Published online Sep 28, 2009. doi: 10.3748/wjg.15.4529
Revised: August 18, 2009
Accepted: August 25, 2009
Published online: September 28, 2009
AIM: To investigate the inhibitory effect of natural taurine (NTau) on portal hypertension (PHT) in rats with experimentally-induced liver cirrhosis (LC).
METHODS: Experimentally-induced LC Wistar rats (20 rats/group) were treated with either oral saline or oral NTau for 6 consecutive weeks. Evaluation parameters included portal venous pressure (PVP), portal venous resistance (PVR), portal venous flow (PVF), splanchnic vascular resistance (SVR) and mean arterial pressure (MAP). Vasoactive substance levels including nitric oxide (NO), nitric oxide synthase (NOS) and cyclic guanosine monophosphate (cGMP) were also measured. Histological investigation of type I and III collagen (COL I and III) and transforming growth factor-β1 (TGF-β1) was also performed.
RESULTS: Treatment with NTau (1) significantly decreased PVP, PVR and PVF, and increased MAP and SVP; (2) markedly increased the vascular compliance and reduced the zero-stress of the portal vein; (3) markedly decreased the amount of NO and cGMP and activity of NOS; and (4) improved the pathological status of the liver tissue and reduced the expression of COL I, COL III and TGF-β1.
CONCLUSION: NTau inhibited the LC-induced PHT by improving hyperdynamic circulation, morphology of liver and biomechanical properties of the portal vein in experimentally-induced LC rats.
- Citation: Liang J, Deng X, Lin ZX, Zhao LC, Zhang XL. Attenuation of portal hypertension by natural taurine in rats with liver cirrhosis. World J Gastroenterol 2009; 15(36): 4529-4537
- URL: https://www.wjgnet.com/1007-9327/full/v15/i36/4529.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4529
Liver cirrhosis (LC)-induced portal hypertension (PHT), also referred to as hepatocirrhotic portal hypertension, is highly susceptible to life-threatening complications such as esophageal and fundus ventriculi variceal bleeding, as well as ascites and hepatic encephalopathy (HE), resulting in high mortality among this group of patients. Currently, several treatment modalities are commonly employed for the management of LC-induced PHT, including systemic drug treatment, surgical intervention, endoscopic ligation, and liver transplantation. Although endoscopic ligation is useful in preventing initial hemorrhage in the upper digestive tract, it is less effective when dealing with recurring bleeding[1]. Transjugular intrahepatic portosystemic stent shunt (TIPSS) can, to a certain extent, reduce various complications of PHT. It may, however, augment the incidence of HE. Therefore, TIPSS is not generally the first therapeutic option for patients with PHT owing to its inability to improve the survival rate[2]. On the other hand, liver transplantation is regarded as the last option to combat advanced liver cirrhosis. However, the inherent risk associated with this radical surgical procedure and the inevitable high costs of organ transplantation, together with the long-standing disturbance of the systemic hemodynamics after transplantation, practically prevents it from being a routinely used method. Presently, long-term pharmacological treatment is still the mainstay for LC-induced PHT. To date, to develop an effective therapeutic approach for the management of LC-induced PHT remains a formidable challenge to many researchers in the field.
Our previous study showed that natural taurine (NTau) markedly inhibited the contraction and collapse of hepatic stellate cells (HSCs)[3]. It has been well established that contraction of HSCs significantly contributes to the initiation and progression of hepatocirrhotic portal hypertension[4,5]. As a continuation of our previous efforts to evaluate the therapeutic potential of NTau for LC-induced PHT, the present study aims at investigating the inhibitory effects of NTau on PHT, specifically focusing on the perspectives of hepatic “forward” and “backward” flow theories as well as on portal venous biomechanics and hemodynamics.
Male specific-pathogen-free (SPF) Wistar rats weighing 215 ± 18 g were provided by the Centre of Experimental Animals, Guangxi Medical University, Nanning, Guangxi Province, China. Immunohistochemical staining kits for type I collagen (COL I), type III collagen (COL III) and transforming growth factor-β1 (TGF-β1) were purchased from the Wuhan Boster Biological Technology Ltd (Wuhan, Hubei Province, China). Assay kits for nitric oxide (NO), nitric oxide synthase (NOS) and cyclic guanosine monophosphate (cGMP) were obtained from the Nanjing Jiangcheng Bioengineering Institute (Nanjing, Jiangsu Province, China). The instruments used in the current project included an electromagnetic flowmeter (MFV-3200; Nihon Kohden, Japan), an 8-channel physiology recorder (RM-6000; Nihon Kohden, Japan), an ultraviolet spectrophotometer (80-2106-20; Pharmacia, UK), a UV-spectrophotometer (9100; Beijing Rayleigh Analytical Instrument Corporation, Beijing, China), and a light microscope (BX51, Olympus, Japan).
The natural taurine (2-aminoethyl-sulfonic acid) used in the present study was extracted from black clams (Meretrix meretrix L.). Briefly, after the clam meat was cleaned and weighed (500 g), it was then minced in an electrical blender (4000 r/min) ten times, with each process lasting for about 10 s. Distilled water (1 L) was then added into the mince and further homogenized for 30 min. The mixture was then boiled in a water bath at 100°C for 30 min, followed by filtering the mixture through four layers of gauze. The residue on the top of the gauze was discarded and the filtrate was then centrifuged to obtain the supernatant, which was then de-acidified with HCl (HCl:H2O = 3). After centrifugation, the proteins were adjusted to pH 10 with NaOH (20%) aqueous solution to yield the de-alkalined proteins. After the pH value was adjusted to 5, the supernatant was further condensed. The other unwanted amino acids and pigments were removed by column chromatography using strong acid cation exchange resin as the solid phase and eluting with distilled water. The resultant NTau was quantitatively measured by high performance liquid chromatography (HPLC) and the purity of the NTau was determined to be 98.8%.
An animal model of LC was established following a previously described protocol[6]. In brief, rats were fed with animal chow consisting of 80% corn flour, 19.5% animal fat and 0.5% cholesterol. The animals were only allowed to drink 15% aqueous alcohol. After an initial subcutaneous injection of a 40% CCl4-olive oil solution at a dose of 5 mL/kg, the subcutaneous injections were repeated once every 3 d at a dose of 3 mL/kg for a total duration of 42 d. All rats were kept at room temperature under 12-h dark/light cycles and received humane care in accordance with the Guidelines of the Guangxi University of Chinese Medicine for the Care of Laboratory Animals.
Forty Wistar rats were randomized into 2 groups, with 20 rats in each group: a model group without NTau treatment (LC - NTau), and a model group treated with NTau (LC + NTau). During establishment of the model, rats in the LC + NTau group were concomitantly administered with 600 mg/kg NTau by gavage once daily, while LC - NTau group received only saline. Another 20 Wistar rats which received only normal animal chow and no CCl4-olive oil solution injection were also used as the normal healthy control (NML) in the experimental design.
Measurement of hemodynamic parameters: Measurement of the hemodynamic parameters was conducted according to the methods described previously[7] and was performed by an experienced technician affiliated to the first author’s research group. Briefly, the animals were fasted for 8 h prior to measurement. Pentobarbital sodium (30 mg/kg) was injected through an ear vein to induce anesthesia. Then, a median epigastric incision was made in each animal which was placed in a supine position. The main portal vein (about 2 cm in length) was dissociated and exposed by blunt dissection. An incision was made followed by placement of an electromagnetic probe with an appropriate caliber connected with the electromagnetic flowmeter into the portal vein to measure the portal venous flow (PVF). Similar catheterizations were made into the portal vein and carotid artery for the measurement of the portal venous pressure (PVP) and mean arterial pressure (MAP) with the aid of a physiology recorder. All data were recorded after the hemodynamic parameters were stabilized. Portal venous resistance (PVR) and splanchnic vascular resistance (SVR) were also calculated separately using the following equations PVR = PVP/PVF and SVR = MAP/PVF, respectively.
Determination of NO, NOS and cGMP in portal venous blood: Heparinized portal venous blood (5 mL) was obtained and centrifuged at 3000 r/min for 10 min. The serum was extracted for determination of the concentrations of NO, NOS and cGMP using the nitrate reductase method, chemical colorimetry and radioimmunoassay respectively according to the manufacturers’ instructions.
Histological investigation of the liver tissue: Twenty-four hours after the last dosing of the experiment, the rats were anesthetized with ethyl ether, and the livers were quickly removed from the etherized animals. Tissue mass of a size measuring about 1 cm × 1 cm × 1 cm was collected from a site about 0.5 cm distant from the hepatic margin of the left lobe and then placed in a 4% paraformaldehyde solution for fixation for 24 h. The tissue mass was dehydrated in increasing concentrations of ethanol. After hydration, wax-impregnation, embedding and sectioning, HE and Masson staining were sequentially performed. Morphological changes and the degree of fiber hyperplasia of the liver tissue in rats with LC were observed under a light microscope. The grading of LC was performed according to the Knodell HAI, Scheuer, METAVIR, modified Ishak HAI and Chevallier grading systems[8,9].
Quantitative detection of COL I, COL III and TGF-β1 in liver tissue: Liver tissue was fixed with 10% formalin and embedded in paraffin. Then, serial sections (4 μm) were cut. Immunohistochemical staining was performed by the Streptavidin-biotin complex (SABC) method. Briefly, the paraffin sections were baked in an oven at 60°C for 1 h and then placed into a pure xylene solution for deparaffinage twice, with each lasting for 15 min. The sections were then placed into a 3% hydrogen peroxide solution at room temperature for 30 min to inactivate endogenous peroxidase, followed by boiling in a 0.01 mol/L citrate buffer under high temperature and pressure conditions for 2 min. After this, the tissues were covered with a normal goat serum blocking buffer and placed in an incubator at 37°C for 30 min. Subsequently, anti-COL I, COL III and TGF-β1 primary antibodies were separately added at a dilution of 1:100 to the tissues. The sections were incubated at 37°C for 30 min. After being kept at 4°C overnight, the sections were washed thoroughly in PBS (5 min × 3 times), and then biotin secondary antibody was added before incubation at 37°C for 30 min. The sections were washed again in PBS (5 min × 3 times) and in distilled water (3 min × 3 times), followed by incubation with avidin-peroxidase at 37°C for 20 min. 3,3′-diaminobenzidine (DAB) was added after smearing. The color developing was monitored under a light microscope. The staining was stopped by washing the sections in distilled water. Following staining with hematoxylin for 1 min and washing in distilled water, the sections were sequentially dehydrated in 95% and 100% ethanol for 5 min each. After air drying, the sections were sealed by neutral gum and observed under a light microscope. Mias-2000 image analysis software (Institute of Image and Graphics, Sichuan, China) was used for quantitative measurement. The ratio of the positive area of COL I, COL III and TGF-β1 to the overall visual field area was calculated.
Determination of biomechanics of the portal vein: After sacrifice of the rats, the main portal vein was immediately removed and connected to a three-way baroceptor. The pressure was amplified by a dynamic electric resistor which was linked to a computer. The biomechanics of the portal vein were evaluated by measuring the corresponding pressure when the relative volume of blood vessels was changed. The vascular compliance, denoted as C, was calculated using the following formula: C = 2πR·ΔR/ΔP, where R represents the radius of the blood vessel, ΔR and ΔP are the change of the radius and portal vein pressure respectively.
To measure the zero-stress state of the blood vessels, cross-sections were made across the portal vein and arterial rings were obtained. A cut along the ventral margin of the arterial ring was made and the expanded angle of the arteriae aorta was observed under an anatomical microscope. The photographic recording was carried out, followed by printing out on paper. The included angle, i.e. the opening angle, which was formed from the midpoint of the inner lining of the arterial ring to the two broken ends of the inner lining, was measured and used to represent changes in the zero-stress state of the blood vessels. In each rat, 5 open angles of the arterial ring were measured and their mean value was calculated.
Data were expressed as mean ± SE. Statistical comparisons among NTau-treated animals, non-treated model controls, and healthy controls were carried out using one-way analysis of variance (ANOVA), followed by post-hoc Dunnett’s test using the appropriate group as the control. Comparison of degrees of LC between groups was conducted by rank sum test. The analysis was performed on the SPSS for Windows (version 14.0). Differences were considered significant at P < 0.05 or P < 0.01.
Table 1 summarizes the hemodynamic data of the normal healthy animals, those having undergone LC induction and LC animals treated with NTau for six consecutive weeks. Compared with the normal healthy control group, the LC rats had very significantly higher PVP (P < 0.01), and the PVF and PVR were also markedly elevated in the experimentally-induced LC animals, while the MAP and SVR were considerably lower in these model animals (P < 0.05). The treatment with NTau significantly attenuated PVP (P < 0.01), PVF and PVR (P < 0.05) when compared with the non-treated model group. Accordingly, the NTau treatment enhanced the MAP and SVR (P < 0.05). These experimental data suggest that NTau was able to improve the hemodynamic conditions in animals with LC.
The effects of NTau on the concentrations of NO and cGMP and NOS activity are summarized in Table 2. When compared with the healthy control group, the amount of NO and cGMP and the activity of NOS were significantly increased in animals with LC. Treatment with NTau caused a significant reduction in NO (P < 0.01) and cGMP (P < 0.05) content in the blood and in the activity of NOS (P < 0.05) when compared with that of the non-treated LC animals. The data derived from this experiment indicate that treatment with NTau significantly mitigates the release and activation of vasoactive substances such as NO, cGMP and NOS.
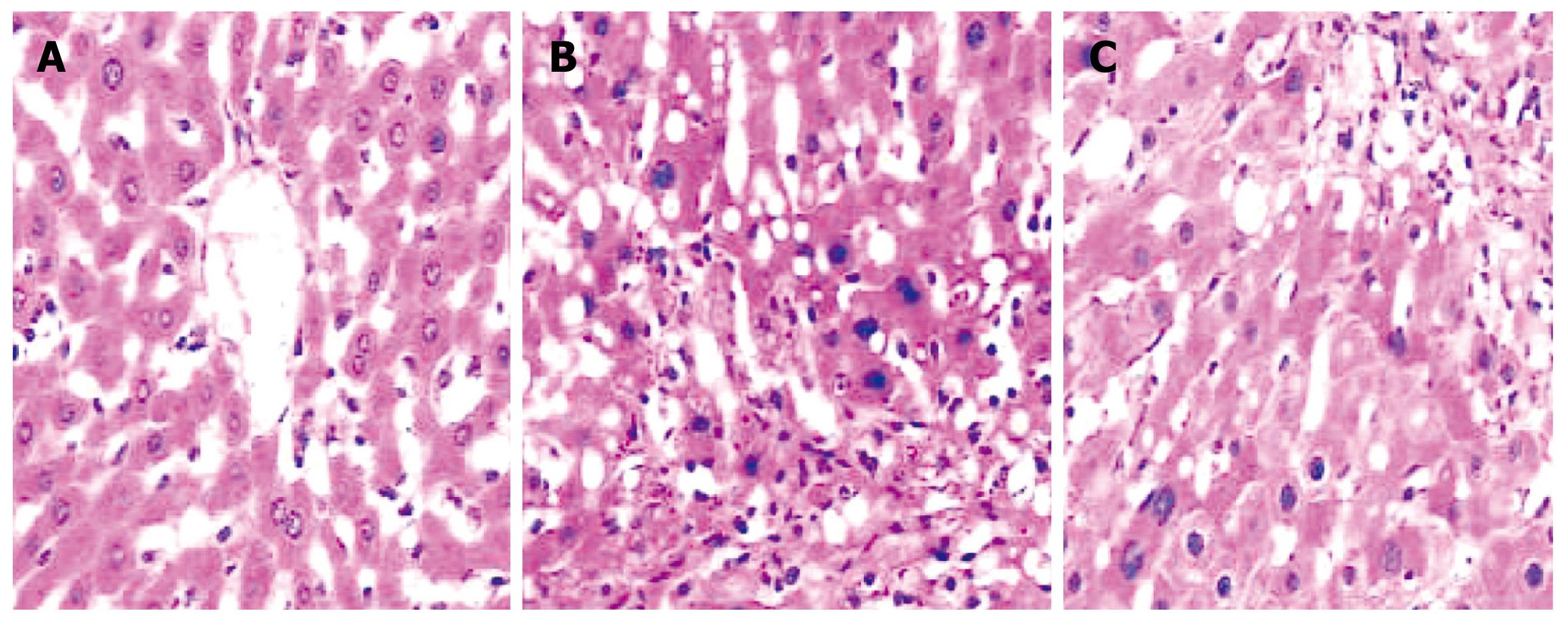
Histological observation of normal rat liver under HE staining was characterized by intact and distinct structure of liver tissue, normal structure of hepatic lobules, and radial distribution of a cord-like arrangement of hepatocytes around the central vein (Figure 1A). In experimentally-induced LC rats, abnormal histological features were observed such as the destruction of the normal structure of hepatic lobules; extensive fibroplasia of interstitial tissue of the liver, which divided the hepatic lobules into different sizes of hepatocellular mass (i.e. formation of pseudo-lobules); extensive fatty degeneration of hepatocytes with some necrosis; and infiltration of many inflammatory cells into the portal areas and hepatic lobules. In the NTau-treated group, the damage of the structure of hepatic lobules was still observed, but with milder fibroplasia, fewer infiltrations of inflammatory cells and only modest foamy degeneration of hepatocytes when compared with the non-treated LC rats. Under Masson staining, only a small amount of collagen was observed in the normal healthy group. In contrast, a large amount of collagen fibrils were found in the LC model rats; however, only thin collagen fibrils were observed in the NTau-treated animals.
The degree of LC in all three animal groups is presented in Table 3. It is evident that the experimental procedure involved in establishing the model, essentially the high fat diet and injection of CCl4, succeeded in inducing LC in all rats, and the degree of LC ranged from SIV to SVI. The treatment with NTau succeeded in ameliorating the extent of LC.
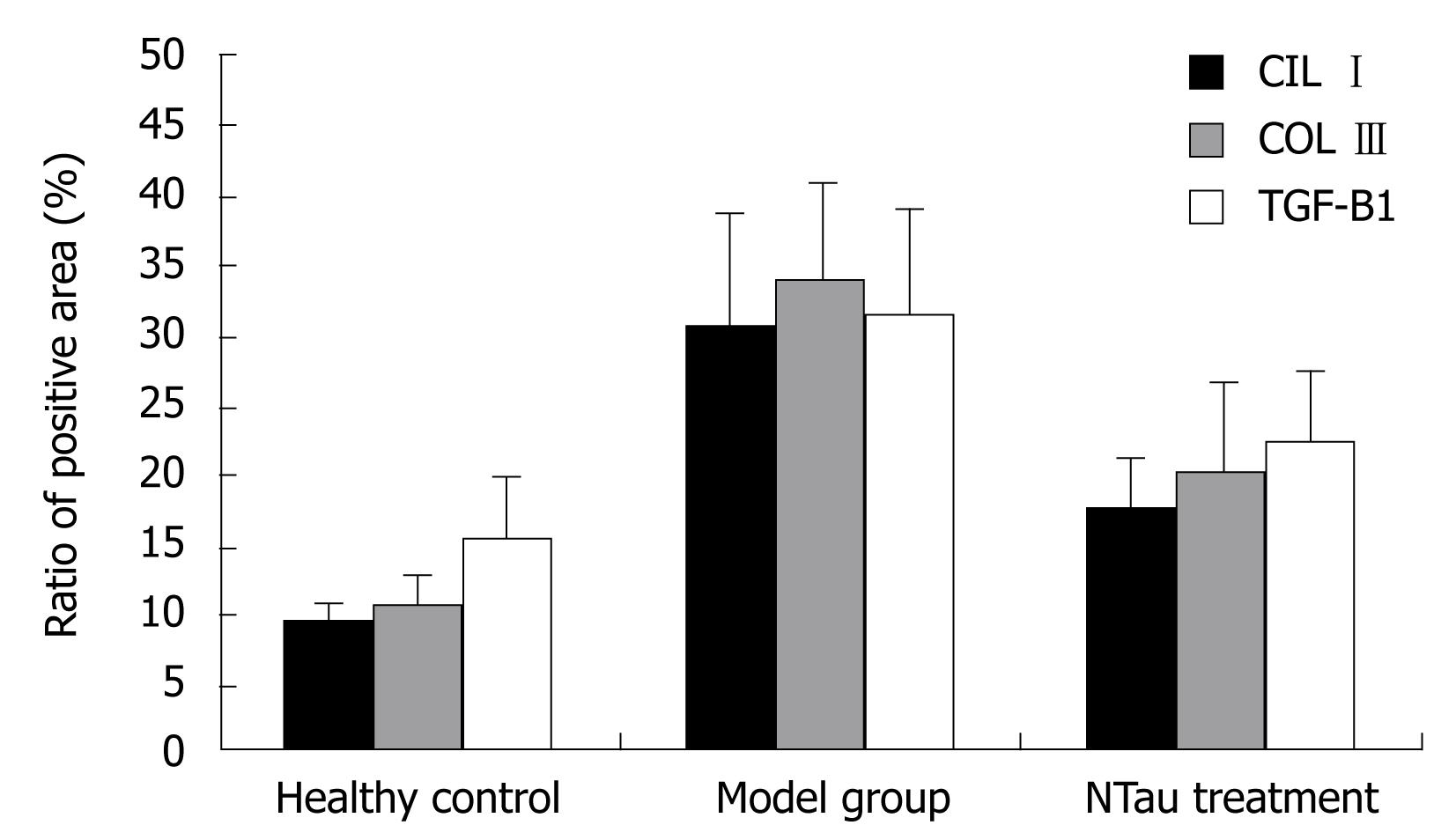
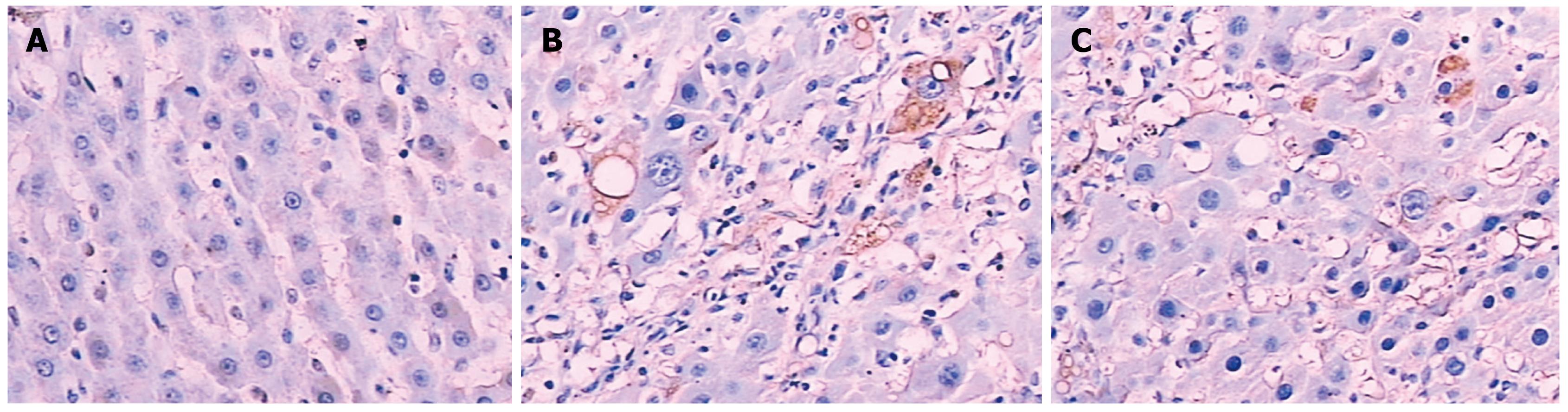
Figure 2 is a bar chart presentation of the ratio of the positive area of COL I, COL III and TGF-β1 in the different animal groups. It is evident that the induction of LC in the model rats significantly increased the amount of COL I, COL III and TGF-β1, with COL I increasing from 9.41 ± 1.36 to 30.63 ± 8.25, COL III from 10.55 ± 2.46 to 33.65 ± 7.52, and TGF-β1 from 15.37 ± 4.62 to 31.28 ± 7.85, respectively. The intervention with NTau was capable of reducing the amount of all these parameters in the LC rats, with COL I decreasing from 30.63 ± 8.25 to 17.58 ± 3.6, COL III from 33.65 ± 7.52 to 20.34 ± 6.41 and TGF-β1 from 31.28 ± 7.85 to 22.17 ± 5.43, respectively. Figures 3, 4, 5 present the immunohistochemical analysis data of the expression of COL I, COL III and TGF-β1 in the liver tissue of the different experimental groups.
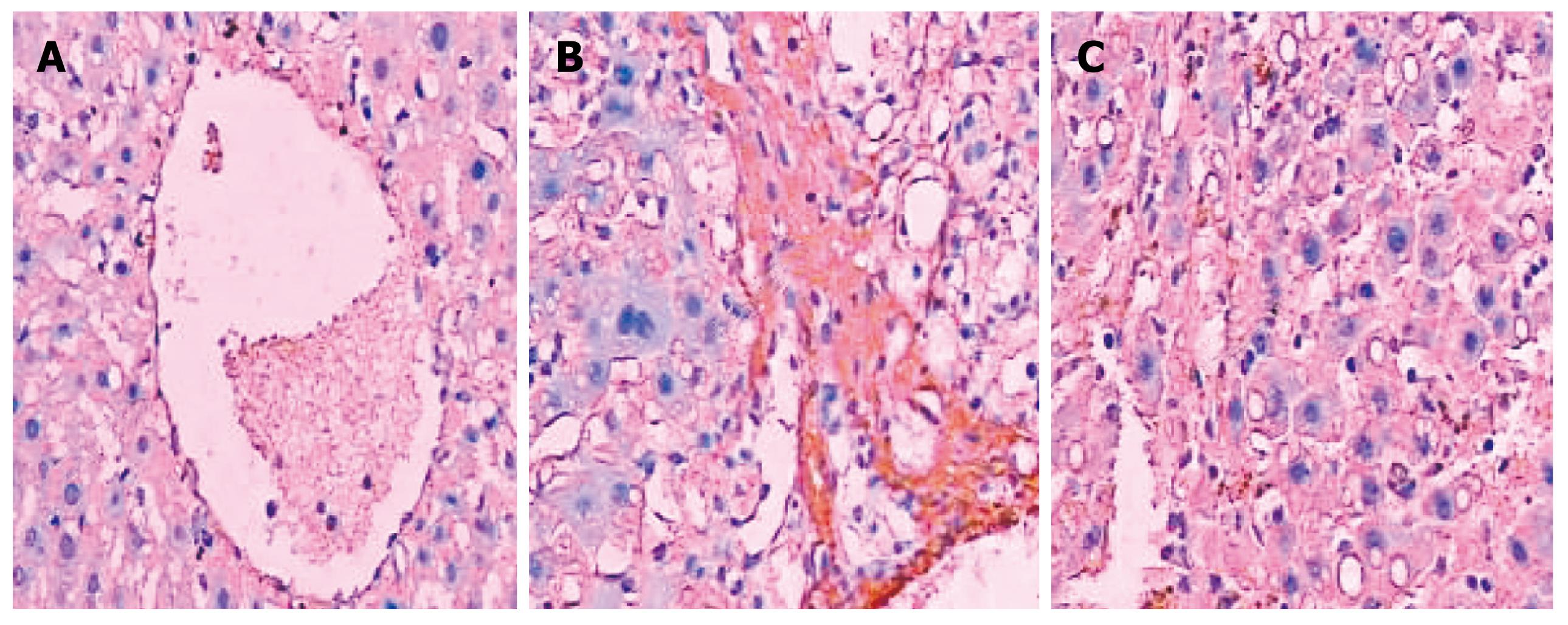
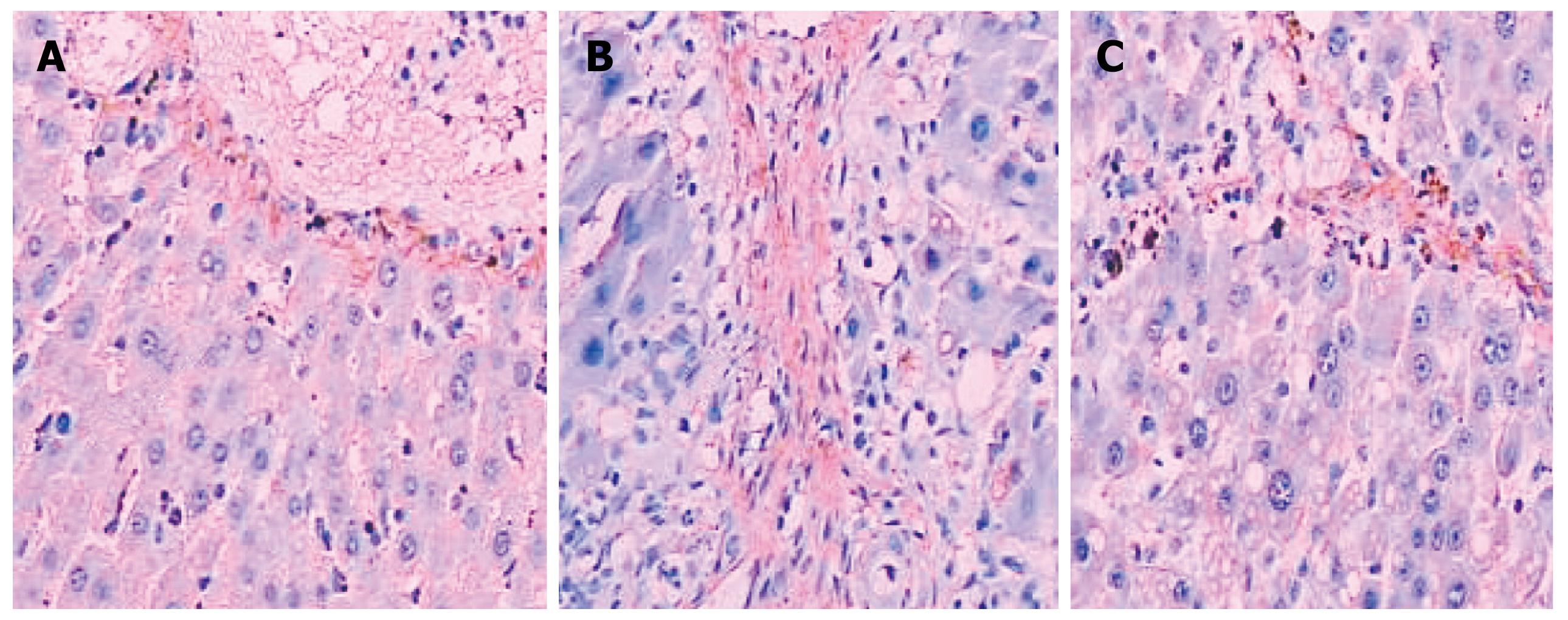
COL I was only slightly expressed in the basement membrane of the central vein of the hepatic lobules in the normal healthy rats. Its expression was significantly elevated in the LC rats with thicker collagen fibrils clearly detectable in the connective tissue surrounding the hepatocytes. Compared with the non-treated model group, the rats in the NTau treatment group had conspicuously reduced expression of COL I and thinner collagen fibrils in the connective tissue surrounding the hepatocytes. There was mild expression of COL III in the interstitial connective tissue surrounding hepatocytes in the normal healthy rats. However, significantly increased and thickened COL III fibrils were observed in the interstitial connective tissue surrounding hepatocytes in the model group. Treatment with NTau was capable of markedly attenuating the expression of COL III, with thinner collagen fibrils being observed. Similarly, TGF-β1 was only expressed mildly in the hepatocellular cytoplasm in normal rats, and there was a strongly positive expression of TGF-β1 in hepatocellular cytoplasm in the LC rats. Similarly to expression of COL I and III, treatment with NTau showed a significant reduction in the expression of TGF-β1 in hepatocellular cytoplasm.
Experiments on portal vein biomechanics showed that, in general, the portal compliance decreased as the pressure in the portal vein increased (Figure 6). Among the different groups of rats, the portal compliance in the model group was significantly lower than that of the normal control group (P < 0.05), while the NTau treatment was able to markedly improve the portal compliance when compared with the non-treated model group (P < 0.05).
In addition, there was a substantial difference (P < 0.01) in the opening angle of the portal ring between the model group (89.23 ± 10.47 degree) and the healthy control group (76.25 ± 9.45 degree). The NTau treatment significantly reduced the opening angle of the portal ring (82.61 ± 6.31) when compared to the animals in the model group (P < 0.05). The direct proportion of zero-stress to opening angle indicated that the LC rats had a very significant increase in zero-stress level, and the NTau treatment was able to mitigate this abnormal elevation.
Taurine, a sulfur-containing β-amino acid [H2N-(CH2)2-SO2OH], is ubiquitously distributed in tissues of mammalian and marine organisms. Taurine was once thought to be a non-functional terminal metabolite of sulfur-containing amino acids in the body. However, recent studies have confirmed that taurine has a wide variety of biological functions including maintaining homeostasis and regulating physiological functions of different systems. Taurine also possesses a wide spectrum of pharmacological activities such as antipyretic, anticonvulsant, antiplatelet-aggregation, hypotensive, immunity-enhancing, liver-protecting and angiotasis-regulating effects[10-17]. It is known that the liver is the main organ for taurine biosynthesis and also an important target organ for taurine’s many biological activities. Interestingly, the amount of taurine in the liver tissue of rats with chronic liver disease falls below the normal range[18].
As a free amino acid, taurine can either be synthesized through chemical reactions or extracted from natural sources. Studies in China and other countries have demonstrated that synthesized taurine may inhibit hepatic fibrosis (HF) by inhibiting collagenation and proliferation of HSCs[19,20]. However, the role of taurine in inhibiting PHT has hitherto not been systematically investigated. Our previous study found that NTau promoted apoptosis of HSCs in a more marked manner than that of synthesized product[21], and that NTau could lower PHT of LC through inhibiting contraction of HSCs[22].
Two seemingly contradictory theories, i.e. the “backward flow” theory and the “forward flow” theory, have been put forward to explain the development of PHT in LC. In the “backward flow” theory, an increase in intrahepatic resistance is thought to be the main reason for the occurrence of PHT. The “forward flow” theory, on the other hand, considers systemic hyperdynamic circulation (SHC) as the primary cause for PHT. Recent studies have shown that both mechanisms are involved in the pathogenesis of PHT[23]. In fact, patients with LC may present both disturbances of blood circulation (i.e. increased resistance of “backward flow” theory) in liver tissue and abnormality in vasoactive substances (i.e. hyperdynamic circulation of “forward flow” theory).
By combining the “backward flow” and the “forward flow” theory together, this would furnish a better understanding of the pathogenesis of PHT[24,25]. In the “forward flow” theory, the pathogenesis of PHT may be primarily attributed to initiation factors, including dilation of peripheral vessels, decrease in peripheral vascular resistance and mean arterial pressure, increase in blood volume, splanchnic blood flow and cardiac output, and development of systemic hyperdynamic circulatory syndrome (HCS)[23,26]. HCS plays an important role in the maintenance of PHT and is also a primary cause for the development of sodium-water retention, ascites, hepato-renal and hepato-pulmonary syndromes. Our experimental study showed that NTau was capable of decreasing portal blood flow and improving systemic HCS by reducing PVF and increasing SVR and MAP. All these experimental observations indicate that NTau is able to reduce portal pressure through acting on the “forward flow” mechanism.
An increase in endogenous vasodilators is regarded as the most significant factor for peripheral arterial vasodilatation. Consequently, an increase of vasodilators may elicit a wide spectrum of pathophysiological alterations including vasodilatation of peripheral and splanchnic tertiary arterioles, decreased resistance, deficiency of effective arterial blood volume, activation of neurohumoral systems for pressure and water-sodium retention, compensatory expansion of plasma volume, increase in returned blood volume and cardiac output, and splanchnic active hyperemia. All these alterations can lead to hyperdynamic systemic and splanchnic circulation. Among the common vasodilators, NO, glicentin, prostacyclin and calcitonin gene-related peptides are the most important ones. NO is produced by L-arginine in the presence of NOS and may exert biological effects by increasing cGMP via activation of guanylate cyclase. It is also an important signaling molecule involved in various physiological processes such as vasodilatation. NO has also been found to be involved in the development of a hyperdynamic circulatory state in LC[27,28]. In our experiments, decreased activity of NOS and reduced amounts of NO and cGMP were observed after the NTau treatment, suggesting that NTau attenuates PHT of LC most probably via regulating the NO system.
In the “backward flow” theory, the most critical initiating determinants of portal pressure include the progression of HF, passive structural disorder in the liver, formation of pseudo-lobules and interrupted supply of blood circulation to hepatocytes, which further contribute to hepatocellular necrosis, proliferation of collagen, and formation of regenerative nodules. Consequently, compression and traction of peripheral vessels and bile duct by regenerative nodules and proliferated collagen fibrils may lead to increased resistance to blood flow of portal and hepatic veins, and subsequently result in PHT[25,29,30]. In our study, the treatment with NTau led to the improvement of the structure of liver tissue and significantly lowered the amounts of COLI and COL III, indicating that NTau can decrease portal pressure by acting on the “backward flow” mechanism, namely by improving the pathological structure of liver tissue and inhibiting HF.
TGF-β1 is a multifunctional peptide with a wide range of potential influences on the growth and differentiation of cells, aggregation of extracellular matrix (ECM), and immune response. It is also one of the mediators that is most closely associated with the production of fibrils. In the occurrence and development of HF, TGF-β1 has an ability to activate HSCs and promote the gene expression of collagen as well as the synthesis and deposition of ECM[31,32]. In our experiments, the level of TGF-β1 in the NTau treatment group was significantly lower than that of the LC model group, suggesting that NTau inhibits the development of HF, possibly through inhibiting the expression of TGF-β1.
Increased resistance to PVF may elicit biomechanical changes in the portal vein by causing vascular reconstruction characterized by thickening of vessel walls, narrowing of vessel lumen and proliferation of smooth muscle cells, resulting in further maintenance and exacerbation of PHT. Such alterations in biomechanics subsequently make treatment of LC-induced PHT ever more difficult[33,34]. Therefore, to achieve a favorable therapeutic response in the treatment of PHT of LC, terminating this vicious circle becomes particularly important, and lowering the portal pressure should not be the sole target for intervention. Vessel wall is a viscoelastic tissue with unique biomechanical properties including creep, stress-relaxation and hysteresis. Compliance and zero-stress state are usually used to describe the biomechanical properties of vessels. Indeed, reduced portal compliance and enlarged opening angle were found in rats with LC. The NTau treatment, on the other hand, was able to improve the portal compliance and decrease the opening angle, indicating that NTau could inhibit the development of LC-induced PHT by improving the biomechanical properties of the portal vein.
The pathomechanism of LC-induced PHT is complex, for it involves SHC, structural alterations in the liver, increased resistance to PVF and biomechanical changes in the portal vein. Our present experimental data derived from the LC rat model unequivocally demonstrate that NTau can inhibit PHT of LC by improving hyperdynamic circulation, structure of liver tissue, and the biomechanical properties of the portal vein by delaying the progression of HF. Given that taurine is an important nutrient in the body with a deficiency in chronic liver diseases, the supplementation of an adequate amount of taurine may improve the functional status of the body. In view of its pharmacological and nutritional values, we believe that treatment with taurine may provide an additional dimension for the management of portal hypertension associated with liver cirrhosis.
Portal hypertension which acts as the main manifestation of patients in the compensatory stage of cirrhosis of the liver is the material cause of death. Treatment of portal hypertension caused by liver cirrhosis can not only enhance the prognosis of the disease but also improve the patients’ quality of life. Hence, the concept of implementing treatment of portal hypertension is gradually attracting more and more attention, changing the passive state of the past which practised symptomatic treatment when complications developed.
Intensive literature review shows that taurine can suppress the course of liver cirrhosis. This project investigates the function of taurine in inhibiting the course of liver cirrhosis and portal hypertension caused by liver cirrhosis from different aspects.
This study proposes the concept of the integration of the three theories for the formation of liver cirrhosis: the backward flow theory, the forward flow theory and the biomechanics of portal vein theory, then systematically explores the inhibiting effect and possible mechanism of action of taurine on portal hypertension caused by liver cirrhosis.
The treatment of portal hypertension mainly embodies medication treatment, surgical treatment, interventional treatment, liver transplant etc. At present, long-term medication treatment takes the center stage. Though there are plenty of medicines available for portal hypertension caused by liver cirrhosis, there is still a lack of effective medication. As a result, there is sound justification for investigating the therapeutic functions of taurine in portal hypertension caused by liver cirrhosis. Pure natural taurine widely exists in marine fauna. The ocean, which occupies 70.8% of the total earth’s surface, is a natural medicinal resource with huge potential. It makes great sense to explore new areas of marine life’s application in the search for new medicinal resources.
Forward flow theory is one of the theories of the formation of portal hypertension caused by liver cirrhosis. The pathogenesis of PHT may be primarily attributed to initiation factors, including dilation of peripheral vessels, decrease in peripheral vascular resistance and mean arterial pressure, increase in blood volume, splanchnic blood flow and cardiac output, and development of systemic hyperdynamic circulatory syndrome. For backward flow theory, the main cause of portal hypertension caused by liver cirrhosis is the formation of diffuse fibrous septa and regenerative nodules in liver which is followed by hepatic sinus narrowing. Then intra-liver vessels constrict and the resistance of blood flow in the portal system increases which results in passive congestion of the portal system. Finally, portal hypertension develops. As vasodilatation increases, the reactivity of vessels to endogenous vascular-constriction substances falls, then functional arteriovenous fistula and portal-systemic shunting develop and result in hyperdynamic circulation over the whole body while blood flow of the portal vein increases.
This is a small but reasonably designed experimental study to examine the impact of natural taurine in a rat model of cirrhosis. The paper particularly concentrates on the effects of this agent on the level of portal hypertension and clearly shows that this is attenuated following repeat dosage by gavage.
Peer reviewer: Alastair D Burt, Professor, Dean of Clinical Medicine, Faculty of Medical Sciences, Newcastle University, Room 13, Peacock Hall, Royal Victoria Infirmary, Newcastle upon Tyne NE1 4LP, United Kingdom
S- Editor Tian L L- Editor Logan S E- Editor Ma WH
| 1. | Bruha R, Petrtyl J, Urbanek P, Svestka T, Kalab M, Marecek Z. [Long-term pharmacological treatment of portal hypertension]. Cas Lek Cesk. 2005;144 Suppl 1:63-66. |
| 2. | Hassoun Z, Pomier-Layrargues G. The transjugular intrahepatic portosystemic shunt in the treatment of portal hypertension. Eur J Gastroenterol Hepatol. 2004;16:1-4. |
| 3. | Liang J, Deng X, WU JY, Yang GY, Huang RB. The effect of natural taurine on hepatic stellate cell of rat. Guangxi Yixue. 2006;28:35-37. |
| 4. | Reynaert H, Thompson MG, Thomas T, Geerts A. Hepatic stellate cells: role in microcirculation and pathophysiology of portal hypertension. Gut. 2002;50:571-581. |
| 5. | Safadi R, Friedman SL. Hepatic fibrosis--role of hepatic stellate cell activation. MedGenMed. 2002;4:27. |
| 6. | Wang YK, Chi BR, Sun B, Wang ZC. Establishment and stability of hepatic cirrhosis rat models. Jilin Daxue Xuebao (Yixue ban). 2005;31:893-895. |
| 7. | Shi B, Zhu L, Zhang ZB, Xie WF, Wu GQ, Liu BY, Chao YX. The changes of biomechanical properties of the portal veins in the rats during the pathogenesis of intrahepatic portal hypertension. J Med Biomech. 2004;19:228-232. |
| 8. | Brunt EM. Grading and staging the histological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatol. 2000;31:241-246. |
| 10. | Marie CB, Mathieu O, Guillaume Q, Maxim M. Taurine-deficient dilated cardiomyopathy in a family of golden retrievers. J Am Anim Hosp Assoc. 2005;41:284-291. |
| 11. | Robert CB, Kwang SK, Andrea JF, Mark DK, Kittleson KA, MacDonald DJ, Maggs JB, Quinton RR. Low plasma taurine concentration in Newfoundland dog is associated with low plasma methionine and cyst(e)ine concentrations and low taurine synthesis. J Nutr. 2006;136:2525-2533. |
| 12. | Yildirim Z, Kilic N, Ozer C, Babul A, Take G, Erdogan D. Effects of taurine in cellular responses to oxidative stress in young and middle-aged rat liver. Ann N Y Acad Sci. 2007;1100:553-561. |
| 13. | Morales I, Dopico JG, Sabate M, Gonzalez-Hernandez T, Rodriguez M. Substantia nigra osmoregulation: taurine and ATP involvement. Am J Physiol Cell Physiol. 2007;292:C1934-C1941. |
| 14. | Hosoi M, Takeuchi K, Sawada H, Toyohara H. Expression and functional analysis of mussel taurine transporter, as a key molecule in cellular osmoconforming. J Exp Biol. 2005;208:4203-4211. |
| 15. | Tabassum H, Parvez S, Rehman H, Dev Banerjee B, Siemen D, Raisuddin S. Nephrotoxicity and its prevention by taurine in tamoxifen induced oxidative stress in mice. Hum Exp Toxicol. 2007;26:509-518. |
| 16. | Bianchi L, Colivicchi MA, Ballini C, Fattori M, Venturi C, Giovannini MG, Healy J, Tipton KF, Della Corte L. Taurine, taurine analogues, and taurine functions: overview. Adv Exp Med Biol. 2006;583:443-448. |
| 17. | Razvodovskii IuE, Doroshenko EM, Prokopchik NI, Smirnov VIu, Ostrovskii SIu. [Hepatoprotective effects of amino acids with branched hydrocarbon chains and taurine in experimental subchronic alcohol intoxication and ethanol withdrawal]. Biomed Khim. 2004;50:64-72. |
| 18. | Warskulat U, Borsch E, Reinehr R, Heller-Stilb B, Monnighoff I, Buchczyk D, Donner M, Flogel U, Kappert G, Soboll S. Chronic liver disease is triggered by taurine transporter knockout in the mouse. FASEB J. 2006;20:574-576. |
| 19. | Chen YX, Zhang XR, Xie WF, Li S. Effects of taurine on proliferation and apoptosis of hepatic stellate cells in vitro. Hepatobiliary Pancreat Dis Int. 2004;3:106-109. |
| 20. | Miyazaki T, Karube M, Matsuzaki Y, Ikegami T, Doy M, Tanaka N, Bouscarel B. Taurine inhibits oxidative damage and prevents fibrosis in carbon tetrachloride-induced hepatic fibrosis. J Hepatol. 2005;43:117-125. |
| 21. | Liang J, Deng X, Yang GY, Huang RB, Pang YS. Study on the effect of natural taurine in hepatic fibrosis of tissue and serum. Guangxi Zhongyi Xueyuan Xuebao. 2006;9:2-4. |
| 22. | Liang J, Deng X, WU JY, Yang GY, Huang RB. Effect of taurine at hepatic stellate cell on portal hypertension. Guangxi Yixue. 2006;28:5-7. |
| 23. | Montano-Loza A, Meza-Junco J. [Pathogenesis of portal hypertension]. Rev Invest Clin. 2005;57:596-607. |
| 24. | Moreau R, Lebrec D. Molecular and structural basis of portal hypertension. Clin Liver Dis. 2006;10:445-457, vii. |
| 25. | Groszmann RJ, Abraldes JG. Portal hypertension: from bedside to bench. J Clin Gastroenterol. 2005;39:S125-S130. |
| 26. | Reichen J, Lebrec D. The future treatment of portal hypertension. Best Pract Res Clin Gastroenterol. 2007;21:191-202. |
| 27. | Shams V, Erkan T, Gumustas MK, Cullu F, Kutlu T, Kaya H, Aydin S, Tumay G. The role of nitric oxide in pediatric patients with portal hypertension. J Trop Pediatr. 2003;49:33-36. |
| 28. | Shah V. Cellular and molecular basis of portal hypertension. Clin Liver Dis. 2001;5:629-644. |
| 29. | Rockey DC. Hepatic fibrosis, stellate cells, and portal hypertension. Clin Liver Dis. 2006;10:459-479, vii-viii. |
| 30. | Li J, Niu JZ, Wang JF, Li Y, Tao XH. Pathological mechanisms of alcohol-induced hepatic portal hypertension in early stage fibrosis rat model. World J Gastroenterol. 2005;11:6483-6488. |
| 31. | Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793-d807. |
| 32. | Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76-99. |
| 33. | Shi B, Zhu L, Zhang ZB, Xie WF, Wu GQ, Liu BY, Chao YX. The changes of biomechanical properties of the portal veins in the rats during the pathogenesis of intrahepatic portal hypertension. J Med Biomech. 2004;19:228-233. |
| 34. | Li T, Yang Z. Research progress of vasculopathy in portal hypertension. World J Gastroenterol. 2005;11:6079-6084. |