Published online Sep 28, 2009. doi: 10.3748/wjg.15.4576
Revised: August 18, 2009
Accepted: August 25, 2009
Published online: September 28, 2009
AIM: To retrospectively evaluate the computed tomography (CT)/magnetic resonance imaging (MRI) imaging features of epithelioid angiomyolipoma of the liver (Epi-HAML), with pathology as a reference.
METHODS: The CT/MRI findings (number, diameter, lobar location, and appearance of lesions) in a series of 10 patients with 12 pathologically proven epithelioid angiomyolipomas of the liver were retrospectively analyzed. The imaging features, including attenuation/signal intensity characteristics, presence of fat, hypervascular, outer rim, and vessels within lesion, were evaluated and compared with that of non-Epi-HAML in 11 patients (13 lesions). The Fisher exact test was used to compare difference in probability of imaging features between the two types.
RESULTS: For 21 patients, CT images of 15 patients and MR images of six patients were available. No patient underwent two examinations. For the 15 patients with a CT scan, all HAML lesions in the two groups (10 Epi-HAML and seven non-Epi-HAML) manifested as hypoattenuation. For the six patients with MRI, all lesions (two Epi-HAML and six non-Epi-HAML) were hypointense on T1WI (fat suppression) and hyperintense on T2WI. There were 10 non-Epi-HAML, but only two Epi-HAML lesions showed the presence of fat, which significantly different between the two types (P = 0.005). On the dynamic contrast enhancement (DCE) imaging, eight Epi-HAML, and 13 non-Epi lesions manifested as hypervascular. Punctate or curved vessels were displayed in 10 Epi-HAML as well as in nine non-Epi lesions and outer rim enhancement could be found with eight Epi-HAML as well as six non-Epi lesions.
CONCLUSION: Little or no presence of adipose tissue was found to be an imaging feature of Epi-HAML, compared with the non-Epi type. In addition, hypervascularity with opacification of central punctiform or filiform vessels on DCE would be a characteristic enhancement pattern for Epi-HAML.
- Citation: Xu PJ, Shan Y, Yan FH, Ji Y, Ding Y, Zhou ML. Epithelioid angiomyolipoma of the liver: Cross-sectional imaging findings of 10 immunohistochemically-verified cases. World J Gastroenterol 2009; 15(36): 4576-4581
- URL: https://www.wjgnet.com/1007-9327/full/v15/i36/4576.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4576
| No. | Age (yr)/sex | Size (cm), segment | Unenhanced CT/MR | Fat | Vascularity | Outer rim | Vessels in lesion |
| 1 | 51/F | 6.5, 4.0 VI/VII, IV | Hypo | No | Hypovascular | Yes | Yes |
| 2 | 42/F | 4.2, I | Hypo | No | Hypovascular | No | No |
| 3 | 35/F | 7.5, VI | Hypo | No | Hypervascular | No | Yes |
| 4 | 36/F | 1.5, IV | Hypo | No | Hypervascular | Yes | Yes |
| 5 | 17/F | 10.0, V/VII/VIII | Hypo | No | Hypervascular | Yes | Yes |
| 6 | 55/F | 5.0, II/III | Hypo | No | Hypervascular | Yes | Yes |
| 71 | 33/F | 6.0, 1.0, II/III/IV, VII | Hypo | Yes | Hypervascular | No | Yes |
| 8 | 36/F | 3.0, IV | Hypo | No | Hypervascular | Yes | Yes |
| 9 | 46/F | 4.0, II/III | T1 hypo, T2 hyper | No | Hypervascular | Yes | Yes |
| 10 | 47/F | 2.5, VI | T1 hypo, T2 hyper | No | Hypervascular | Yes | Yes |
| No. | Age (yr)/sex | Size (cm), segment | Unenhanced CT/MR | Fat | Vascularity | Outer rim | Vessels in lesion |
| 1 | 45/F | 6.0, VI/VII | Hypo | Yes | Hypervascular | No | No |
| 2 | 34/F | 4.5, II/III | Hypo | No | Hypervascular | No | Yes |
| 3 | 37/F | 5.5, VIII/IV | T1 hypo, T2 hyper | Yes | Hypervascular | Yes | Yes |
| 4 | 40/F | 12.0, VII/VIII/IV | T1 hypo, T2 hyper | Yes | Hypervascular | Yes | Yes |
| 2.0, 1.0, II/III | |||||||
| 5 | 50/F | 6.5, IV | T1 hypo, T2 hyper | Yes | Hypervascular | Yes | Yes |
| 6 | 43/M | 2.0, VI | Hypo | Yes | Hypervascular | No | No |
| 7 | 47/M | 2.0, IV | Hypo | Yes | Hypervascular | No | No |
| 8 | 46/M | 3.0, VIII | Hypo | Yes | Hypervascular | No | Yes |
| 9 | 44/F | 4.0, II | Hypo | No | Hypervascular | No | No |
| 10 | 50/F | 3.0, VI | Hypo | Yes | Hypervascular | No | Yes |
| 11 | 21/F | 12.0, VI/VI | T1 hypo, T2 hyper | No | Hypervascular | Yes | Yes |
| Epi-HAML, n = 12 (10) | Non-Epi-HAML, n = 13 (11) | P value | |
| Fat | 2 (1) | 10 (8) | 0.005 |
| Hypervascular | 8 (8) | 13 (11) | 0.082 |
| Vessels in lesions | 10 (9) | 9 (7) | 0.645 |
| Outer rim | 8 (7) | 6 (4) | 0.428 |
Hepatic Angiomyolipoma (HAML) is a rare benign tumor and the etiology is unclear. Some cases have been associated with the tuberous sclerosis complex (TSC). HAML belongs to a family of tumors that have collectively been called ‘‘PEComa’’[1-3]. Histologically, HAML is composed of a heterogeneous mixture of blood vessels, smooth muscle, and adipose cells, of varying proportions and distributions, not only among different tumors, but from area to area within the same tumor. Thus, according to the line of differentiation and the predominance of tissue components, HAML is usually subcategorized into mixed, lipomatous (≥ 70% fat), myomatous (≤ 10% fat), and angiomatous types. The smooth muscle cell component is the most specific for the diagnosis. Depending on the dominant cell type, HAML can be subcategorized into epithelioid, spindle, and intermediate forms[1]. Epithelioid AML (Epi-AML) was first described in the liver in 2000[4], diagnosis can be difficult, for example the differentiation of Epi-AML from hepatocellular carcinoma and the metastatic sarcomatoid variant of renal cell carcinoma. Only one case report of Epi-AML has been mentioned or reviewed in the literature[4-7]. In this paper, 10 immunohistochemically verified cases were retrospectively analyzed and the CT/MR imaging findings were summarized and compared with those of non-epi-HAML.
The imaging examinations of 21 cases (10 Epi-HAML and 11 non-Epi-HAML) with pathologically proven HAML were included in this study. Proof of diagnosis was based on findings at liver resection and pathological manifestations, including immunohistochemical staining. Cases were collected from one university hospital over a seven-year period and were identified by reviewing pathology databases. In our patients, AML were incidentally detected on cross-sectional imaging performed for various reasons, such as abdominal pain (n = 15), suspected gallbladder stone (n = 3), or urinary (n =3) diseases. Institutional review board approval and patient consent were not required for this retrospective study because patient privacy was maintained and patient care was not impacted.
Helical multiphasic CT was performed in 15 patients using a Siemens Sensation 16 (Siemens Medical Solutions) or a PHILIPS Marconi MX8000 4 slice CT unit with 5 to 7 mm contiguous sections. After non-enhanced acquisitions of the liver, patients underwent helical multiphase CT that included both hepatic arterial phase and portal venous phase imaging (30-35 s and 80-85 s, respectively), after i.v. infusion of 90-100 mL nonionic contrast material (iopromide, Ultravist 300, Bayer Schering Pharma). Contrast material was injected at a rate of 3 mL/s with a power injector (Envision CT, MEDRAD).
MRI was performed in six patients using 1.5-T MR units (Magnetom avanto, Siemens Medical Solutions) with the combination of a phased-array body coil and spine array coil for signal reception. Baseline MR images, including a respiratory-navigated T2-weighted turbo spin-echo sequence [TR/TE, 2000/104 ms; slice thickness, 7 mm; flip angle, 150°; matrix, 207 (phase) × 384 (read); FOV, 33-38 cm] and a breath-hold T1-weighted fast low angle shot (FLASH) sequence [TR/TE, 112/4.76 ms; slice thickness, 7 mm flip angle, 70°; matrix, 114 (phase) × 256 (read); FOV, 33-38 cm]. Dynamic imaging, breath-hold T1-weighted FLASH sequence was performed using the following parameters: TR/TE, 230/2.47 ms; flip angle, 70°; matrix, 135 (phase) × 256 (read); effective slice thickness, 7 mm; and FOV, 33-38 cm. Dynamic imaging was performed before and after administration of gadopentate dimeglumine (Magnevist; Bayer Schering Pharma), consisting of late arterial (delay time 20-25 s), portal (70-90 s), and equilibrium (180 s) phases. The contrast-enhanced imaging was acquired after a bolus injection of 30-35 mL of contrast with a fixed delay. The contrast material was injected into the antecubital vein at a rate of 2.5 mL/s via a power injector (Spectris, Medrad, Indianola, PA, USA). Three dynamic phases were repeated for 18-21 s during a single breath-hold.
Imaging studies were evaluated on film by two abdominal radiologists (with experience ranging from 5 to 10 years) in consensus. Readers were not blinded to the pathology results.
The following imaging criteria were analyzed: number of lesions; lesion diameter; lesion location according to the hepatic segment numbering system of Couinaud; attenuation at non-enhanced CT, classified as hypoattenuating, isoattenuating, or hyperattenuating to the adjacent liver parenchyma; signal intensity characteristics of the lesions at non-enhanced T1-weighted (including T1WI and T1WI with fat suppression) and T2-weighted MRI; presence of fat tissue, hypoattenuating foci (-20 to -120 Hu) on non-enhanced CT images or hyperintense on T1WI but hypointense on T1WI with fat suppression; enhancement pattern at contrast-enhanced CT or MRI with regard to three-phase dynamic enhancement; presence of the central vessels in lesion at contrast-enhanced imaging; and presence of outer rim enhancement.
All tissues were reviewed independently by one pathologist. Histopathological diagnosis was made according to the World Health Organization’s classification of tumors of the liver and intrahepatic bile ducts[8]. The most important diagnostic criterion was the presence of HMB-45-positive myoid cells. All tumor tissues had been fixed in neutral buffered formalin and were routinely embedded in paraffin. Hematoxylin-eosin stained sections were evaluated and immunohistochemical studies were performed on representative blocks by the EnVision Plus system (DAKO, Glostrup, Denmark) with a panel of antibodies (HMB-45, SMA, S-100, CD34, A103, MSA, Vimentin, CD68, CD117, HepPar-1, AFP, AE1/AE3, and CK8).
Statistical analysis was performed by using software (Intercooled Stata, version 9.0 for Windows, 2005; Stata Corp, College Station, TX, USA). We used the Fisher exact test to compare probability of these imaging features for Epi-HAML and non-Epi lesion. A P value less than 0.05 was considered statistically significant.
The data are summarized in Tables 1 and 2. Age at diagnosis of hepatic angiomyolipoma varied between 17 and 55 years (mean 40.7 years). In the Epi-HAML group, there was history of TSC for one patient, and a combination of left kidney AML for another one. Among 10 patients, eight had solitary lesions and two had two lesions. Lesions had a mean diameter of 4.6 (range, 1.5-10.0). In the non-Epi-HAML group, there was right kidney AML in one patient, 10 had solitary lesions, and one had three lesions. Lesions had a mean diameter of 4.9 (range, 1.0-12.0).
The CT/or MR images of 21 patients (CT images for 15 patients and MR for six) were available for retrospective analysis. The results are show in Tables 1-3.
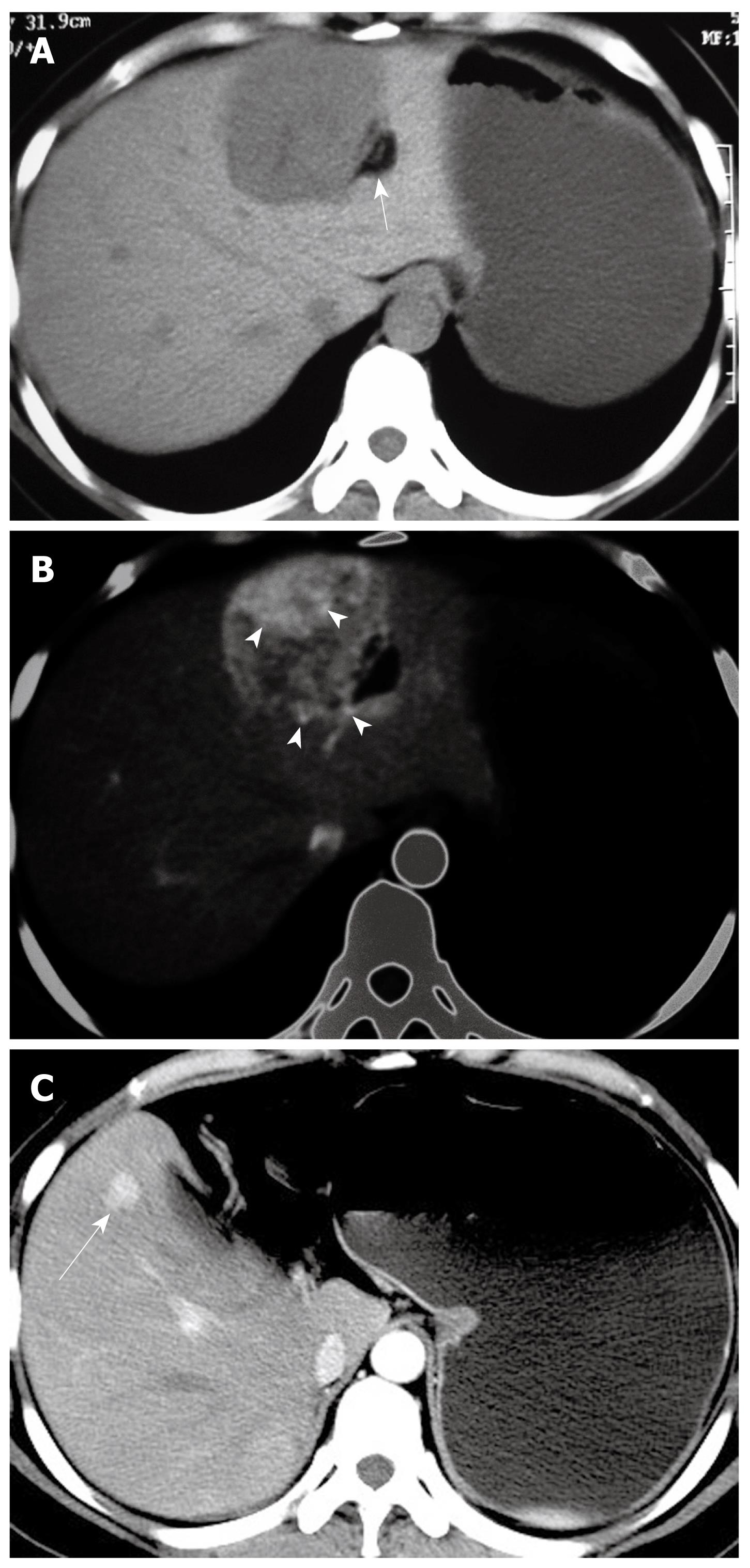
At non-enhanced CT, 10 Epi-HAML and seven non-Epi HAML were hypoattenuating to the surrounding liver. Two Epi-HAML and six non-epi lesions were hypointense on T1WI (fat suppression) and hyperintense on T2WI. The presence of fat was detected in 10 non-Epi HAML but only two Epi type lesions (Figure 1) and there were significant differences between the two lesion types (P < 0.01, Table 3).
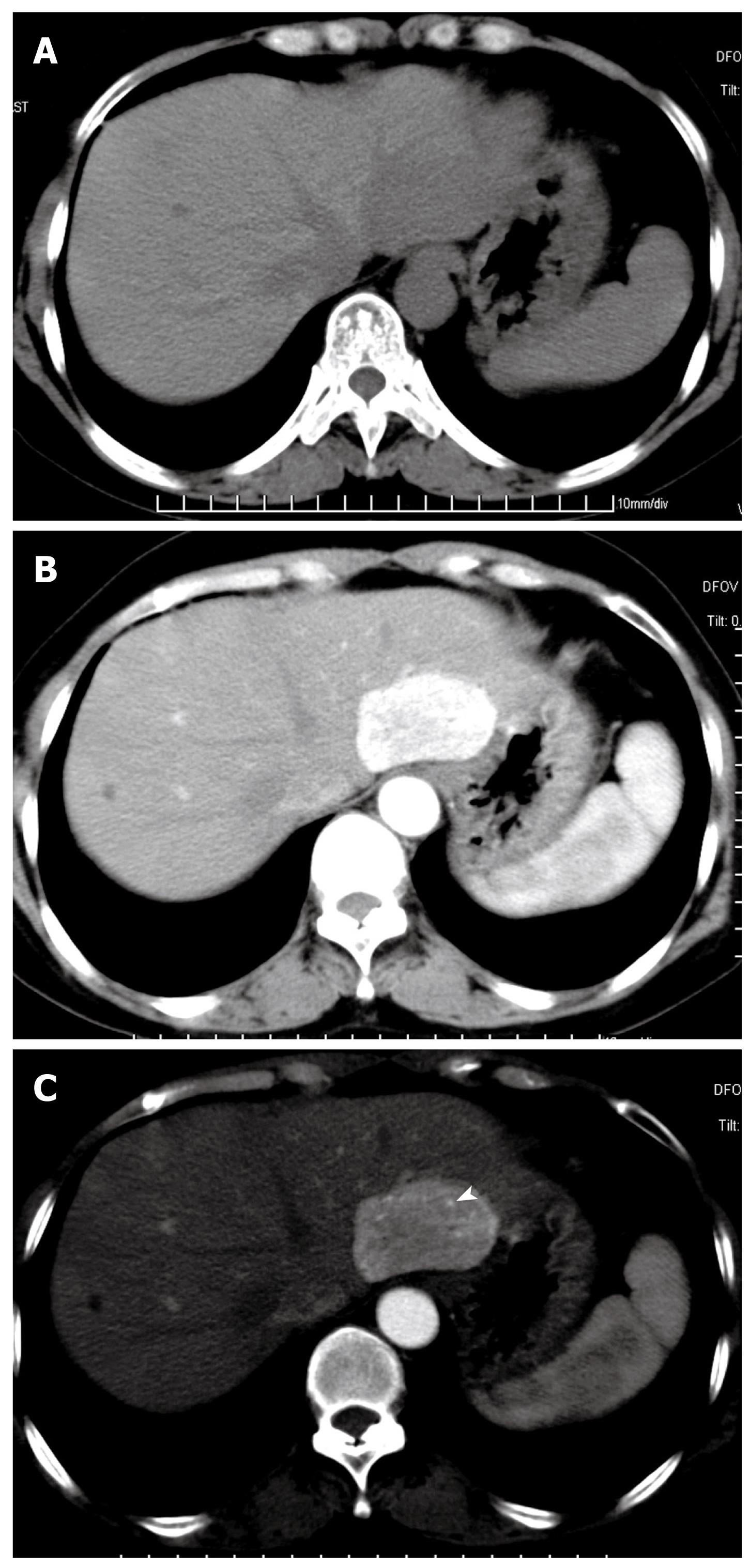
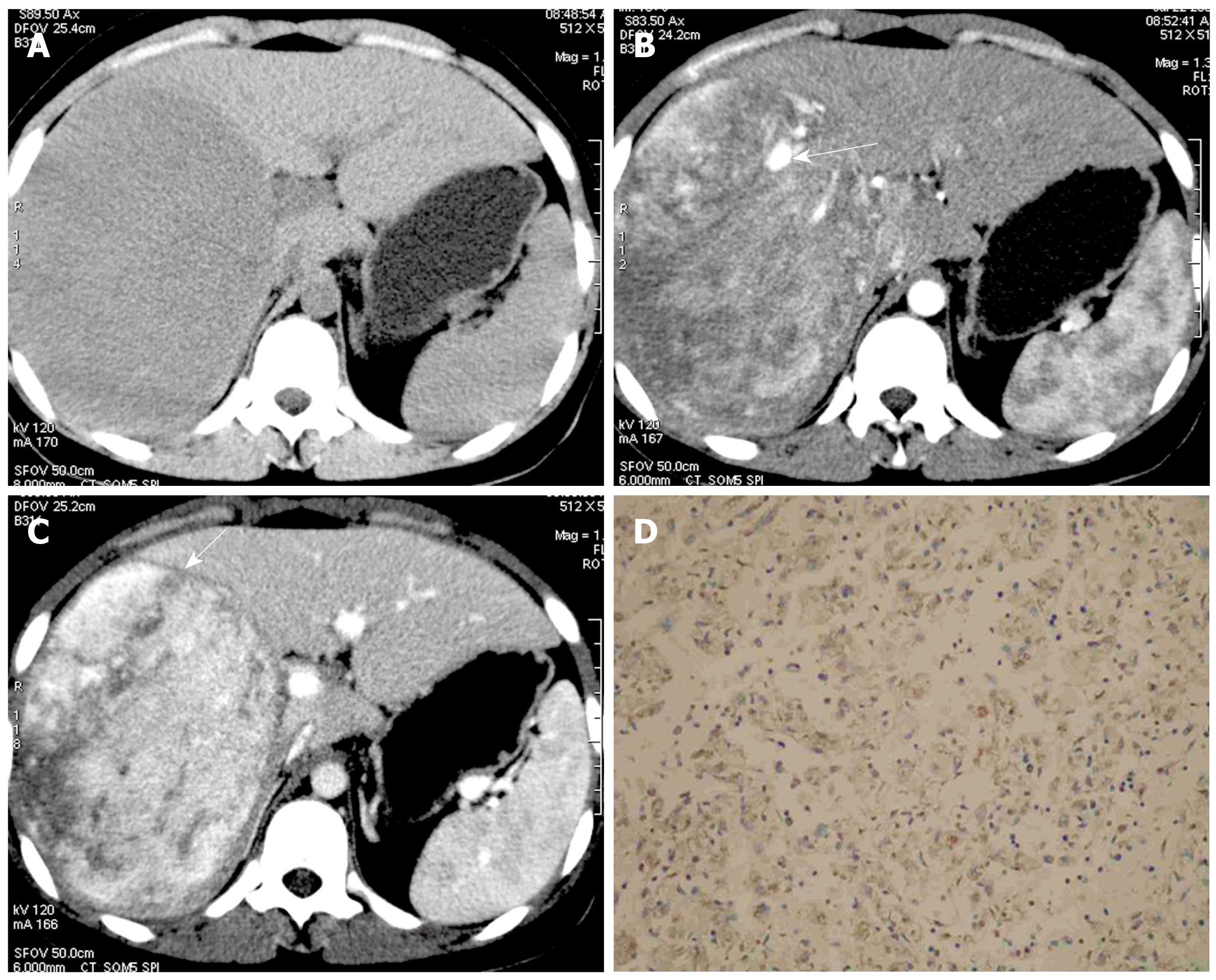
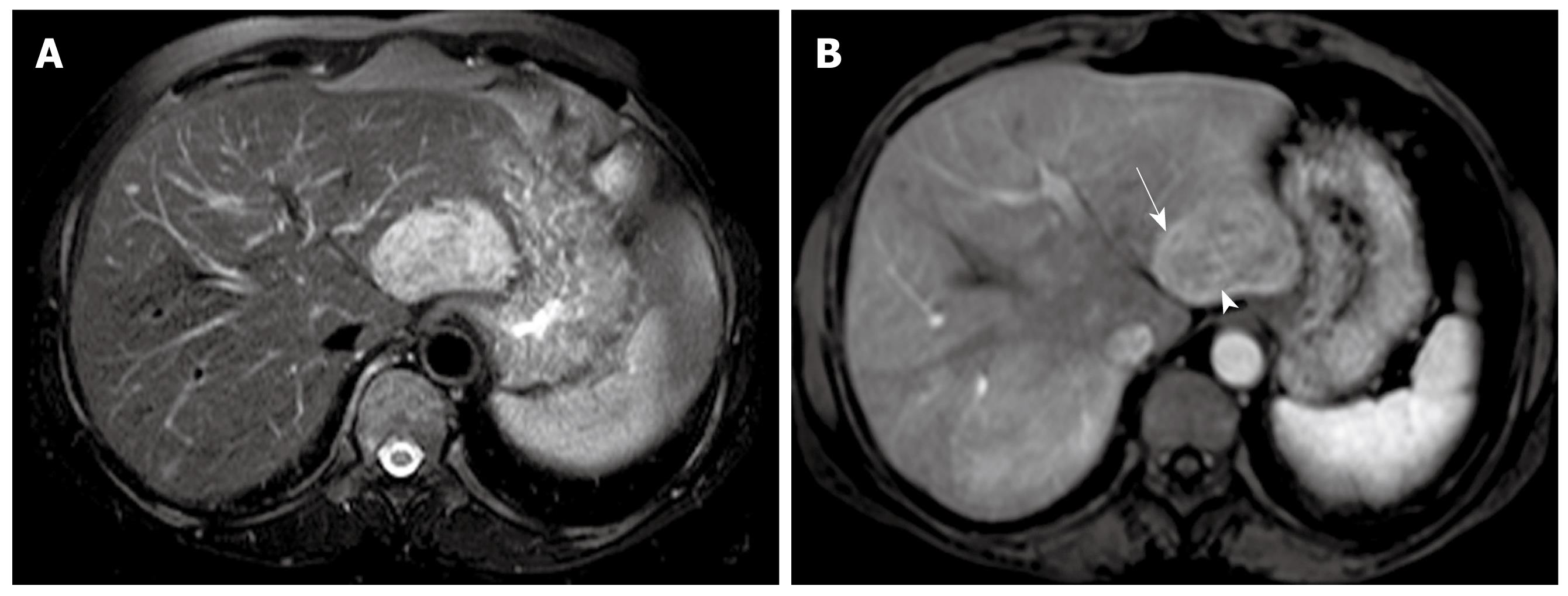
On arterial phase, eight Epi-HAML and 13 non-Epi-HAML showed obvious enhancement (Figures 1, 2, 3). Punctate or curved vessels could be seen within 10 Epi-HAML as well as nine non-Epi type lesions (Figures 1-4) on arterial or/and portal phase. Outer rim enhancement could be found in eight Epi-HAML as well as in six non-Epi-HAML on enhanced imaging (Figures 3 and 4).
Immunohistochemical studies showed that tumor cells were positive for HMB-45 and A103, but negative for cytokeratin (HepPar-1, AFP, AE1/AE3, CK8.) in all cases. Smooth muscle actin (SMA) and desmin staining were weak to moderate in epithelioid cells. MSA staining was similar to desmin but was more weak when positive. The endothelial cells lining the blood vessels were positive for CD34. CD117 and S-100 were negative in all cases.
Imaging and clinical follow-up was available in 10 patients with Epi-HAML and ranged from one to six years. Recurrent hepatic lesions were found in one patient (Figure 1), pubic bone destruction and metastatic nodule in body soft tissue were proved with biopsy in another.
Previous literatures showed variable imaging appearances for HAML[9-12]. The imaging characteristics of HAML are correlated with its histological components, and demonstration of blood vessels and mature adipose tissue are the most important radiographic features. Color Doppler sonography shows a punctiform or filiform vascular distribution pattern. Contrast-enhanced CT shows marked enhancement of the soft tissue components in the arterial phase. MRI is the most specific imaging entity for the detection of lipomatous components; however, because hepatic AML can have such variation in the amount of adipose tissue present, detection varies based on the percentage of the lesion that is composed of adipose tissue. MRI findings include hypointensity or hyperintensity on T1WI, slight hyperintensity on T2WI, dense enhancement in the arterial phase, and hypointensity in the delayed phase.
In our study, most of the Epi-AML tumors (10/12) were completely devoid of adipose tissue, which is the characteristic finding compared with that of non-Epi type. The result was consistent with a previous report[1]. After i.v. contrast administration, there was obvious enhancement on the arterial phase for most of these lesions, suggesting that Epi-AML was a hypervascular tumor. Most lesions (seven) manifested as hypoattenuation or hypointensity on portal/equilibrium phase. By adjusting the window width and level, punctate or curved vessels could be seen within 10 lesions on enhanced scanning. In addition, intact or discrete outer rim enhancement within 8 lesions was observed. According to the pathology specimens and literature report, it is not true capsular but a pseudocapsule, which is composed of the compressed liver parenchyma and sparse fibrosis tissues with small vessels, resulting in delayed enhancement on late phase[13]. A similar manifestation can be found in other hypervascular lesion, such as hepatocellular carcinoma(HCC) and focal nodular hyperplasia (FNH). The distinction between these lesions can be difficult. However, the enhancement patterns were somewhat different among Epi-AML, HCC, and FNH. Most of the HCC enhanced markedly in the arterial phase and decreased rapidly on the portal venous/equilibrium phase. Additionally, capsule could be found in most of the HCC, so the margins of HCC were more clear than those of AML in the portal venous phase, a suggestive finding for correct diagnosis[14]. The enhancement pattern of FNH was similar to AML, in both of them prolonged enhancement could be shown in the portal venous phase. However, most of FNH enhanced homogeneously on the arterial phase except the central scar, which was characteristic of FNH and could be enhanced on the portal venous phase/delayed phase[15].
Five cases of hepatic malignant angiomyolipoma have been reported[5,16-19]. Strict histology criteria for defining hepatic angiomyolipoma as malignant have not been put forward, but it should be suspected, especially in tumors with many mitoses. Those with necrosis and significant cellular pleomorphism might show aggressive behavior. There is clinical evidence of aggressive behavior such as recurrence and metastases beyond the liver, for two cases in these 10 Epi-AML cases. However, there are no characteristic findings for malignant HAML on cross-sectional imaging. Therefore, although most HAMLs are biologically benign, this tumor should be considered to have malignant potential, especially for Epi-AML. So resection and careful follow-up are recommended.
It is important to recognize the limitations of our study. Firstly, the study is retrospective; secondly, the numbers reported are limited and no one patient had both CT and MRI. A further limitation is that not every patient underwent regular imaging follow-up and documentation was incomplete.
In summary, little or no adipose tissue was found to be an imaging feature of Epi-HAML, vs non-Epi-HAML. In addition, hypervascularity with opacification of central punctiform or filiform vessels on DCE would be a characteristic enhancement pattern for Epi-HAML.
Hepatic Angiomyolipoma (HAML) is a rare benign tumor and belongs to a family of tumors that have collectively been called ‘‘PEComa’’. The immense variability of the morphological appearance is due to the different heterogeneous mixtures of vessels, epithelioid cells, and lipocytes. As a specific form, the epithelioid HAML (Epi-HAML) has characteristics in pathology and diagnosis that can be difficult, e.g. differentiation of Epi-AML from hepatocellular carcinoma and the metastatic sarcomatoid variant of renal cell carcinoma.
Previous studies showed variable imaging appearances for HAML. The imaging characteristics of HAML are correlated with its histological components. Both CT and MRI, for the most part, do not allow the definitive differentiation of HAML from hepatocellular carcinoma, adenoma, liposarcoma, lipoma, hamartoma, and sometimes even from focal nodular hyperplasia, especially if the fat content is low. The Epi-HAML has morphological characteristics of tumor cells. In addition, the tumors are devoid of fat or only scattered fat cells distribution in pathology. However, the imaging features of Epi-HAML have not been unequivocally described and evaluated. In this study, the authors summarized CT/MRI imaging features of Epi-HAML and compared with those of non-Epi-HAML.
Previous reports have showed demonstration of mature adipose tissue is the most important radiographic features and highlighted the importance in the diagnosis of HAML, in particular for mixed, lipomatous types of HAML. This is the first study to report that blood vessels are also an imaging feature in Epi-HAML. Furthermore, the study would suggest that little or no adipose tissue might be imaging characteristics of Epi-HAML, versus non-Epi-HAML.
By understanding the imaging characteristics, this study could help radiologists familiarize themselves with the appearance of Epi-HAML, and improve the confidence in the diagnosis of incidental liver tumor, especially in cases where the fat content is low and the interpretation of histological findings is difficult.
Immunohistochemistry is a method of analyzing and identifying cell types based on the binding of antibodies to specific components of the cell. The most important diagnostic criterion for HAML is the presence of HMB-45-positive myoid cells.
The manuscript reported the imaging characteristics of Epi-HAML, only a few reports had been published about the lesion before. It will be helpful for radiologists to obtain knowledge of the lesion.
Peer reviewer: Xiao-Peng Zhang, Department of Radiology, Peking University School of Oncology, Beijing Cancer Hospital & Institute, No.52 Haidian District, Beijing 100142, China
S- Editor Tian L L- Editor Stewart GJ E- Editor Lin YP
| 1. | Tsui WM, Colombari R, Portmann BC, Bonetti F, Thung SN, Ferrell LD, Nakanuma Y, Snover DC, Bioulac-Sage P, Dhillon AP. Hepatic angiomyolipoma: a clinicopathologic study of 30 cases and delineation of unusual morphologic variants. Am J Surg Pathol. 1999;23:34-48. |
| 2. | Xu AM, Zhang SH, Zheng JM, Zheng WQ, Wu MC. Pathological and molecular analysis of sporadic hepatic angiomyolipoma. Hum Pathol. 2006;37:735-741. |
| 3. | Jiang TA, Zhao QY, Chen MY, Wang LJ, Ao JY. Diagnostic analysis of hepatic angiomyolipoma. Hepatobiliary Pancreat Dis Int. 2005;4:152-155. |
| 4. | Yamasaki S, Tanaka S, Fujii H, Matsumoto T, Okuda C, Watanabe G, Suda K. Monotypic epithelioid angiomyolipoma of the liver. Histopathology. 2000;36:451-456. |
| 5. | Dalle I, Sciot R, de Vos R, Aerts R, van Damme B, Desmet V, Roskams T. Malignant angiomyolipoma of the liver: a hitherto unreported variant. Histopathology. 2000;36:443-450. |
| 6. | Tryggvason G, Blöndal S, Goldin RD, Albrechtsen J, Björnsson J, Jónasson JG. Epithelioid angiomyolipoma of the liver: case report and review of the literature. APMIS. 2004;112:612-616. |
| 7. | Garcia TR, Mestre de Juan MJ. Angiomyolipoma of the liver and lung: a case explained by the presence of perivascular epithelioid cells. Pathol Res Pract. 2002;198:363-367. |
| 8. | Hirohashi S, Blum HE, Ishak KG. Tumours of the liver and intrahepatic bile ducts. Pathology and genetics. Tumours of the digestive system. World Health Organization classification of tumours. Lyon: IARC Press 2000; 157-202. |
| 9. | Prasad SR, Wang H, Rosas H, Menias CO, Narra VR, Middleton WD, Heiken JP. Fat-containing lesions of the liver: radiologic-pathologic correlation. Radiographics. 2005;25:321-331. |
| 10. | Yoshimura H, Murakami T, Kim T, Nakamura H, Hirabuki N, Sakon M, Wakasa K, Inoue Y. Angiomyolipoma of the liver with least amount of fat component: imaging features of CT, MR, and angiography. Abdom Imaging. 2002;27:184-187. |
| 11. | Yan F, Zeng M, Zhou K, Shi W, Zheng W, Da R, Fan J, Ji Y. Hepatic angiomyolipoma: various appearances on two-phase contrast scanning of spiral CT. Eur J Radiol. 2002;41:12-18. |
| 12. | Högemann D, Flemming P, Kreipe H, Galanski M. Correlation of MRI and CT findings with histopathology in hepatic angiomyolipoma. Eur Radiol. 2001;11:1389-1395. |
| 13. | Chang JC, Lee YW, Kim HJ. Preoperative diagnosis of angiomyolipoma of the liver. Abdom Imaging. 1994;19:546-548. |
| 14. | Iannaccone R, Piacentini F, Murakami T, Paradis V, Belghiti J, Hori M, Kim T, Durand F, Wakasa K, Monden M. Hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: helical CT and MR imaging findings with clinical-pathologic comparison. Radiology. 2007;243:422-430. |
| 15. | Mortelé KJ, Praet M, Van Vlierberghe H, de Hemptinne B, Zou K, Ros PR. Focal nodular hyperplasia of the liver: detection and characterization with plain and dynamic-enhanced MRI. Abdom Imaging. 2002;27:700-707. |
| 16. | McKinney CA, Geiger JD, Castle VP, Ruiz RE, Strouse PJ. Aggressive hepatic angiomyolipoma in a child. Pediatr Hematol Oncol. 2005;22:17-24. |
| 17. | Mizuguchi T, Katsuramaki T, Nobuoka T, Nishikage A, Oshima H, Kawasaki H, Kimura S, Satoh M, Hirata K. Growth of hepatic angiomyolipoma indicating malignant potential. J Gastroenterol Hepatol. 2004;19:1328-1330. |
| 18. | Croquet V, Pilette C, Aubé C, Bouju B, Oberti F, Cervi C, Arnaud JP, Rousselet MC, Boyer J, Calès P. Late recurrence of a hepatic angiomyolipoma. Eur J Gastroenterol Hepatol. 2000;12:579-582. |
| 19. | Nguyen TT, Gorman B, Shields D, Goodman Z. Malignant hepatic angiomyolipoma: report of a case and review of literature. Am J Surg Pathol. 2008;32:793-798. |