on the predictive value of this cut-off point, up to 95% of children and 53% of adults would be correctly diagnosed without biopsy. Despite GFDs and decreased tTG antibody levels, 25% of the adults did not recover from villous atrophy during the second year after diagnosis.
CONCLUSION: Strongly positive tTG antibody titers might be sufficient for CD diagnosis in children. However, duodenal biopsy cannot be avoided in adults because disease presentation and monitoring are different.
- Citation: Vivas S, Ruiz de Morales JG, Riestra S, Arias L, Fuentes D, Alvarez N, Calleja S, Hernando M, Herrero B, Casqueiro J, Rodrigo L. Duodenal biopsy may be avoided when high transglutaminase antibody titers are present. World J Gastroenterol 2009; 15(38): 4775-4780
- URL: https://www.wjgnet.com/1007-9327/full/v15/i38/4775.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4775
| ≤ 2 yr | 3-14 yr | 15-35 yr | > 35 yr | |
| Marsh 1 | 0 | 0/2 | 0/9 (0) | 0/27 (0) |
| Marsh 2 | 0 | 1/1 (100) | 0/19 (0) | 0/15 (0) |
| Marsh 3a | 9/10 (90) | 22/24 (92) | 29/43 (67) | 41/57 (72) |
| Marsh 3b | 28/28 (100) | 20/21 (95) | 22/24 (92) | 15/20 (75) |
| Marsh 3c | 10/10 (100) | 1/1 (100) | 7/7 (100) | 6/6 (100) |
| No. of biopsies avoided | 47/48 (98) | 44/49 (90) | 58/102 (57) | 62/125 (50) |
Serological assessment is the first step in celiac disease (CD) diagnosis, and wide availability of serological antibodies allows for easy CD testing[1]. IgA anti-endomysial antibodies (EMAs) are used as the “gold standard” for CD screening because of their high sensitivity and specificity. A high correlation between EMA titer and duodenal histopathology has also been reported[2-4]. More recently, human anti-tissue transglutaminase (tTG) antibodies have also shown to be correlated with mucosal damage and are used widely in CD screening. However, neither antibody is detected in patients with minor mucosal changes (Marsh types I-II and IIIa) and IgA deficiency may be ruled out when using IgA type antibodies.
In the clinical setting, a patient with positive serological results requires duodenal biopsy to confirm CD diagnosis. However, a definitive diagnosis is only made when a response to gluten-free diet (GFD) is present[5]. Furthermore, duodenal biopsy has several pitfalls: (1) at least four forced biopsies are needed to achieve good readability; (2) poorly oriented or inadequate biopsies may not be useful for diagnosis; and (3) it is an invasive procedure, both in children and adults. In the last few years, a more prominent role for a definitive diagnosis based solely on serological assays has been proposed. In pediatric populations, strongly positive tTG antibody results (≥ 100 U) showed a high specificity for Marsh type 3a or greater changes[6,7]. This predictive value of high tTG antibody titers has also been reported in a retrospective cohort of adult and pediatric CD patients[8]. Based on these studies, some authors have proposed to start a GFD for those patients with high tTG antibody levels and to perform a duodenal biopsy only when the patient’s symptoms do not improve after a GFD.
The primary objective of the present work was to analyze the predictive value of a finding of high tTG antibody titers for the presence of duodenal atrophy at the time of diagnosis in adult and pediatric CD patients. In addition, the possibility that avoiding duodenal biopsy in these 2 groups of CD patients was explored.
The study was approved by the Research and Ethical Committees of the participating hospitals.
Adult and pediatric CD patients were recorded prospectively from 2000 to 2008 at two tertiary centers in the North of Spain: Hospital Universitario Central de Asturias and Hospital de León. Pediatric and adult gastroenterology units at both centers have specialty CD clinics. Patients were referred from primary care settings or from other medical specialties for diagnosis and follow-up. Subjects were referred for evaluation of clinical complaints suggestive of CD, had positive family history or belonged to some high-risk group for CD.
None of the patients included had a previous diagnosis of CD before they attended at our clinic and were on a free diet (gluten containing diet). They were informed regarding the suspicion of illness and gave informed consent to perform the complementary studies. The pediatric population included children ≤ 14 years and adults were ≥ 15 years old. A life time of 14 years was selected to discriminate children from adults because pediatric patients were below this age in our hospitals. The elapsed time between serological assay and duodenal biopsy was always < 8 wk.
The clinical spectrum was divided into two categories according to the main symptoms that led to diagnosis: (1) typical or classical, clinical malabsorption, chronic diarrhea or failure to thrive (children ≤ 2 years); and (2) atypical or oligosymptomatic, abdominal pain, iron deficiency anemia, chronic hypertransaminasemia, growth failure (children ≥ 3 years) or screening of risk groups or familial study.
Quantitative detection of human IgA class tTG antibody used the same commercial kits (Phadia Diagnostics, Uppsala, Sweden) in both centers. The manufacturer reference ranges for positive results were values > 10 U. From our experience, a cut-off value of 4 U showed a diagnostic value similar to IgA anti-endomysial antibodies[9,10]. A cut-off level > 30 U tTG antibody was considered strongly positive for additional evaluations. IgA level was determined together with tTG antibodies and IgA deficiency patients were not included.
Adult CD patients were typed for HLA-DQ2 (DQA1*0501 and DQB1*0201 alleles) and DQ8 (DQA1*03 and DQB1*0302 alleles) by polymerase chain reaction. HLA genotype was performed only when duodenal biopsy showed inflammatory lesions (Marsh 1 and 2), in order to confirm CD diagnosis.
Biopsies from the distal duodenum (minimum of four forceps biopsies) were obtained by upper gastrointestinal endoscopy. Two pathologists experienced with CD diagnosis reviewed the histopathological specimens in each center and classified them according to Marsh’s criteria as modified by Oberhuber et al[11]. Type 3 specimens (any degree of villous atrophy) were considered characteristic of CD. Marsh 1 and 2 lesions were considered nonspecific, but consistent with CD diagnosis if serology was positive. When serology was negative, HLA-DQ was typed and symptoms evaluated with a GFD. Only those cases that were positive for HLA-DQ2 and DQ8, together with the disappearance of symptoms were included as definitive CD cases. Those cases with Marsh 3 lesions and negative serology were evaluated for an alternative diagnosis that could explain the histological abnormalities.
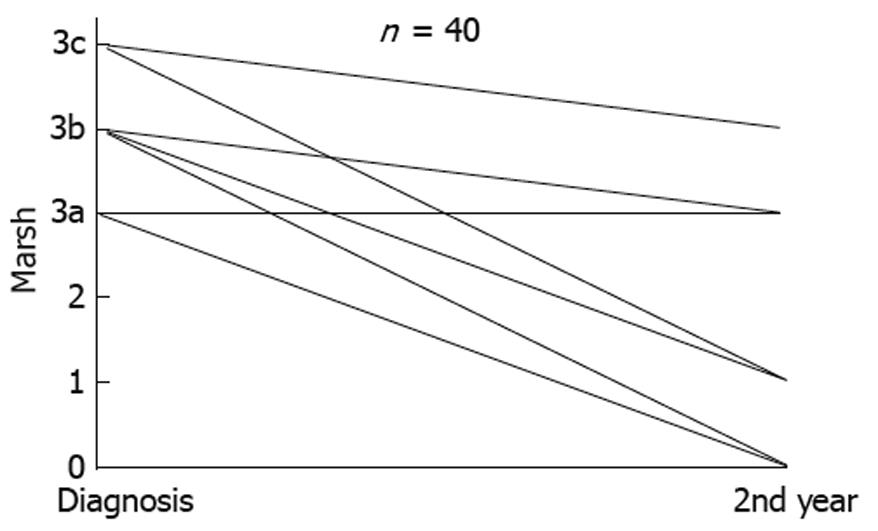
A second biopsy was performed in a selected cohort of 40 patients at Hospital de Leon to evaluate duodenal atrophy recovery. All of them showed, at the beginning, Marsh 3 lesions, and the second biopsy was performed during the second year after diagnosis, in patients taking a GFD, who were asymptomatic and with normal tTG antibody level.
Data were analyzed using SPSS version 13.0. Categorical variables were expressed as numbers and percentages and quantitative variables as mean ± SD. Categorical variables were analyzed by cross-tabulations using a χ2 test with a continuity correction test when necessary. Differences between groups for quantitative variables were assessed by Student’s t test or ANOVA. A non-parametric Mann-Whitney U test was used when the groups values deviated from a normal curve. Associations between quantitative variables were assessed by Pearson correlation test or Spearman rank correlation test. P < 0.05 was selected to reject the null hypothesis by two-tailed tests. Multivariate logistic regression was used to determine independent associations between histopathological and serological or clinical data. Analysis of receiver operating characteristics (ROC) curve was used to evaluate cut-off points for tTG antibodies as a predictor of Marsh scores.
A total of 324 patients who fulfilled the established CD diagnostic criteria comprised the study population. The pediatric population included 97 children (mean age: 4.5 years; range: 1-14 years) and 227 adult CD subjects (mean age: 39 years; range: 15-80 years). Female/male ratio was 1.7 for children and 2.6 for adults (P = 0.06).
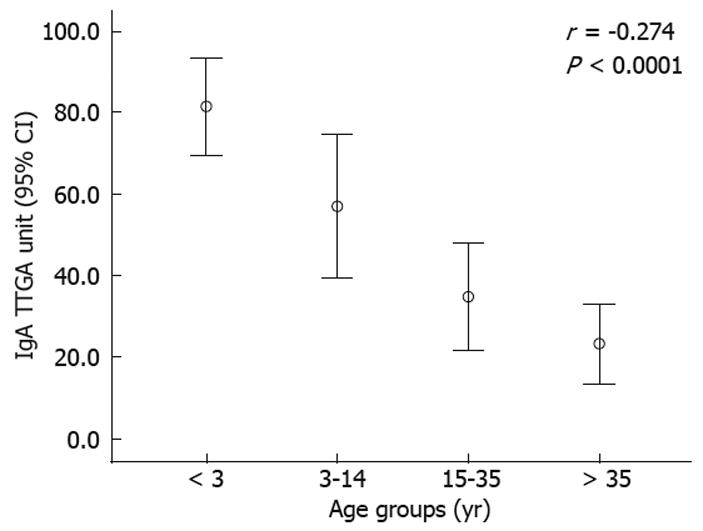
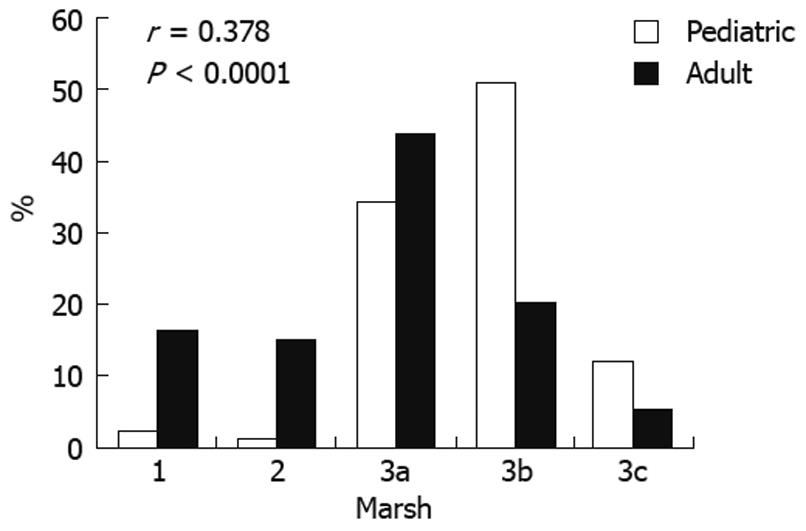
A typical CD presentation was observed for 64/97 (66%) children vs 82/227 (36%) adults (P < 0.0001). Age-related differences in tTG antibody titers and histopathology were found. An inverse relationship of tTG antibody titers at diagnosis with increasing patient age was found (Figure 1). Higher levels were seen in children aged ≤ 2 years and lower titers in adults > 35 years. A trend towards less severe histopathology with increasing age at diagnosis was observed (Figure 2). Marked villous atrophy (Marsh 3b and 3c) was present in 63% of children vs 26% of adults (P < 0.0001).
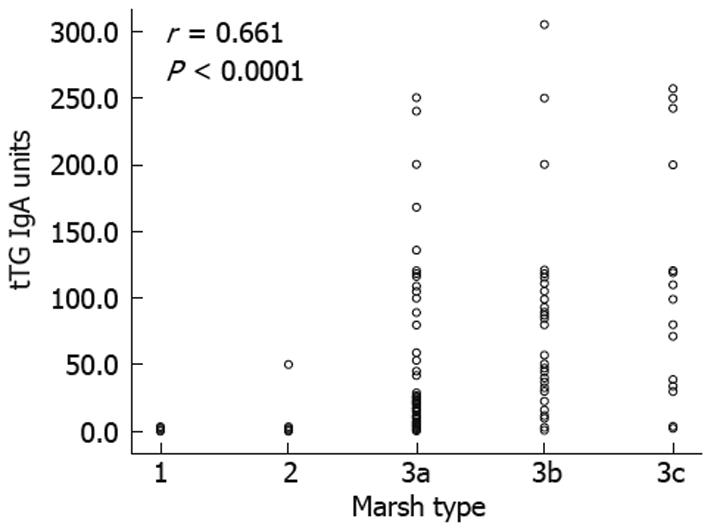
The levels of tTG antibody were correlated significantly with Marsh types in the entire population (Figure 3) (r = 0.661, P < 0.0001), and separately for the pediatric (r = 0.633, P < 0.001) and adult (r = 0.574, P < 0.0001) groups. Mean tTG antibody levels showed a progressive increase that was associated with higher Marsh types. Seventy-three patients showed Marsh types 1 and 2 (three were children and the remaining 70 were adults). In the pediatric group, only one Marsh type 2 patient showed tTG antibody titer < 30 U. Negative tTG antibody results were found for 46/73 (63%) Marsh types 1 and 2 CD subjects (all were adults). Twelve of 132 (9%) Marsh 3a CD patients had negative tTG antibody results (all were also adults). In contrast, none of the Marsh 3b and 3c patients had negative serology results. A definitive CD diagnosis was confirmed in this subgroup with minor mucosal changes and normal tTG antibody levels on the basis of clinical response to GFD, follow-up, and HLA-DQ2 or DQ8 compatibility.
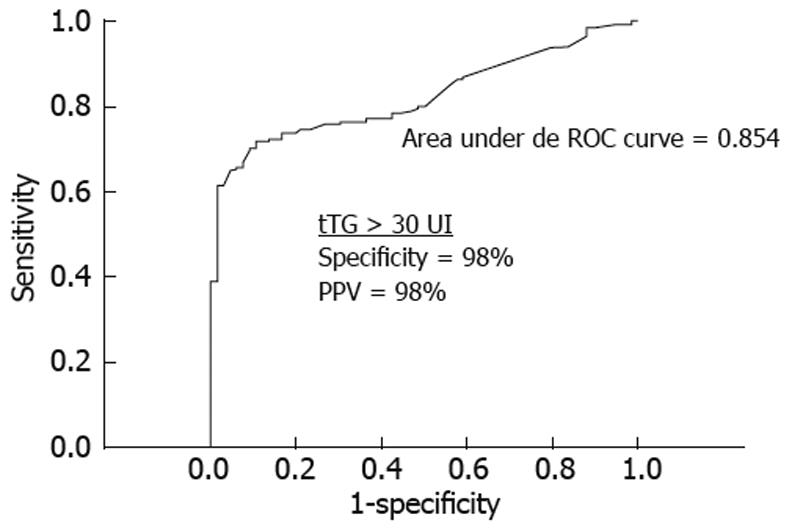
Strongly positive tTG antibody titers (> 30 U) were present in 102 of 132 (77%) Marsh 3a patients, 79/95 (83%) Marsh 3b patients, and 24/24 (100%) Marsh 3c patients. Multiple logistic regression analysis showed that only the tTG antibody titer was an independent predictor for Marsh 3 lesions, but the clinical presentation type and patient age were not. As shown in Figure 4, at the cut-off point of ≥ 30 U tTG antibody, ROC curve analysis provided the highest area under the curve. Increasing this limit may increase the specificity and positive predictive value, but may decrease the area under the curve and sensitivity.
If we had considered a cut-off point of 30 U tTG antibody to predict atrophy (Marsh 3), we would have avoided 212/324 (65%) biopsies. However, if we had considered children and adults separately, 95% of children, but only 53% adults with true atrophy, would have avoided biopsy (Table 1). Furthermore, in 40 adults with CD with an initial biopsy of Marsh 3 type, a second biopsy was performed during the second year after diagnosis. Ten (25%) showed persistence of villous atrophy despite a correct GFD, being symptom free, and normalization of tTG antibody levels (Figure 5). One patient developed refractory type II CD after an initial biopsy compatible with Marsh type 3c[12].
A positive correlation between tTG antibody serum level and duodenal histopathology has been described previously for pediatric and adult CD populations[6,8,13]. Although some of these early studies employed guinea pig rather than human tTG as an antigen[13,14], the higher sensitivity and specificity have rendered the latter as the worldwide standard.
The pathogenesis of gluten-induced small-intestinal changes and atrophy is not understood fully[15]. It is considered to be T-cell mediated. However, recent in vitro studies[16,17], and a study that reported anti-tTG2 IgA deposits in a morphologically normal jejunum before systemic detection of tTG antibody predicted overt CD with atrophy[18], suggest a pathogenic role for antibodies.
The study population in the present study was selected on the basis of a prospective clinical CD diagnosis. However, previous similarly designed studies have used retrospective laboratory results to select positive tTG levels[6,8]. It is more likely that the results presented in our study are more easily generalized to other populations than those based on retrospective laboratory results. Furthermore, we also describe differences between adult and pediatric populations that were not analyzed in previous studies. These differences confirm our previously reported results in a single center CD population[19]. The higher tTG antibody titers and the more severe Marsh grades that were observed consistently in the pediatric population are two important points to consider when looking for correlations and predictions. Although tTG antibody levels were correlated with duodenal histopathology in the adult and pediatric populations, the higher percentage of Marsh type 3 lesions observed in children makes a high antibody titer especially interesting for CD prediction in children.
The choice of an upper cut-off limit of tTG antibody to predict accurately CD or Marsh type 3 lesions may depend on the commercial kit used for tTG IgA ELISA. On the basis of previous experience[9,10], we considered 30 U as the cut-off that resulted in the highest predictive value. The same cut-off value has also been adopted by other authors who have used the same commercial kit[20]. This value showed the highest area under the ROC curve for Marsh type 3 histology in the entire CD population. Other series have considered a cut-off value > 100 U tTG antibody for a better Marsh type 3 prediction[6-8]. The cut-off should probably be standardized in each laboratory based on experience with different kits.
Our series established CD diagnosis based on clinical judgment and expertise. This accounts for the high percentage of Marsh type 1 and 2 adult patients that were included, most of whom showed negative serology or low tTG antibody levels. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition and European Society for Pediatric Gastroenterology, Hepatology and Nutrition guidelines indicate that Marsh type 1 or 2 changes are less specific or, perhaps, unlikely to be included in CD[21]. However, in adult CD populations, atypical presentations with milder symptomatology and minor histopathology changes are described more frequently[19,22]. There is evidence that CD patients who show only increased intraepithelial lymphocyte numbers, without mucosal atrophy, may show clinical features similar to those with Marsh type 3 lesions, and can also develop nutritional deficiencies and malabsorption[23,24]. Any effort aimed at recognizing the presence of latent or occult minimally symptomatic CD would be the key reason to look for subtle abnormalities behind the symptoms[25-27].
The primary reason to continue using biopsy as the definitive step in CD diagnosis is the possibility of false-positive serological results. All the cases in our series were diagnosed finally with CD, therefore, this possibility could not be explored. For some other series, a high positive tTG antibody result has not always been associated with a final CD diagnosis[28]. However, these reports may be of questionable value to us for several reasons: (1) they were small series that lacked a prospective follow-up of tTG positive patients; (2) false-negative duodenal biopsies may be observed in CD (biopsy is not correctly performed, pathologist does not have experience with CD, or patchy histopathology lesions may be present); and (3) guinea pig tTG antibody, which lacks specificity, was used in some of the studies. Although tTG antibody positivity may appear in other gastrointestinal and liver inflammatory disorders, to date, strong positive results have not been described for such conditions[29-32]. In addition, many of these patients may have coexisting CD[33-35].
Based on the observed high predictive value of high tTG antibody titers in our series, 65% of the biopsies may not have been necessary. An important clinical difference was observed between adult and pediatric populations, in that only 53% of adults vs 95% of children would have properly avoided biopsy. The results for the adult population are concordant with a similar recent study[20]. In 25% of our adult patients, mucosal recovery was not achieved in the second year after diagnosis on a GFD, despite normalization of serology and symptomatology. In fact, a slow mucosal recovery and persistent villous atrophy on a strict GFD in adult CD have been reported previously[36-38]. Although asymptomatic, these patients without mucosal recovery may be at risk for subsequent severe complications[36,39]. We believe that a follow-up biopsy may be important for detecting these individuals, and thus, should be mandatory. From our experience, this follow-up can be important for the correct identification of refractory CD.
Although we did not perform follow-up biopsies in children, the available results coincide with the idea that most pediatric CD patients recover from villous atrophy shortly after starting a GFD[38,39]. Thus, for children it would be less important to perform an initial biopsy, as histological follow-up appears not to be necessary.
Overall, our results confirm the high diagnostic accuracy and predictive value of serological tests. We suggest that because of the high predictive value of tTG antibody for mucosal atrophy, duodenal biopsy may not always be necessary. In children, CD diagnosis may only require clinical and serological features, thus avoiding an invasive procedure, and starting an earlier GFD. In contrast, for adults, CD presentation and monitoring are different, thus rendering necessary a histopathological confirmation in all the cases at diagnosis, and in some selected cases at follow-up on a GFD. Future CD guidelines may take into account these age-related differences.
Duodenal biopsy remains the gold standard for celiac disease (CD) diagnosis. However, it has several pitfalls and requires an invasive procedure in children. In the past few years, a more prominent role for a definitive diagnosis based solely on serology has been proposed. The predictive value of high levels of anti-tissue transglutaminase (tTG) antibodies has also been reported in retrospective CD cohorts. Based on these studies, some authors have proposed to start a gluten-free diet (GFD) for those patients with high tTG antibody levels, without duodenal biopsy.
There is no agreement to start a GFD without biopsy to confirm mucosal atrophy. There are age-related differences in CD diagnosis that may be taken into account to evaluate the predictive value of tTG antibody for mucosal atrophy.
This study revealed that tTG antibody is correlated with Marsh types, and is an independent predictor for Marsh type 3 lesions in children and adults. However, adults with CD showed less severe atrophy and lower tTG antibody titers than children. Moreover, a significant proportion of adult patients did not recover from atrophy during follow-up. These age-related differences have not been defined clearly in previous studies, and they are associated with the predictive value of tTG antibody for duodenal atrophy.
These results suggest that high tTG antibody titers may be sufficient for CD diagnosis in children, but a biopsy may be necessary to diagnose and monitor adults patients. Starting a GFD without a confirmatory biopsy may be possible in selected cases.
CD is characterized by intolerance to ingested gluten in susceptible individuals. Immunologically mediated inflammation of the small intestine mucosa and the consequent atrophy is now the main point for diagnosis. Serological testing is the first step for diagnosis and tTG antibody is available widely as a screening method.
In this interesting study, the authors investigated the predictive value of tTG antibody (cut-off value 30 U) for establishment of villous atrophy in children and adults suffering from CD. The serum parameter was correlated with histopathological findings in duodenal biopsies. The authors demonstrated that disease presentation and monitoring are different between children and adults. The increase in tTG antibody has a better predictive value in children. In conclusion, the authors recommend histopathological confirmation of tTG antibody serum levels in all adults, whereas in children with > 30 U tTG antibody, histopathological confirmation can be omitted.
Peer reviewer: Nikolaus Gassler, Professor, Institute of Pathology, University Hospital RWTH Aachen, Pauwelsstrasse 30, 52074 Aachen, Germany
S- Editor Tian L L- Editor Kerr C E- Editor Lin YP
| 1. | Rodrigo L. Celiac disease. World J Gastroenterol. 2006;12:6585-6593. |
| 2. | Abrams JA, Diamond B, Rotterdam H, Green PH. Seronegative celiac disease: increased prevalence with lesser degrees of villous atrophy. Dig Dis Sci. 2004;49:546-550. |
| 3. | Sategna-Guidetti C, Pulitanó R, Grosso S, Ferfoglia G. Serum IgA antiendomysium antibody titers as a marker of intestinal involvement and diet compliance in adult celiac sprue. J Clin Gastroenterol. 1993;17:123-127. |
| 4. | Tursi A, Brandimarte G, Giorgetti G, Gigliobianco A, Lombardi D, Gasbarrini G. Low prevalence of antigliadin and anti-endomysium antibodies in subclinical/silent celiac disease. Am J Gastroenterol. 2001;96:1507-1510. |
| 5. | Hill ID, Dirks MH, Liptak GS, Colletti RB, Fasano A, Guandalini S, Hoffenberg EJ, Horvath K, Murray JA, Pivor M. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:1-19. |
| 6. | Donaldson MR, Firth SD, Wimpee H, Leiferman KM, Zone JJ, Horsley W, O'Gorman MA, Jackson WD, Neuhausen SL, Hull CM. Correlation of duodenal histology with tissue transglutaminase and endomysial antibody levels in pediatric celiac disease. Clin Gastroenterol Hepatol. 2007;5:567-573. |
| 7. | Barker CC, Mitton C, Jevon G, Mock T. Can tissue transglutaminase antibody titers replace small-bowel biopsy to diagnose celiac disease in select pediatric populations? Pediatrics. 2005;115:1341-1346. |
| 8. | Donaldson MR, Book LS, Leiferman KM, Zone JJ, Neuhausen SL. Strongly positive tissue transglutaminase antibodies are associated with Marsh 3 histopathology in adult and pediatric celiac disease. J Clin Gastroenterol. 2008;42:256-260. |
| 9. | Vivas S, Ruiz de Morales JM, Martinez J, González MC, Martín S, Martín J, Cechini C, Olcoz JL. Human recombinant anti-transglutaminase antibody testing is useful in the diagnosis of silent coeliac disease in a selected group of at-risk patients. Eur J Gastroenterol Hepatol. 2003;15:479-483. |
| 10. | Fernández ML, Vivas S, Ruiz de Morales JM, Marugán JM. [Usefulness of anti-transglutaminase antibodies in the diagnosis of celiac disease]. Gastroenterol Hepatol. 2005;28:437-440. |
| 11. | Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol. 1999;11:1185-1194. |
| 12. | Vivas S, Ruiz de Morales JM, Ramos F, Suárez-Vilela D. Alemtuzumab for refractory celiac disease in a patient at risk for enteropathy-associated T-cell lymphoma. N Engl J Med. 2006;354:2514-2515. |
| 13. | Tursi A, Brandimarte G, Giorgetti GM. Prevalence of antitissue transglutaminase antibodies in different degrees of intestinal damage in celiac disease. J Clin Gastroenterol. 2003;36:219-221. |
| 14. | Hoffenberg EJ, Bao F, Eisenbarth GS, Uhlhorn C, Haas JE, Sokol RJ, Rewers M. Transglutaminase antibodies in children with a genetic risk for celiac disease. J Pediatr. 2000;137:356-360. |
| 15. | Schuppan D. Current concepts of celiac disease pathogenesis. Gastroenterology. 2000;119:234-242. |
| 16. | Esposito C, Paparo F, Caputo I, Rossi M, Maglio M, Sblattero D, Not T, Porta R, Auricchio S, Marzari R. Anti-tissue transglutaminase antibodies from coeliac patients inhibit transglutaminase activity both in vitro and in situ. Gut. 2002;51:177-181. |
| 17. | Freitag T, Schulze-Koops H, Niedobitek G, Melino G, Schuppan D. The role of the immune response against tissue transglutaminase in the pathogenesis of coeliac disease. Autoimmun Rev. 2004;3:13-20. |
| 18. | Korponay-Szabó IR, Halttunen T, Szalai Z, Laurila K, Király R, Kovács JB, Fésüs L, Mäki M. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut. 2004;53:641-648. |
| 19. | Vivas S, Ruiz de Morales JM, Fernandez M, Hernando M, Herrero B, Casqueiro J, Gutierrez S. Age-related clinical, serological, and histopathological features of celiac disease. Am J Gastroenterol. 2008;103:2360-2365; quiz 2366. |
| 20. | Hill PG, Holmes GK. Coeliac disease: a biopsy is not always necessary for diagnosis. Aliment Pharmacol Ther. 2008;27:572-577. |
| 21. | Troncone R, Bhatnagar S, Butzner D, Cameron D, Hill I, Hoffenberg E, Maki M, Mendez V, de Jimenez MZ. Celiac disease and other immunologically mediated disorders of the gastrointestinal tract: Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39 Suppl 2:S601-S610. |
| 22. | Santaolalla R, Fernández-Bañares F, Rodríguez R, Alsina M, Rosinach M, Mariné M, Farré C, Salas A, Forné M, Loras C. Diagnostic value of duodenal antitissue transglutaminase antibodies in gluten-sensitive enteropathy. Aliment Pharmacol Ther. 2008;27:820-829. |
| 23. | Esteve M, Rosinach M, Fernández-Bañares F, Farré C, Salas A, Alsina M, Vilar P, Abad-Lacruz A, Forné M, Mariné M. Spectrum of gluten-sensitive enteropathy in first-degree relatives of patients with coeliac disease: clinical relevance of lymphocytic enteritis. Gut. 2006;55:1739-1745. |
| 24. | Ciclitira PJ. Does clinical presentation correlate with degree of villous atrophy in patients with celiac disease? Nat Clin Pract Gastroenterol Hepatol. 2007;4:482-483. |
| 25. | Green PH. Where are all those patients with Celiac disease? Am J Gastroenterol. 2007;102:1461-1463. |
| 26. | Kaukinen K, Collin P, Mäki M. Latent coeliac disease or coeliac disease beyond villous atrophy? Gut. 2007;56:1339-1340. |
| 27. | Kaukinen K, Mäki M, Partanen J, Sievänen H, Collin P. Celiac disease without villous atrophy: revision of criteria called for. Dig Dis Sci. 2001;46:879-887. |
| 28. | Freeman HJ. Strongly positive tissue transglutaminase antibody assays without celiac disease. Can J Gastroenterol. 2004;18:25-28. |
| 29. | Clemente MG, Musu MP, Frau F, Lucia C, De Virgiliis S. Antitissue transglutaminase antibodies outside celiac disease. J Pediatr Gastroenterol Nutr. 2002;34:31-34. |
| 30. | Di Tola M, Sabbatella L, Anania MC, Viscido A, Caprilli R, Pica R, Paoluzi P, Picarelli A. Anti-tissue transglutaminase antibodies in inflammatory bowel disease: new evidence. Clin Chem Lab Med. 2004;42:1092-1097. |
| 31. | Lo Iacono O, Petta S, Venezia G, Di Marco V, Tarantino G, Barbaria F, Mineo C, De Lisi S, Almasio PL, Craxì A. Anti-tissue transglutaminase antibodies in patients with abnormal liver tests: is it always coeliac disease? Am J Gastroenterol. 2005;100:2472-2477. |
| 32. | Germenis AE, Yiannaki EE, Zachou K, Roka V, Barbanis S, Liaskos C, Adam K, Kapsoritakis AN, Potamianos S, Dalekos GN. Prevalence and clinical significance of immunoglobulin A antibodies against tissue transglutaminase in patients with diverse chronic liver diseases. Clin Diagn Lab Immunol. 2005;12:941-948. |
| 33. | Tursi A, Giorgetti GM, Brandimarte G, Elisei W. Crohn's disease and celiac disease: association or epiphenomenon? Eur Rev Med Pharmacol Sci. 2006;10:127-130. |
| 34. | Hernandez L, Johnson TC, Naiyer AJ, Kryszak D, Ciaccio EJ, Min A, Bodenheimer HC Jr, Brown RS Jr, Fasano A, Green PH. Chronic hepatitis C virus and celiac disease, is there an association? Dig Dis Sci. 2008;53:256-261. |
| 35. | Caprai S, Vajro P, Ventura A, Sciveres M, Maggiore G. Autoimmune liver disease associated with celiac disease in childhood: a multicenter study. Clin Gastroenterol Hepatol. 2008;6:803-806. |
| 36. | Kaukinen K, Peräaho M, Lindfors K, Partanen J, Woolley N, Pikkarainen P, Karvonen AL, Laasanen T, Sievänen H, Mäki M. Persistent small bowel mucosal villous atrophy without symptoms in coeliac disease. Aliment Pharmacol Ther. 2007;25:1237-1245. |
| 37. | Tursi A, Brandimarte G, Giorgetti GM, Elisei W, Inchingolo CD, Monardo E, Aiello F. Endoscopic and histological findings in the duodenum of adults with celiac disease before and after changing to a gluten-free diet: a 2-year prospective study. Endoscopy. 2006;38:702-707. |
| 38. | Bardella MT, Velio P, Cesana BM, Prampolini L, Casella G, Di Bella C, Lanzini A, Gambarotti M, Bassotti G, Villanacci V. Coeliac disease: a histological follow-up study. Histopathology. 2007;50:465-471. |
| 39. | Wahab PJ, Meijer JW, Mulder CJ. Histologic follow-up of people with celiac disease on a gluten-free diet: slow and incomplete recovery. Am J Clin Pathol. 2002;118:459-463. |