INTRODUCTION
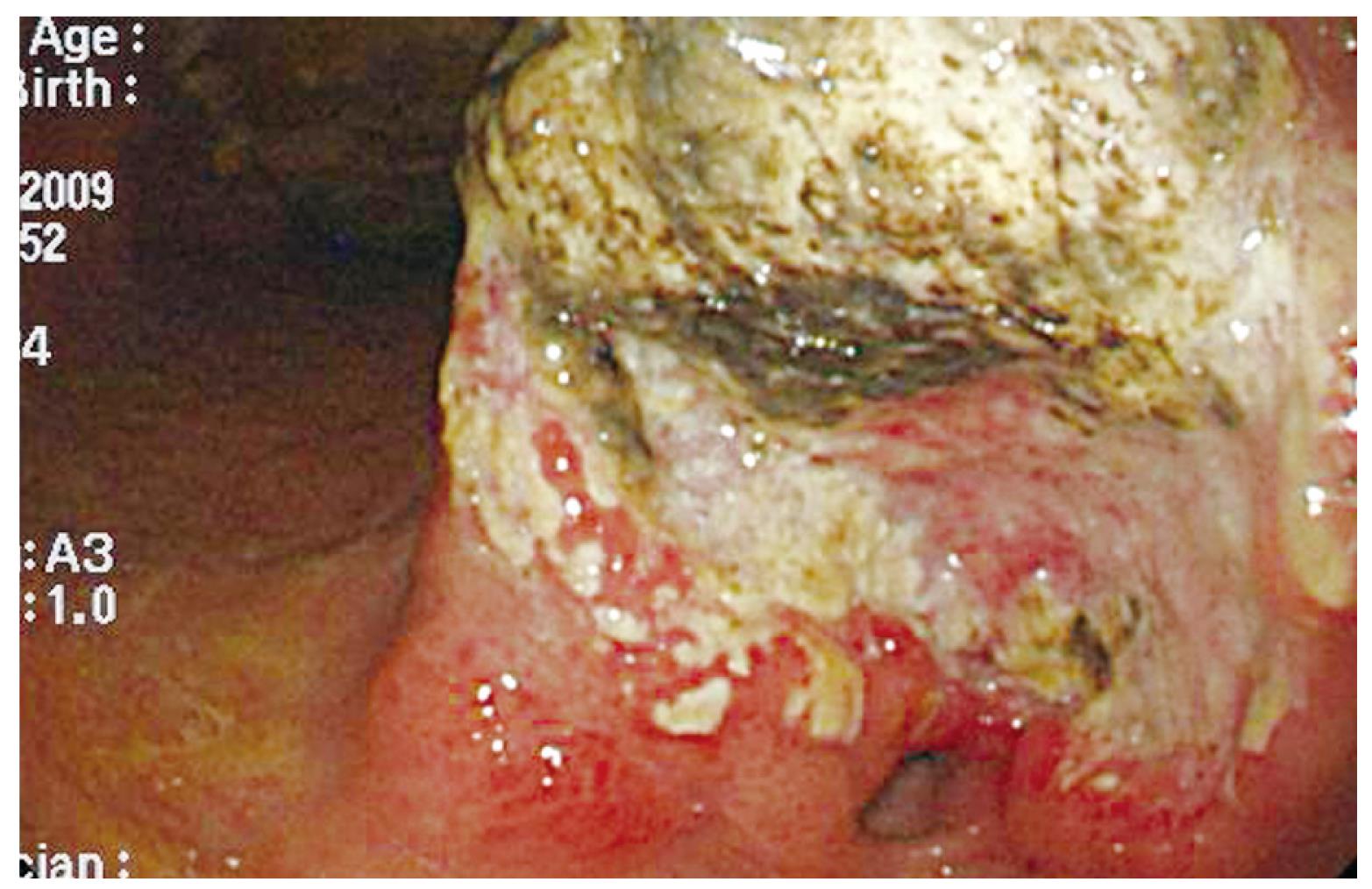
Figure 1 The esophagogastroduodenoscopy (EGD) showed an ulcerated, annular lesion over the gastric antrum.
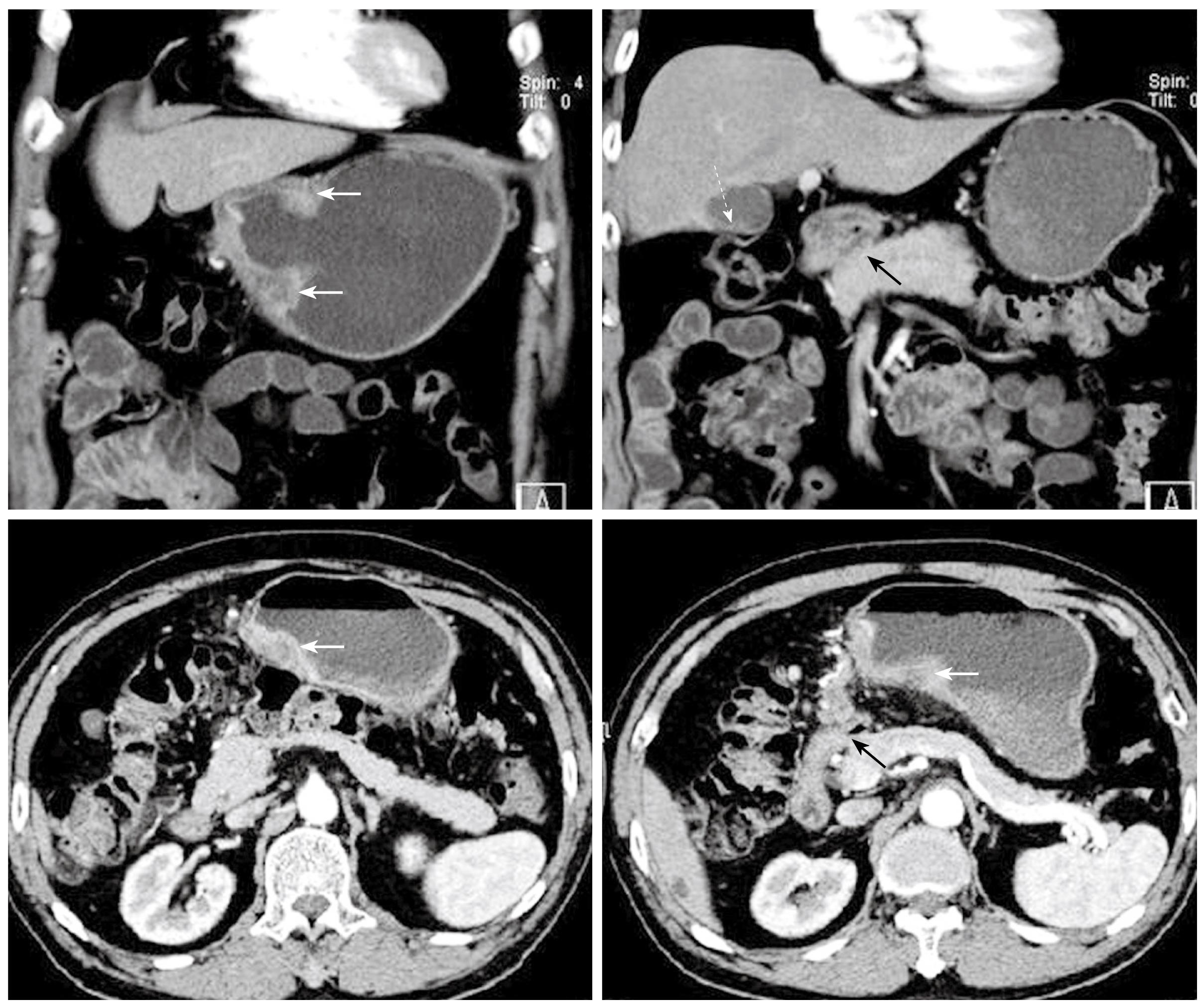
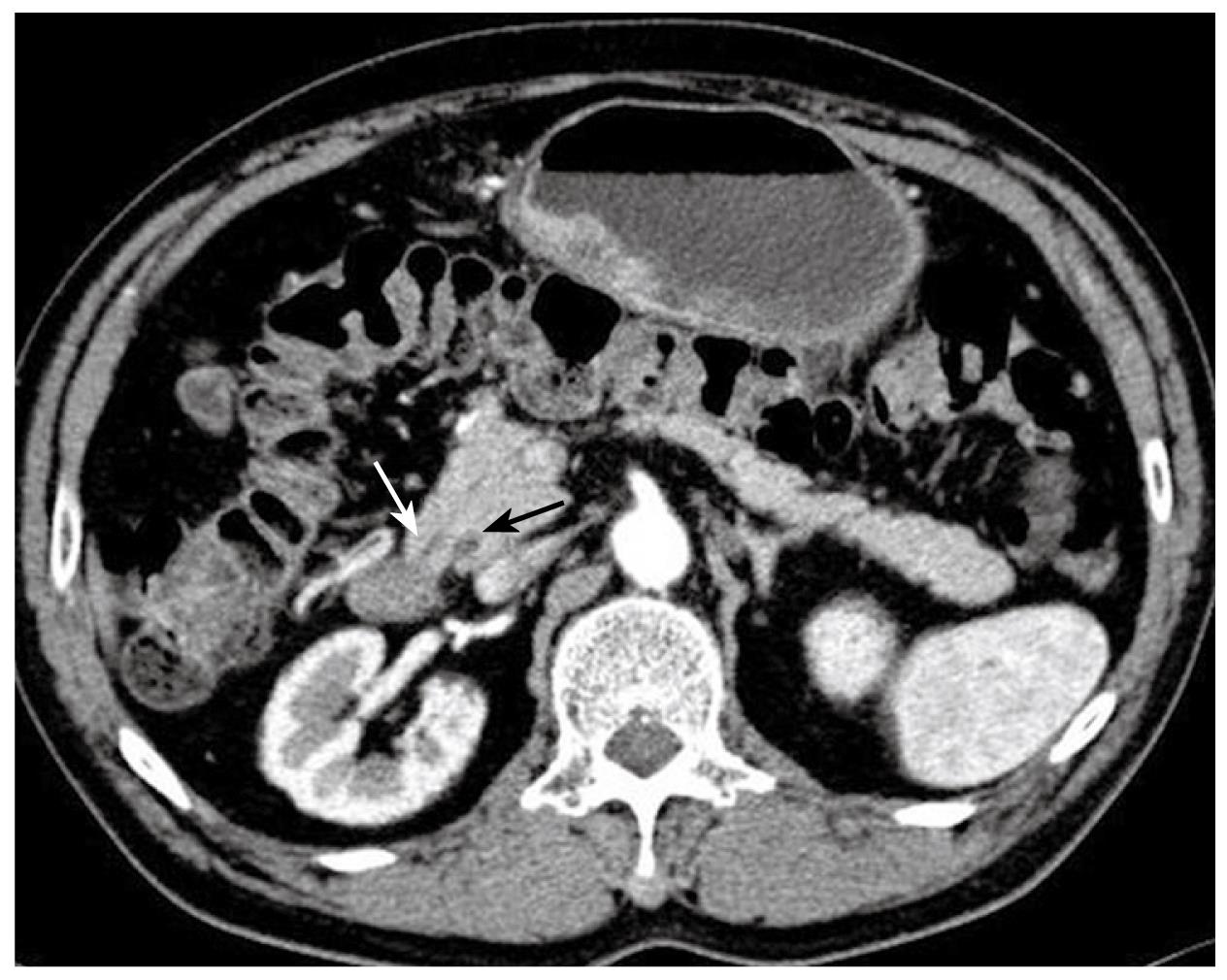
Figure 2 Preoperative abdominal CT images in coronary and transverse sections.
The white solid arrows indicate diffuse wall thickening at the gastric antrum. The white dotted arrow indicates a contracted gallbladder with eccentric wall thickening. The black solid arrows point to the apparent deformity of the duodenal bulb with adhesion to the head of the pancreas. There is no evidence of intra-abdominal metastasis in this study.
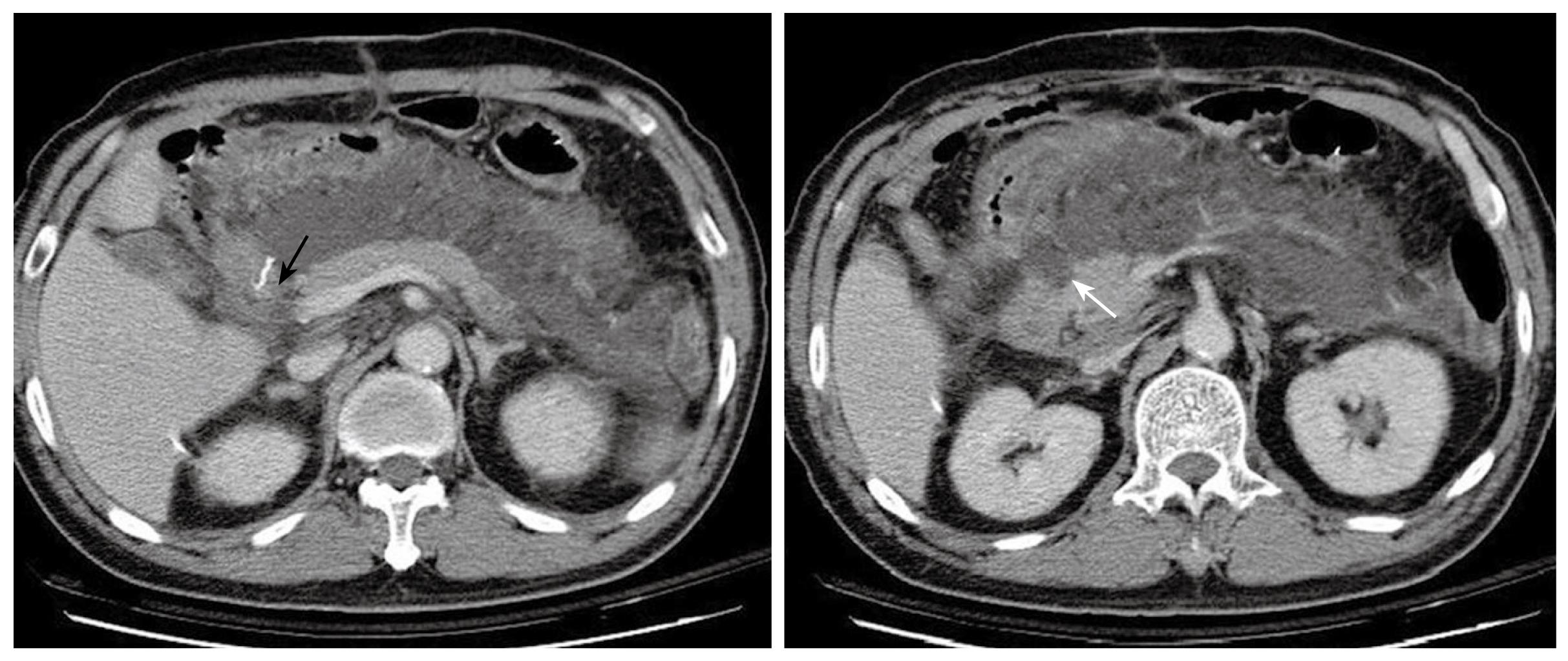
Figure 3 Abdominal CT 8 d after the first operation demonstrates massive fluid accumulation in the peripancreatic area and the lesser sac.
The homogenous fluid extends to the retroperitoneal space. The status of the duodenal stump (black arrow) cannot be clearly assessed. The pancreas is well enhanced and enlarged, and the head shows an uneven and infiltrative margin (white arrow).
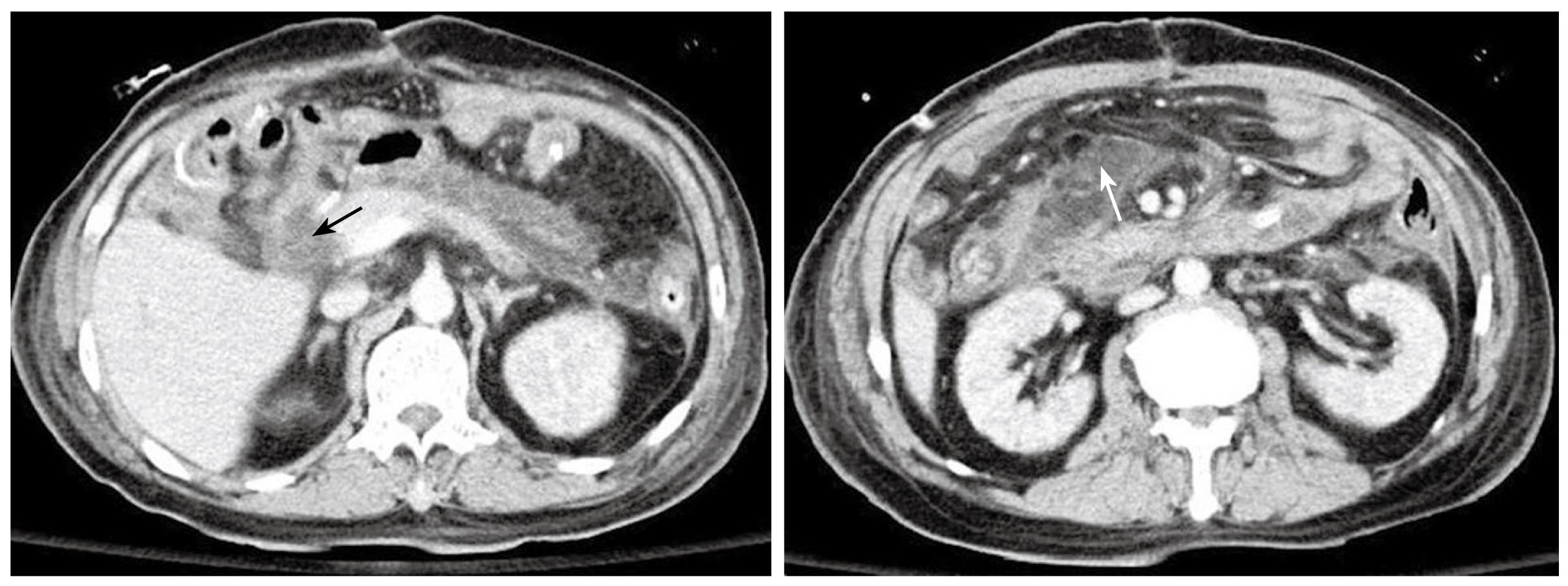
Figure 4 Follow-up abdominal CT after the second operation.
Severe fat stranding is seen. An edematous duodenal stump is observed without a clear fat plane surrounding it. This is highly suspicious of a stump leak (black arrow). The loculated fluid with heterogeneous changes indicates the possibility of an abscess formation (white arrow).
Figure 5 The preoperative CT scan.
The dominant dorsal duct (duct of Santorini) drains into the minor papilla (white arrow). The CBD is seen in the section (black arrow) but the ventral duct (duct of Wirsung) can not be traced. These features are consistent with pancreas divisum.
Although gastrectomies for lesions of the stomach and duodenum have been performed safely for more than one hundred years, major complications such as anastomosis leakage, duodenal stump leakage and post-operative bleeding cannot be completely avoided even by experienced surgeons. In the past, most gastrectomies were performed for benign gastric or duodenal ulcer. One of the complications associated with these procedures was post-gastrectomy acute pancreatitis (PGAP). The pathogenesis of this condition is hypothesized to be related to pancreatic parenchymal injury secondary to severe adhesion of peripancreatic tissue, compromised pancreatic micro-circulation, hyperpressure of the duodenum, and edema or spasm of the major papilla[1,2]. The incidence of PGAP has decreased in recent years, coinciding with the dramatic reduction in the need for surgical intervention of complicated peptic ulcer disease in the advent of improved medical and endoscopic management. Gastrectomies nowadays are performed mainly for patients with gastric malignancy. The “pancreatitis-like” presentation is mostly seen in patients with afferent loop obstruction after gastrectomies, mostly following Billroth II reconstruction. The true PGAP might be related to the extended lymph node dissection and resection of adjacent organs (such as splenectomy and distal pancreatectomy) in radical gastrectomies for gastric cancer[3-5]. However, the correlation between pancreatic anomalies and PGAP has not been well documented in the literature. This article, therefore, reports a patient with concurrent advanced gastric cancer and pancreas divisum (PD) who had PGAP and died from multiple organ failure post-operatively.
CASE REPORT
A 70-year-old gentleman with a medical background of hypertension and asthma, presented with a 3 mo history of poor appetite, 10 kg weight loss, postprandial fullness and nausea. There was, however, no history of tarry stool, hematemesis, fever or abdominal pain. The physical examination did not reveal any palpable abdominal mass, lymphadenopathy, anemia, or jaundice. All laboratory data, including biochemistry examination and hemogram, were within normal ranges except for a low hemoglobin level (10.3 g/dL). Esophagogastroduodenoscopy (EGD) was performed and one ulcerative mass over the posterior wall of antrum was identified (Figure 1). The histopathological examination of the biopsy of the gastric lesion confirmed that it was an adenocarcinoma. Dynamic CT was arranged for pre-operative staging. It revealed several cystic lesions of the liver and possible deformity of the duodenal bulb without definite evidence of metastatic disease (Figure 2). In addition, the gallbladder was contracted with suspicious wall thickening and heterogeneous content. At laparotomy, one unsuspected nodule was seen in segment 3 of the liver. The lesion was confirmed to be a metastatic liver nodule by frozen section. At the time of the operation, severe adhesion between the head of the pancreas and the peripyloric tissue was noted. As a result, a palliative subtotal gastrectomy with Billroth II gastrojejunostomy was performed due to partial outlet obstruction.
Post operatively, the patient had low-grade fever and persistent epigastric pain without jaundice, tarry stools, or active upper gastrointestinal hemorrhage. The laboratory tests on the 3rd postoperative day demonstrated a raised white cell count with left shift (11 500/μL), a platelet count of 176 000/μL and a hemoglobin of 9.5 g/dL. Serum biochemistry revealed a normal liver function and renal function but a raised C-reactive protein (CRP) of 193.27 mg/L. Slightly elevated amylase (185 U/L) and normal lipase (39 U/L) level were also noted. Chest X ray and urinary analysis were obtained and disclosed no pneumonia or urinary tract infection. Although the drainage fluid was clear, there was some yellowish and cloudy discharge from the abdominal wound. We collected the fluid for microbial analysis only and culture, which grew a yeast-like organism. Fever persisted despite the administration of intravenous antibiotics. Abdominal CT arranged on the 8th postoperative day demonstrated irregular pancreatic contours with diffuse enlargement and fluid accumulation in the lesser sac with retroperitoneal extension (Figure 3), suggestive of either an acute pancreatitis or duodenal stump leakage. The laboratory tests on the 10th postoperative day demonstrated a white cell count with marked left shift (8400/μL, 86% in segment form and 10% in band form), a decreased platelet count of 58 000/μL, a raised CRP of 247.95 mg/L, and normal serum level of pancreatic enzymes (amylase level was 79 U/L and lipase level was 26 U/L). In view of continuing clinical deterioration associated with peritonitis and intra-abdominal sepsis from a possible anastomosis leak rather than acute pancreatitis (according to the normal amylase and lipase levels), the patient underwent an exploratory laparotomy instead of percutaneous drainage of the fluid 10 d after the first operation. At laparotomy, colorless turbid fluid accumulation was found at the same regions as reported on the abdominal CT scan. There was no evidence of bile leak, and the duodenal stump and anastomosis were intact. The necrotic tissues around the cavity of the loculated fluid collection had a similar appearance to fat necrosis in acute pancreatitis. Limited debridement was performed and a sump drain tube was placed for post-operative irrigation and drainage.
Bile-stained fluid was noted in the sump drain 2 d after the second operation, and the biochemistry study of the drainage fluid disclosed lower amylase and lipase levels (14 U/L and 9 U/L). Blood culture grew Bacteroides fragilis and hemogram showed progressive pancytopenia. A repeat CT scan suggested possible duodenal stump leakage with abscess formation (Figure 4). Despite total parenteral nutrition, hemodialysis for acute renal failure, and sump drain irrigation-drainage with intravenous antibiotics for septicemia and intra-abdominal infection, the patient eventually died of profound septic shock and multiple organ failure on day 30 after the first operation. A retrospective review of the patient’s abdominal CT demonstrated a previously undiagnosed, asymptomatic PD (Figure 5).
DISCUSSION
Gastrectomy is a commonly practiced procedure for both benign and malignant lesions of the stomach. Major complications such as postoperative bleeding, anastomotic leak and delayed gastric emptying are well documented[6]. In the past, most gastrectomies were performed for benign gastric or duodenal ulcer. The incidence of PGAP might be as high as 40.8% and the mortality rates in this group of patients ranged from 12.6% to 62.5%[1,2]. Recently, gastrectomies have mostly been performed for gastric malignancies, and the complication and mortality rates are much less because of improved surgical technique and postoperative care. The incidence of PGAP nowadays, although difficult to accurately estimate, has been reported to be less than 5%[7]. The mortality rate, however, can be up to 33.3%-50%[8,9] and is higher than acute pancreatitis of other etiologies[10]. Chen et al[3] reported a higher incidence of PGAP in patients having total gastrectomy compared with other types of gastrectomies (7.4% vs 0.8%), with a 33.3% mortality rate. Another study reported higher PGAP rate in patients undergoing extended lymph node dissection[4]. Despite the various advantages of laparoscopically assisted gastrectomy (LAG), the rates of acute pancreatitis after LAG have also been shown to range from 0.7% to 2.3%[8,9,11]. The poorer prognosis of PGAP might be related to postoperative immuno-suppression, increased pancreatitis-related hemorrhage because of extensive soft tissue/lymph node dissection, or anastomotic leak secondary to necrotizing pancreatitis and intra-abdominal sepsis.
PGAP may be a result of several factors. The stomach/duodenum might adhere to the underlying pancreas because of severe peptic ulceration or desmoplastic change from malignancy. Sometimes, the pancreas might actually be the base of the ulcer. Injury to the pancreatic parenchyma by electrocauterization or traction, microcirculation compromise, and direct injury to the pancreatic duct might occur during gastrectomy[7,11]. Other causes of PGAP such as duodenal hyperpressure[1] and postoperative spasm of the major papilla[2] have been hypothesized, but there is a general lack of evidence in the literature to substantiate these theories. Although some studies suggested insertion of a nasogastric tube or a duodenostomy tube after gastrectomy for decompression and releasing duodenal hyperpressure, Hsu et al[12] reported no difference in PGAP in spite of NG tube insertion. On the contrary, the “pancreatitis-like” presentation (such as hyperamylasemia and epigastric pain) caused by afferent loop obstruction can be cured under careful management of operations or decompression procedures[7,13]. The condition should be taken into consideration because the prognosis, timing of intervention and treatment options are far different from PGAP without A-loop obstruction.
The diagnosis of acute pancreatitis is mainly dependent on suggestive clinical features and laboratory studies. Physical examination may be variable and non-specific in postoperative patients because of pain and symptoms related to the operation. Elevated lipase levels 3-fold or more above the normal range appearing within 48 h is the most reliable test[14]. However, normal serum pancreatic enzyme levels cannot exclude acute pancreatitis absolutely[14]. Abdominal CT scan is particularly helpful in making a definitive diagnosis and excluding other differential diagnoses such as anastomotic leak, intra-abdominal abscess and hemorrhage[15]. However, the anatomical distortion post-operatively often makes the interpretation of CT images difficult[16]. This is exemplified by the present case report whereby a definitive diagnosis of PGAP was delayed because of equivocal pancreatic enzymes and non-specific CT findings of intra-abdominal fluid collection.
PD is a common congenital anomaly of the pancreas, with an incidence rate of 4% to 10%[17-19]. Anatomically, the dorsal duct (duct of Santorini) becomes the dominant duct draining the majority of the pancreatic juice through the minor papilla. The ventral duct (duct of Wirsung) does not fuse with the dorsal duct and only drains a small portion of the pancreatic head through the major papilla. The diagnosis of PD is made with endoscopic retrograde pancreatography (ERP) or magnetic resonance pancreatography (MRP). However, with the advent of multi-detector CT scans, high sensitivity and specificity for diagnosis of PD can be achieved by CT images in some particular conditions[20-22]. The CT features consistent with PD include the presence of the dominant dorsal duct sign (the dorsal duct being larger than the ventral duct, or a missing ventral duct) and the miss-communication between the two pancreatic ducts[20]. Both of these features were observed on the patient’s CT images retrospectively (Figure 5). About 5% to 45.5% of patients with PD present with pancreatitis or chronic abdominal pain[23,24]. The pathogenesis of this is attributable to the “relative stenosis” of the minor papilla as it drains the majority of the pancreatic juice through a small opening[24], resulting in an increase in the intra-ductal pressure of the dorsal duct system (20-28 mmHg vs 8-14 mmHg in normal pressure of major papilla)[25]. In addition, the unusual anatomical arrangement of the sphincter of the minor papilla could also contribute to the development of intra-ductal hypertension leading to pancreatitis[26]. Kamisawa et al[27] reported the high incidence of pancreatitis or pancreatic-type abdominal pain in patients with dominant duct of Santorini but without pancreas divisum.
According to these findings, it is reasonable to suppose that patients with PD might be more sensitive to minor injury of the pancreas and disturbance of its microcirculation. Once developed, it might progress precipitously to a more severe form and patients are slower to gain full recovery. Prophylactic administration of octreotide in the post-operative setting has been suggested in an attempt to prevent the development of PGAP[7]. The roles of preoperatively prophylactic cannulation of minor papilla, stent placement, and sphincterotomy have not been reported yet. The potential benefits of these invasive procedures in patients with PD or dominant duct of Santorini undergoing gastrectomy warrant further investigation.
In conclusion, PGAP is a less frequent post-gastrectomy complication in the current era. However, diagnosis and intervention should be made as early as possible because of the relatively high mortality rate associated with PGAP. Theoretically, patients with pancreas divisum or dominant duct of Santorini might connect to PGAP but the absolute relationship should be explored. Although serum pancreatic enzymes play an important role in the diagnosis of acute pancreatitis, imaging study such as an abdominal CT scan is particularly helpful in patients at high risk for PGAP. The roles and benefits of prophylactic octreotide, preoperative cannulation/stenting of the minor papilla, and sphincterotomy of minor papilla have not been well documented and would therefore warrant further investigation.