Published online Sep 28, 2009. doi: 10.3748/wjg.15.4511
Revised: September 2, 2009
Accepted: September 9, 2009
Published online: September 28, 2009
AIM: To analyze the pathogenetic role and potential clinical usefulness of the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor 2 (HER2) in patients with advanced biliary tract cancer (BTC).
METHODS: EGFR and HER2 expression was studied in biopsy samples from 124 patients (51% women; median age 64.8 years), with advanced BTC diagnosed between 1997 and 2004. Five micrometers sections of paraffin embedded tissue were examined by standard, FDA approved immunohistochemistry. Tumors with scores of 2+ or 3+ for HER2 expression on immunochemistry were additionally tested for HER2 gene amplification by fluorescence in situ hybridisation (FISH).
RESULTS: 34/124 patients (27.4%) had gallbladder cancer, 47 (37.9%) had intrahepatic BTC and 43 (34.7%) had extrahepatic or perihilar BTC. EGFR expression was examined in a subset of 56 samples. EGFR expression was absent in 22/56 tumors (39.3%). Of the remaining samples expression was scored as 1+ in 12 (21.5%), 2+ in 13 (23.2%) and 3+ in 9 (16%), respectively. HER2 expression was as follows: score 0 73/124 (58.8%), score 1+ 27/124 (21.8%), score 2+ 21/124 (17%) and score 3+ 4/124 (3.2%). HER2 gene amplification was present in 6/124, resulting in an overall amplification rate of 5%.
CONCLUSION: Our data suggest that routine testing and therapeutic targeting of HER2 does not seem to be useful in patients with BTC, while targeting EGFR may be promising.
- Citation: Harder J, Waiz O, Otto F, Geissler M, Olschewski M, Weinhold B, Blum HE, Schmitt-Graeff A, Opitz OG. EGFR and HER2 expression in advanced biliary tract cancer. World J Gastroenterol 2009; 15(36): 4511-4517
- URL: https://www.wjgnet.com/1007-9327/full/v15/i36/4511.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4511
| Patients (n = 124) | n (%) |
| Age (yr) | |
| Range | 32.8-84.8 |
| Median | 64.8 |
| mean ± SD | 63.4 ± 11.0 |
| Male:female | 61:63 (49:51) |
| Gallbladder cancer | 33 (26.6) |
| Mass forming type | 47 (37.9) |
| Intraductal growth type | 44 (35.5) |
| Histological type | |
| Well differentiated | 8 (6.5) |
| Moderately differentiated | 80 (64.5) |
| Poorly differentiated | 36 (29) |
| Stage1 | |
| I | 9 (7.3) |
| II | 20 (16.1) |
| III | 31 (25) |
| IV | 64 (51.6) |
| Chemotherapy | 62 (50) |
| Partial response | 9 (14.5) |
| Stable disease | 27 (43.5) |
| Progressive disease | 26 (42) |
| Parameter | EGFR 2+ and 3+ tumors | P | HER2 2+ and 3+ tumors | P |
| Gallbladder cancer | 5/13 | 8/34 | ||
| Mass forming type | 6/24 | 0.088 | 7/47 | 0.517 |
| Intraductal growth type | 11/19 | 10/43 | ||
| Histological type | ||||
| Well differentiated | 2/3 | 1/8 | ||
| Moderately differentiated | 16/39 | 0.511 | 20/80 | 0.202 |
| Poorly differentiated | 4/14 | 4/36 | ||
| Stage1 | ||||
| I | 3/4 | 4/9 | ||
| II | 2/6 | 0.317 | 4/20 | 0.059 |
| III | 8/17 | 9/31 | ||
| IV | 9/29 | 8/64 | ||
| Chemotherapy | ||||
| Partial response | 2/3 | 4/9 | ||
| Stable disease | 3/13 | 0.296 | 4/27 | 0.156 |
| Progressive disease | 5/13 | 5/26 |
Biliary tract cancer (BTC) is a heterogeneous tumor entity consisting of an intrahepatic mass forming type cholangiocarcinoma, perihilar Klatskin tumors, extrahepatic BTC, also termed intraductal growth type, and gallbladder cancer. More than 50% of tumors are diagnosed at an advanced stage with these patients having a dismal prognosis with a mean overall survival of 7-8 mo[1,2]. Chemotherapy is widely used but of only little benefit. Therefore, new treatment options are urgently needed. Growth factor inhibitors or antibodies and small molecules such as erlotinib, gefitinib, cetuximab, panitumumab, trastuzumab and lapatinib targeting the epidermal growth factor receptor (EGFR) or the human epidermal growth factor receptor 2 (HER2) have been successfully used for the treatment of colorectal, breast, lung and head and neck cancers among others[3-7]. While there are some case reports and a phase II trial reporting promising results targeting EGFR in BTC there are no data regarding the use of trastuzumab or lapatinib also targeting HER2[8-10]. For pancreatic carcinoma, a malignancy somewhat related to BTC, trastuzumab has been successfully used in one study and at least one further multicenter study is currently under way[11,12].
EGFR and HER2 are receptor tyrosine kinases encoded by proto-oncogenes. Growth factors such as epidermal growth factor (EGF) or transforming growth factor (TGF) bind to these receptors at their extracellular ligand-binding domain and initiate intracellular signalling cascades, leading to tumor cell proliferation, migration, invasion, resistance to apoptosis and angiogenesis[3,4]. In an experimental tumor model a high proportion of ErbB-2 (HER2) transgenic mice develop BTC, suggesting a role of ErbB-2 signalling in biliary carcinogenesis[13].
Overall, overexpression of EGFR and HER2 in tumor cells has been associated with a poor prognosis, but also offers the therapeutic option of pharmacologically targeting these receptors. To date, EGFR and HER2 overexpression has been reported in up to about 80% of BTC, mostly in small patient cohorts[14-19]. Nevertheless, there are no data regarding the correlation of immunohistochemical scores, determined by standardized methods, with clinical findings, including the overall survival of patients with advanced BTC treated by chemotherapy. The aim of this study, therefore, was to asses the clinical significance of the expression of EGFR and HER2 proteins and their potential as therapeutic targets in advanced BTC.
Expression of EGFR and HER2 was analyzed in biopsy samples from 124 patients with advanced or relapsed, unresectable BTC (51% women, median age 64.8 years). The patients had been consecutively diagnosed at the Tumorzentrum Ludwig Heilmeyer-Comprehensive Cancer Center Freiburg between 1997 and 2004, and were followed for a median of 71 mo. All BTC cases were histologically proven adenocarcinoma. Written informed consent was obtained from all patients. Primary gastrointestinal cancers other than BTC were excluded by upper endoscopy, colonoscopy and a multislice computed tomography (CT) scan according to the consensus guidelines published in 2002[20]. Sixty-one patients (49.2%) had been treated by chemotherapy and could be restaged for response. The chemotherapy regimens were based on 5-fluorouracil or gemcitabine, often in combination with cisplatin or oxaliplatin. The treatment response was measured by CT, magnetic resonance imaging or ultrasound according to the standard World Health Organization (WHO) criteria (WHO, 1979). The patients subgroup analyzed for EGFR was randomly selected. The different tumor types were similar between the subgroup and the whole study population. The baseline characteristics of the study population are given in Table 1.
Biopsy samples were fixed in 10% formalin, embedded in paraffin, cut in 5 μm sections and stained with hematoxylin/eosin for histological typing and grading. Immunohistochemistry (IHC) was performed on adjacent freshly cut deparaffinized sections using the peroxidase-labelled streptavidin-biotin technique, EGFR pharmDx™ for EGFR and Dako REAL™ detection system for HER2 staining (both Dako Glostrup, Denmark). Immunostaining was performed according to the package insert of the two FDA-approved detection systems, using only the supplied reagents and procedures.
IHC results were scored independently by two pathologists (Waiz O and Schmitt-Graeff A) blind to all clinical data. As recommended by the manufacturer additional tissue controls were performed along with the cell line controls.
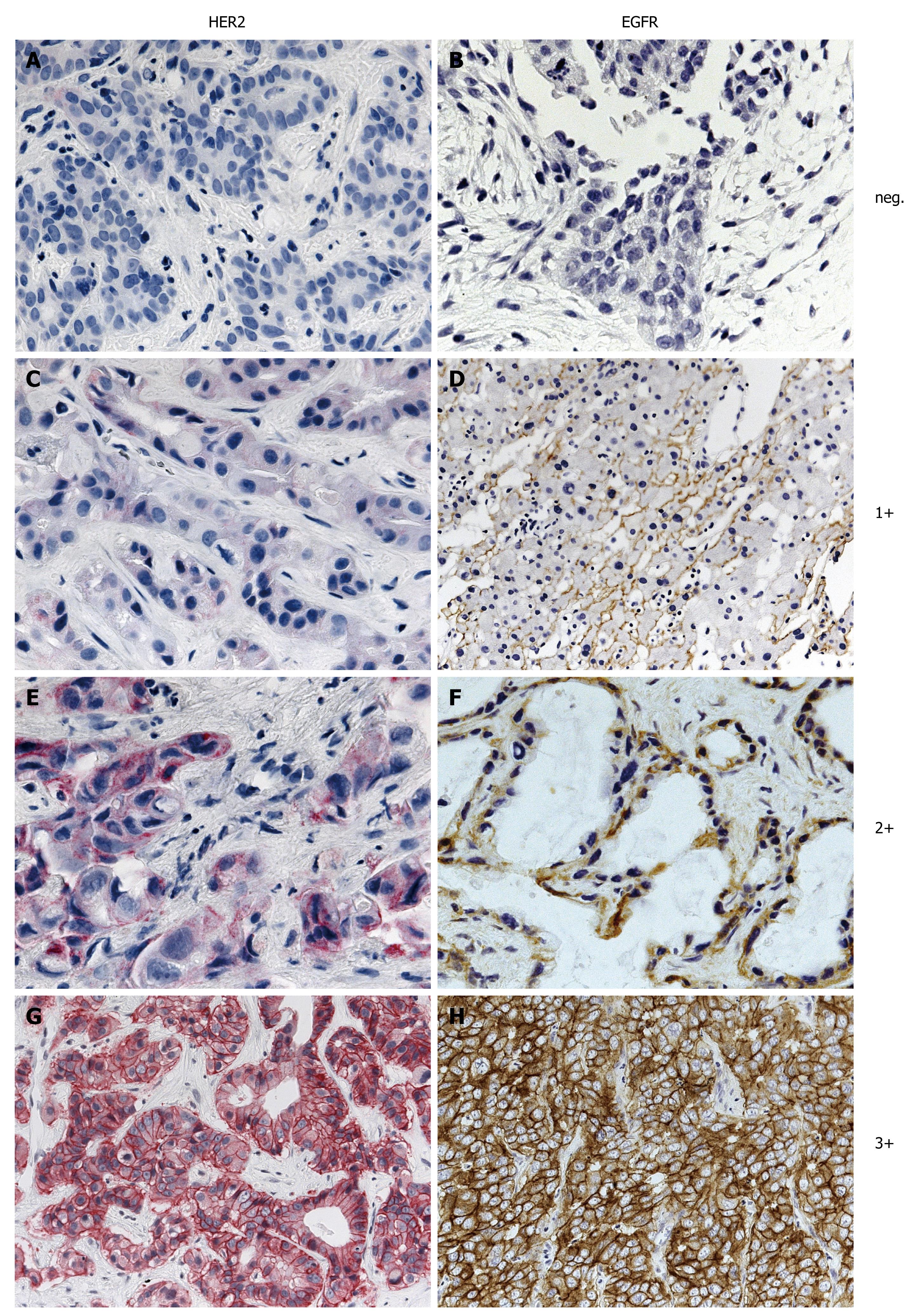
The EGFR pharmDx™ kit is based on the dextran technology. First sections were treated with proteinase K at 37°C for 5 min (epitope retrieval) prior to incubation with the EGFR antibody for 1 h at room temperature. Following incubation with the primary antibody, the visualization reagent consists of both labeled polymer and HRP (Horseradish peroxidase). Subsequently added chromogen DAB+ results in a visible reaction product (brown) at the antigen site. Staining was performed using the Dako Autostainer® (Dako Glostrup, Denmark) automated system. As recommended for the interpretation of EGFR pharmDx™, both the intensity and percentage of tumor cells exhibiting membranous and/or cytoplasmic staining were assessed. If specific membrane staining was observed in less than 1% of tumor cells, the specimen was reported to be EGFR negative. The four categories negative, weak (1+), moderate (2+) and intense (3+) were used as recommended by the manufacturer and approved by the FDA (Figure 1).
The detection of HER2 was performed with heat induced epitope retrieval (HIER) in 10 mmol/L citrate buffer (Target Retrieval Solution, pH 6.1, Dako) in a water bath at 98°C for 45 min and anti-HER2 primary antibody (Polyclonal Rabbit Anti Human c-erbB-2 oncoprotein, Dako Glostrup, Denmark). Following incubation with the primary antibody, the visualization is based on the sequential application of biotinylated link antibody and streptavidin labeled with alkaline phosphatase antibody. Subsequently added chromogen Fast RED results in a visible reaction product (red) at the antigen site. HER staining was also performed using the Dako Autostainer® (Dako Glostrup, Denmark). Tumors were counted negative if less than 10% of carcinoma cells were stained, irrespective of membrane signal intensity. If 10% or more of carcinoma cells showed membrane staining, the staining intensity was classified as weak (1+), moderate (2+) or intense (3+) as recommended by the manufacturer and approved by the FDA (Figure 1). Cytoplasmic staining was discounted. Images provided by DAKO were used to define these staining categories.
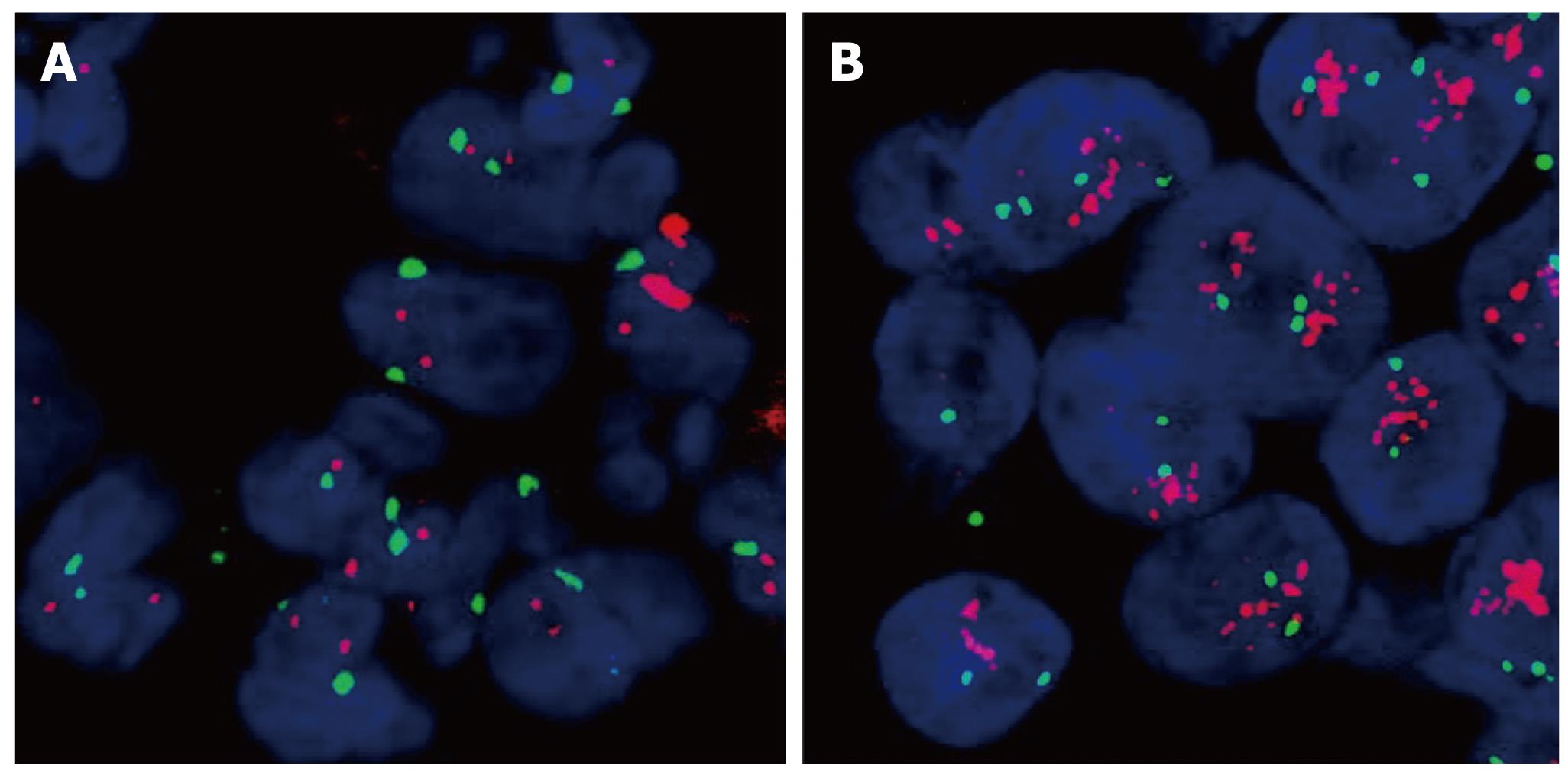
The PathVysion™ detection kit (Abbott, Illinios, USA) was used for FISH analysis in cases of IHC expression scores 2+ or 3+ for HER2. FISH staining was carried out according to the manufacturer’s manual. The presence of gene amplification was determined using the supplied fluorescence-labelled DNA probe for chromosomal locus 17q11.2-q12 for the detection of HER2. FISH-stained sections were scanned at × 1000 magnification and in each of three separate carcinoma areas. Twenty nuclei were analyzed. HER2 and chromosome 17 copy number were counted for all cells and the ratio of HER2 to chromosome 17 was calculated. A normal HER2:17 ratio was defined as ≤ 2; a ratio > 2 was interpreted as gene amplification. A normal HER2 copy number was attested at < four signals per cell (Figure 2).
Data are presented as mean ± SD or medians with range for continuous variables and as absolute and relative frequencies for categorical variables. Overall survival estimates and median survival times were calculated by the Kaplan-Meier method followed by Cox models estimating relative risks. All statistical tests were two-sided using a 5% significance level. SASS software was used for the calculations.
From the 124 patients examined 34 (27.4%) had gallbladder cancer, 47 (37.9%) had intrahepatic mass forming type BTC (IHCC) and 43 (34.7%) had extrahepatic or perihilar, intraductal growth type BTC (EHCC). Because EGFR expression was expected to be present two fold more frequently than HER2 expression in BTC only a subset of 56 samples was examined. EGFR expression was negative in 39.3% (22/56) of the patients. Weak (1+) positive EGFR staining was found in 21.5% (12/56), moderate (2+) in 23.2% (13/56) and intense (3+) in 16% (9/56) of the tumors. Overall 39.2% of the samples showed EGFR overexpression (scores 2+ and 3+), being significantly (P = 0.028) more frequent in EHCC (57.9%) than in IHCC (25%). HER2 expression was as follows: 72/124 (58%) were negative, 26 (21%) 1+, 22 (18%) 2+ and 4 (3%) 3+. Representative examples of EGFR and HER2 immunohistochemical staining and in situ hybridization are shown in Figures 1 and 2.
A close correlation between treatment response and gene amplification has been shown for HER2 in previous studies[4]. However, unlike the case of trastuzumab and HER2 in breast cancer, EGFR gene amplification detected by FISH has not been approved as being as useful for deciding on an EGFR targeted therapy yet. Therefore, we did not study EGFR gene amplification in our patient cohort. Regarding HER2, based on published data and the manufacturer’s recommendation, tumors with no or 1+ HER2 immunostaining were not further investigated for gene amplification. Of the 124 patients samples tested 25 were examined for HER2 gene amplification. HER2 FISH was performed in 2+ and 3+ samples and was successfully performed in all but one tumor examined. All specimens exhibiting 3+ immunostaining (4/4) showed gene HER2 amplification while amplification was present in 2/21 (10%) of 2+ samples. Taken together, HER2 gene amplification could be detected in 6/124 (5%) tumors.
Among the 124 patients 80 (64.5%) had moderately differentiated tumors, 36 (29%) had poorly differentiated and 8 (6.5%) had well differentiated tumors. The majority of patients (64/124, 51.6%) had stage IV disease, 31 (25%) had stage III, 20 (16.1%) stage II and 9 stage I (7.3%). The patients had not undergone surgery because of unresectability, comorbidity or patients’ wish. Half of the patients (62/124) had been treated with chemotherapy, resulting in tumor control in 59% (14.7% PR, 44.3% SD). Median overall survival was 13 mo with a median OS of 14 mo for patients treated with chemotherapy compared to 9 mo for patients not treated with chemotherapy. There was no statistical association between protein expression and grade, stage, overall survival and treatment response for EGFR and HER2, respectively. The frequencies of EGFR and HER2 overexpression and clinicopathological variables are summarized in Table 2. In univariate analysis EGFR and HER2 expression could not be shown to be of prognostic relevance for overall survival (P = 0.06 and P = 0.49).
Expression of the two ErbB family growth factor receptors EGFR and HER2 has been intensively studied in different tumor entities and led to the use of targeted therapy with specific inhibitors or antibodies of these receptors in colorectal, breast, lung as well as head and neck cancer[4]. To date in other cancers monoclonal antibodies and small molecule tyrosine kinase inhibitors such as cetuximab, trastuzumab, erlotinib, gefitinib and lapatinib are under investigation. Expression of EGFR and HER2 as potential therapeutic targets has been reported in various tumors[4,7,21,22]. For BTC, data for EGFR and HER2 overexpression have been presented in mostly small patient cohorts[14,17,19,23]. Recently Yoshikawa et al[24] described an unselected large cohort of 236 cases of resected BTC. In this study, we investigated EGFR and HER2 expression in a large cohort of patients with advanced, unresectable BTC.
In BTC the percentage of EGFR overexpressing tumors in previously reported series ranged from 8.1% to 81%. Yoshikawa et al[24] showed EGFR overexpression in 26.4% of EHCC and 17.7% of IHCC. Similarly, in our study EGFR overexpression was more frequent in EHCC (57.9%) than in IHCC (25%, P = 0.028). In these reports by Ito et al[23] and Yoshikawa et al[24] EGFR overexpression was associated with biological aggressiveness of BTC. In addition, there is increasing evidence that EHCC and IHCC respond differently to chemotherapy suggesting different biological properties of these two tumor subtypes of “cholangiocarcinoma”[25,26].
HER2 overexpression and amplification has been found in a range between 5% and 76% in BTC[14-19,24,27]. This wide range may in part reflect the lack of standardized methodologies used within the different studies to assess HER2 status. In our cohort with advanced, unresectable BTC, HER2 overexpression was present in 20% and treatment relevant HER amplification in 5%. Some authors suggest that HER2 overexpression is due to gene deregulation rather than gene amplification because in some reports there is no strict correlation between protein expression and gene amplification[19]. Overall, the highest concordance between HER2 expression and gene amplification was demonstrated for breast cancer[7,28,29]. Our data suggest that in advanced BTC with high HER2 expression there is also a good correlation between overexpression and amplification, since all samples with a 3+ HER2 score showed gene amplification as compared to only 2/21 (10%) with a 2+ HER2 score. Similar correlations have been reported for other malignancies, including breast cancer with a tendency towards lower therapeutic relevant expression rates with improved, standardized detection methods. It has to be assumed that earlier reports overestimated HER2 expression in BTC, explaining the discrepancy between HER2 expression and gene amplification[28,30,31]. Despite the fact that HER2 transgenic mice frequently develop BTC, HER2 does not seem to play a major role in human biliary tract malignancies. This animal tumor model may therefore be of limited value as a model for human BTC.
In recent reports in early stage, resected BTC overall HER2 amplification was about 5%-10%[24,32]. In our cohort of patients with advanced BTC, HER2 amplification was merely 5%. Since targeted therapy with the anti-HER2 antibody trastuzumab in breast cancer is only effective when the HER2/neu receptor is overexpressed via gene amplification HER2 seems not to be a promising target in BTC. Therapeutic trials targeting HER2 should therefore not further be initiated.
Since EGFR expression does not predict its therapeutic usefulness, future clinical trials have to evaluate the advantage of Anti-EGFR therapy in comparison with standard treatment in patients with BTC.
In summary, our findings demonstrate that EGFR overexpression is frequent in BTC, especially in EHCC. In contrast, HER2 overexpression and gene amplification is a rare event. While therapeutic targeting of HER2 seems to be not promising, future clinical trials in patients with BTC should focus on EGFR.
The epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor 2 (HER2) are involved in the carcinogenesis of many malignancies. Therapeutic molecules targeting EGFR and HER2 have been successfully used for the treatment of colorectal, breast, lung and head and neck cancers among others. It is unknown if EGFR and HER2 are overexpressed in advanced biliary tract cancer (BTC) and therefore may serve as therapeutic targets in these cancers.
As the so called targeted therapies are most effective when the corresponding receptor or signalling pathway is activated, previous studies have focused on EGFR and HER2 in various tumors. There are conflicting data about overexpression of EGFR and HER2 in BTC and about the therapeutic importance of the two growth factor receptors in these tumors. Because of the low incidence, clinical trials on BTC are difficult and mostly performed in small patient cohorts. A possible selection bias might additionally explain these conflicting data.
While EGFR is significantly overexpressed in advanced BTC, HER2 overexpression and amplification is rare and therefore seems not to play a role in the carcinogenesis of BTCs.
Because of these expression data on EGFR and HER2 in BTC, and the correlation of expression and therapeutic effectiveness, especially with HER2 in other tumors, future clinical trials in BTC should focus on EGFR as a therapeutic target.
EGFR and HER2 are receptor tyrosine kinases encoded by proto-oncogenes. Growth factors such as epidermal growth factor or transforming growth factor bind to these receptors and initiate tumor cell proliferation, migration, invasion, resistance to apoptosis and angiogenesis. BTC is a heterogeneous tumor entity with rising incidence, consisting of intrahepatic mass forming type cholangiocarcinoma, perihilar Klatskin tumors, extrahepatic bile duct tumors, also termed intraductal growth type cholangiocarcinoma, and gallbladder cancer.
Harder et al describe low expression and gene amplification of HER2 in biopsy samples of advanced BTC, as assessed by immunohistochemistry and fluorescence in situ hybridisation, respectively. The authors suggest that deviating numbers of some earlier studies may be due to non-standardized techniques. It is convincing that in the present paper results for protein expression and gene amplification correlated well.
Peer reviewer: Dr. Bart Rik De Geest, Center for Molecular and Vascular Biology, Katholieke Universiteit Leuven, Campus Gasthuisberg, Herestraat 49, Leuven 3000, Belgium
S- Editor Tian L L- Editor O’Neill M E- Editor Zheng XM
| 1. | Malhi H, Gores GJ. Review article: the modern diagnosis and therapy of cholangiocarcinoma. Aliment Pharmacol Ther. 2006;23:1287-1296. |
| 2. | Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366:1303-1314. |
| 3. | Ménard S, Casalini P, Campiglio M, Pupa SM, Tagliabue E. Role of HER2/neu in tumor progression and therapy. Cell Mol Life Sci. 2004;61:2965-2978. |
| 4. | Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39-51. |
| 5. | Hudis CA. Trastuzumab adds to adjuvant chemotherapy for resected HER2-positive breast cancer. Nat Clin Pract Oncol. 2006;3:12-13. |
| 6. | Press MF, Lenz HJ. EGFR, HER2 and VEGF pathways: validated targets for cancer treatment. Drugs. 2007;67:2045-2075. |
| 7. | Ooi A, Takehana T, Li X, Suzuki S, Kunitomo K, Iino H, Fujii H, Takeda Y, Dobashi Y. Protein overexpression and gene amplification of HER-2 and EGFR in colorectal cancers: an immunohistochemical and fluorescent in situ hybridization study. Mod Pathol. 2004;17:895-904. |
| 8. | Huang TW, Wang CH, Hsieh CB. Effects of the anti-epidermal growth factor receptor antibody cetuximab on cholangiocarcinoma of the liver. Onkologie. 2007;30:129-131. |
| 9. | Bralet MP, Bellin MF, Guettier C, Adam R, Paule B. Response to cetuximab and gemcitabine-oxaliplatin in an advanced case of intrahepatic cholangiocarcinoma. Clin Oncol (R Coll Radiol). 2006;18:426. |
| 10. | Sprinzl MF, Schimanski CC, Moehler M, Schadmand-Fischer S, Galle PR, Kanzler S. Gemcitabine in combination with EGF-Receptor antibody (Cetuximab) as a treatment of cholangiocarcinoma: a case report. BMC Cancer. 2006;6:190. |
| 11. | Büchler P, Reber HA, Eibl G, Roth MA, Büchler MW, Friess H, Isacoff WH, Hines OJ. Combination therapy for advanced pancreatic cancer using Herceptin plus chemotherapy. Int J Oncol. 2005;27:1125-1130. |
| 12. | Büchler P, Reber HA, Büchler MC, Roth MA, Büchler MW, Friess H, Isacoff WH, Hines OJ. Therapy for pancreatic cancer with a recombinant humanized anti-HER2 antibody (herceptin). J Gastrointest Surg. 2001;5:139-146. |
| 13. | Kiguchi K, Carbajal S, Chan K, Beltrán L, Ruffino L, Shen J, Matsumoto T, Yoshimi N, DiGiovanni J. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 2001;61:6971-6976. |
| 14. | Aishima SI, Taguchi KI, Sugimachi K, Shimada M, Sugimachi K, Tsuneyoshi M. c-erbB-2 and c-Met expression relates to cholangiocarcinogenesis and progression of intrahepatic cholangiocarcinoma. Histopathology. 2002;40:269-278. |
| 15. | Altimari A, Fiorentino M, Gabusi E, Gruppioni E, Corti B, D'Errico A, Grigioni WF. Investigation of ErbB1 and ErbB2 expression for therapeutic targeting in primary liver tumours. Dig Liver Dis. 2003;35:332-338. |
| 16. | Chow NH, Huang SM, Chan SH, Mo LR, Hwang MH, Su WC. Significance of c-erbB-2 expression in normal and neoplastic epithelium of biliary tract. Anticancer Res. 1995;15:1055-1059. |
| 17. | Endo K, Yoon BI, Pairojkul C, Demetris AJ, Sirica AE. ERBB-2 overexpression and cyclooxygenase-2 up-regulation in human cholangiocarcinoma and risk conditions. Hepatology. 2002;36:439-450. |
| 18. | Terada T, Ashida K, Endo K, Horie S, Maeta H, Matsunaga Y, Takashima K, Ohta T, Kitamura Y. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325-331. |
| 19. | Ukita Y, Kato M, Terada T. Gene amplification and mRNA and protein overexpression of c-erbB-2 (HER-2/neu) in human intrahepatic cholangiocarcinoma as detected by fluorescence in situ hybridization, in situ hybridization, and immunohistochemistry. J Hepatol. 2002;36:780-785. |
| 20. | Khan SA, Davidson BR, Goldin R, Pereira SP, Rosenberg WM, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Thursz MR, Wasan H. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002;51 Suppl 6:VI1-VI9. |
| 21. | Ooi A, Kobayashi M, Mai M, Nakanishi I. Amplification of c-erbB-2 in gastric cancer: detection in formalin-fixed, paraffin-embedded tissue by fluorescence in situ hybridization. Lab Invest. 1998;78:345-351. |
| 22. | Takehana T, Kunitomo K, Suzuki S, Kono K, Fujii H, Matsumoto Y, Ooi A. Expression of epidermal growth factor receptor in gastric carcinomas. Clin Gastroenterol Hepatol. 2003;1:438-445. |
| 23. | Ito Y, Takeda T, Sasaki Y, Sakon M, Yamada T, Ishiguro S, Imaoka S, Tsujimoto M, Higashiyama S, Monden M. Expression and clinical significance of the erbB family in intrahepatic cholangiocellular carcinoma. Pathol Res Pract. 2001;197:95-100. |
| 24. | Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, Hirohashi S, Shibata T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418-425. |
| 25. | Nehls O, Oettle H, Hartmann JT, Hofheinz RD, Hass HG, Horger MS, Koppenhöfer U, Hochhaus A, Stieler J, Trojan J. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer. 2008;98:309-315. |
| 26. | Harder J, Riecken B, Kummer O, Lohrmann C, Otto F, Usadel H, Geissler M, Opitz O, Henss H. Outpatient chemotherapy with gemcitabine and oxaliplatin in patients with biliary tract cancer. Br J Cancer. 2006;95:848-852. |
| 27. | Voravud N, Foster CS, Gilbertson JA, Sikora K, Waxman J. Oncogene expression in cholangiocarcinoma and in normal hepatic development. Hum Pathol. 1989;20:1163-1168. |
| 28. | Yau TK, Sze H, Soong IS, Hioe F, Khoo US, Lee AW. HER2 overexpression of breast cancers in Hong Kong: prevalence and concordance between immunohistochemistry and in-situ hybridisation assays. Hong Kong Med J. 2008;14:130-135. |
| 29. | Carlson RW, Moench SJ, Hammond ME, Perez EA, Burstein HJ, Allred DC, Vogel CL, Goldstein LJ, Somlo G, Gradishar WJ, Hudis CA, Jahanzeb M, Stark A, Wolff AC, Press MF, Winer EP, Paik S, Ljung BM. HER2 testing in breast cancer: NCCN Task Force report and recommendations. J Natl Compr Canc Netw. 2006;4 Suppl 3:S1-S22; quiz S23-S24. |
| 30. | Kobayashi M, Ooi A, Oda Y, Nakanishi I. Protein overexpression and gene amplification of c-erbB-2 in breast carcinomas: a comparative study of immunohistochemistry and fluorescence in situ hybridization of formalin-fixed, paraffin-embedded tissues. Hum Pathol. 2002;33:21-28. |
| 31. | Hirashima N, Takahashi W, Yoshii S, Yamane T, Ooi A. Protein overexpression and gene amplification of c-erb B-2 in pulmonary carcinomas: a comparative immunohistochemical and fluorescence in situ hybridization study. Mod Pathol. 2001;14:556-562. |