INTRODUCTION
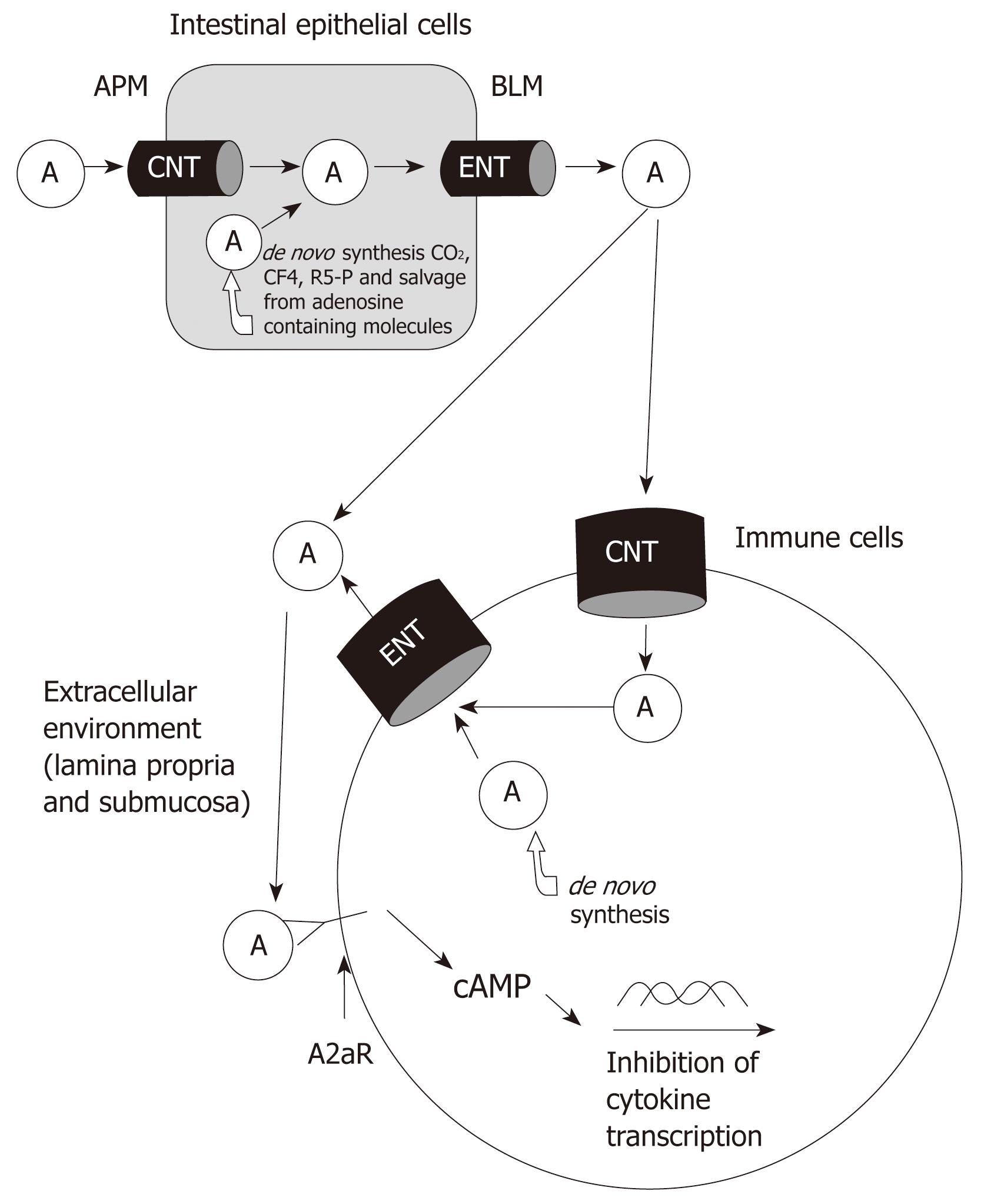
Figure 1 Pathways and roles of adenosine.
Absorbed and de novo synthesized adenosine delivered to extracellular space. Adenosine binds to A2aR of immune cells and activates signaling pathways to inhibit the production of inflammatory mediators. A: Adenosine; APM: Apical membrane; BLM: Basolateral membrane; CNT: Concentrative nucleoside transporter; ENT: Equilibrative nucleoside transporter.
Adenosine is a purine molecule necessary for normal cell metabolism and growth. Recently, adenosine has been recognized as a potential anti-inflammatory molecule. In general, cellular adenosine is produced by both de novo synthesis and by absorption from the diet into the body through transporters in the gastrointestinal tract. It is thought that activation of adenosine receptors deactivates the synthesis of critical components necessary for activation of chronic inflammatory diseases, including inflammatory bowel disease (IBD). Many reviews have focused on the general aspects of adenosine activation of its receptors in inflamed tissues. This review focuses on the identification of the role of intestinal epithelial cell adenosine transporters during IBD.
CURRENT UNDERSTANDING OF INFLAMMATORY BOWEL DISEASES
IBD, including Crohn’s disease (CD) and ulcerative colitis (UC), is a common and lifelong disabling gastrointestinal disease[1,2]. It has highest incidence and prevalence in the developed countries. The worldwide incidence varies greatly with that of UC ranging from 0.5-24.5/100 000 and that of CD ranging from 0.1-16/100 000 in different populations. There are more than 2 million IBD patients in the United States[3]. The precise mechanism of IBD is still unknown. CD and UC differ in their histological presentation and cytokine profile. The accumulated data indicate that IBD results from a complex interplay of genetic, environmental, and immunologic factors. The presence of one or more genetically determined defects leads to an over-reaction of the host mucosal immune system to normal constituents of the mucosal microflora. The genetically determined alterations of gut epithelial barrier function enhance exposure of the mucosal immune system to microflora components. The over-reaction causes either a Th1-type T cell-mediated inflammation (Crohn’s disease) or a Th2-type T cell-mediated inflammation (ulcerative colitis). Multiple cytokines are released in the inflammatory process. The most important factors are tumor necrosis factor (TNF)-α, interleukin (IL)-1, interferon (INF)-γ, IL-6, 12, 13 and 17, monocyte chemotactic protein (MCP)-1 and IL-8[4]. These cytokines attract and activate neutrophils, eosinophils, mast/plasma cells and macrophages. These inflammatory cells produce large amounts of unstable chemical species such as reactive oxygen species (ROS) or oxyradicals (i.e. superoxide anions, hydrogen peroxide, hydroxyl radicals, peroxynitrite), resulting in tissue injury[5,6].
Since the cause of IBD is still unknown, currently available treatments for the disease are non-specific and may cause side effects such as osteoporosis and suppression of the immune system. Many patients respond and maintain remission with existing therapy. Thus, at present, there is no cure for IBD. But for some patients, the available therapeutic options for IBD are still inadequate. The conventional treatments use corticosteroids, mesalamine, and immunosuppressants. These either nonspecifically block downstream inflammatory events, such as the secretion of cytokines and activation of immunocytes and neutrophils, or increase tissue adenosine levels, regardless of the nature of the underlying T cell response that generated these events. These agents have been used for treatment of mild and moderate IBD with some success for many years despite shortcomings and toxicities. The newer therapies using biologics, such as antibodies against TNF-α and α-integrin molecules, eliminate a specific major inflammatory cytokine or act by disrupting accumulation of cells at areas of inflammation. Both strategies have been successful in subsets of IBD patients but have also been associated with significant complications including fatal infections[7-12].
Emerging treatments are being developed to target the hierarchy of the inflammatory cytokine effect including IL-12/IL-23[13], IFN-γ[14], IL-6[15], and IL-10 levels[16]. Several antibodies currently on clinic trial include: anti-IL-12p40, an antibody against IL-12 and IL-23, the master cytokines underlying the Th1 response, for Crohn’s disease[13]; anti-IL-23p19, a potentially useful treatment for patients with resistance to anti-TNF therapy which acts by targeting IL-23 and IL-17 rather than IL-12 and IFN-γ in experimental colitis. Other approaches to the treatment of IBD currently under investigation are leukocytapheresis to eliminate effector cells[17,18], administration of probiotics, use of GM-CSF to enhance innate immune function[19], administration of microbe-derived agents or intestinal parasites to activate the innate immune system by inducing counter-regulatory immune responses to quell established inflammation[20], administration of anti-CD3 antibodies[21], autologous hematopoietic stem cell transplant[22], extracorporeal photophoresis to restore immunoregulation[23], and adipose stem cell infusion[24].
ADENOSINE MODULATES CHRONIC INFLAMMATION IN IBD
Adenosine exerts broad biologic effects, including smooth muscle contraction, neurotransmission in the peripheral and central nervous systems, platelet aggregation, pain, exocrine and endocrine secretion, lipolysis, glycogenesis, immune system development and response (e.g. severe combined immunodeficiency is due to lack of adenosine-deaminase), cardiac conduction and contractility, and anti-inflammation[25]. It has long been reported that adenosine, a purine nucleoside that is released at injured and inflamed sites, plays a central role in the regulation of inflammatory responses and in limiting inflammatory tissue destruction[26]. Early after the injurious or infectious signal, high concentrations of extracellular adenosine favor a transition from neutrophil infiltration to macrophage recruitment, to facilitate a highly efficient specific immune response carried out by macrophages. At later stages of immune or inflammatory processes, adenosine contributes to the resolution of inflammation, both by down-regulating macrophage activation and by advancing Th2- vs Th1-cell response[26]. Some anti-inflammatory and immunomodulating drugs, such as salicylates, methotrexate and purine analogs like 6-MP and cyclosporine, exert their therapeutic actions in inflammatory diseases, at least in part, by decreasing intracellular adenosine 5’-triphosphate (ATP) concentrations and increasing extracellular adenosine levels[27].
There are numerous reports that have demonstrated the ability of adenosine to exert anti-inflammatory actions in a variety of animal models. The anti-inflammatory effects can be achieved by increasing intracellular or extracellular adenosine levels through the mechanism of either enhanced production or inhibition of adenosine catabolism. The majority of work has been focusing on inhibition of adenosine catabolism or direct activation of adenosine receptors. A recent article by Antonioli et al[28] reported that inhibition of adenosine deaminase can attenuate mucosal inflammation in experimental colitis through the mechanism of reducing mucosal myeloperoxidase activity, production of malondialdehyde and TNF-α levels as well as plasma TNF-α and interleukin-6 levels. Other studies have demonstrated that adenosine acting on the A2a receptor of T-lymphocytes can selectively suppress the expression of pro-inflammatory cytokines while sparing anti-inflammatory activity mediated by IL-10 and TGF-β[29]. The tissue injury and inflammation in mice with enteritis induced by Clostridium difficile toxin A can be alleviated by a new A2a receptor agonist, ATL 313, through the mechanism of inhibiting neutrophil infiltration, TNF-α production and adenosine deaminase activity[30]. Adenosine can down-regulate neutrophil functions by decreasing their adhesion, degranulation, and oxidant activities[25]. An increase in endogenous adenosine levels by inhibition of adenosine kinase ameliorates colitis by suppression of IFN-γ in colonic tissue and CD69 expression in splenocytes, as well as maintaining tissue integrity by reducing energy demand, increasing nutrient availability, and modulating the immune system[31]. At a molecular level, adenosine has been demonstrated to be a negative regulator of NF-κB and MAPK signaling in human intestinal epithelial cells[32]. Based on these findings, the adenosine system can represent a very promising target for therapies of inflammatory bowel diseases.
PHYSIOLOGY OF ADENOSINE METABOLISM BY INTESTINAL EPITHELIUM CELLS
Intracellular adenosine level is maintained by constant synthesis and degradation, as well as by trans-membrane transport through nucleoside transporters. The intracellular adenosine is produced by de novo synthesis from amino acids, CO2, carbon-1-tetrahydrofolate and ribose-5-phosphate, salvage of endogenous adenine, dephosphorylation of ATP, ADP and AMP, as well as by transportation of exogenous nucleobases and nucleosides through concentrative nucleoside transporters (CNTs) or equilibrative nucleoside transporters (ENTs). Adenosine is metabolized to inosine by adenosine-deaminase, to either an end product uric acid or phosphorylated to ATP by adenosine-kinase or diffused into the extracellular space via ENTs[33,34].
Most tissues and cells have de novo synthesis capacity to produce adenine endogenously from amino acids, CO2 and carbon-1-tetrahydrofolate for their own use. However, some tissues and cells either lack or have very limited de novo synthesis capacity. These tissues and cells rely largely on exogenous nucleoside supply and salvage of endogenous nucleobases and nucleosides. Bone marrow, lymphocytes, leukocytes and intestinal epithelial cells are among them[35-39]. The liver is the major organ supplying the nucleobases to these tissue and cells. The dietary nucleobases and nucleosides are absorbed by intestinal villous epithelial cells and degraded to end products such as uric acid. The uric acid is brought to the liver by blood and is taken up by hepatocytes and transformed into nucleobases. The synthesized nucleobases are released into the blood stream and carried to the tissues.
The intestinal epithelial cells have a very limited capacity for de novo synthesis; the villous cells can directly use the absorbed dietary nucleosides but the cryptal cells depend on blood supply. The impact of lack or limitation of nucleoside supply to cryptal cells on epithelial repair and barrier function during IBD has not been fully investigated[40,41]. It is reasonable to speculate that poor absorption in the intestine or suppression of synthesis in the liver will ultimately result in disruption of epithelial barrier function in chronic bowel inflammatory diseases like IBD, which in turn will lead to over-exposure of the innate immune system to intraluminal bacterial antigens and cause persistent inflammation or exacerbation of the diseases.
In general, the extracellular adenosine is produced by dephosphorylation of ATP by an enzymatic cascade consisting of Ntpases and ecto-5’-nucleotidase (Ecto 5’NTase), and direct diffusion of intracellular adenosine through ENTs. It is removed by enzymatic degradation by adenosine deaminase to inosine or by adenosine kinase to AMP. It can also be transported back into cells by membranous transporters like CNTs/ENTs. The extracellular adenosine level is believed to be lower than 1 μmol/L in normal tissue but can be as high as 100 μmol/L in inflamed or ischemic tissues[25]. Only a high adenosine level can exert immunomodulatory and immunosuppressive effects. Luminal adenosine level is estimated to be 5 mmol/L in normal intestine, while it is 6 mmol/L in inflamed intestine due to ATP and adenosine secretion in inflammatory and other cell types[42].
It is not entirely clear which cell types are the most important producer of extracellular adenosine, but endothelial cells, neutrophils, nerve terminal and epithelial cells have been identified in the literature[43,44]. Extracellular adenosine binds to adenosine receptors (AR) 1, 2a, 2b and 3, all of which are expressed on the surface of immune cells. Low level expression of A1R is demonstrated in small intestine. A2bR is the only receptor expressed in epithelial cells of cecum and colon. A3R can be detected in jejunum and proximal colon[45,46]. Adenosine receptors are members of the G protein-coupled family of receptors[47]. A1 and A3 receptors are usually coupled with Gi proteins that inhibit adenylate cyclase, whereas the A2aR and A2bR receptors are coupled with Gs proteins that activate adenylate cyclase. Several studies have demonstrated that adenosine attenuates intestinal inflammation predominantly through the effects of the A2aR receptor of neutrophils and T-lymphocytes[29,30,48]. However, Yang et al[49] found that activation of the A2bR can also have anti-inflammatory effects, using a gene knock-out method to delete this gene in order to show a pro-inflammatory phenotype.
MOLECULAR MECHANISM OF ADENOSINE TRANSPORT
The significance of exogenous adenosine transport by intestinal epithelial cells in the treatment of IBD and its impact on epithelial cell barrier function has not been explored. Concentrative nucleoside transporters (CNTs) have been identified as the major transporters for absorption of exogenous nucleosides from the diet. Three distinct CNTs (CNT1, CNT2 and CNT3) that exhibit different substrate specificity have been cloned and characterized from humans, rats and mice[34]. CNT1 predominantly transports pyrimidines. CNT2 transports purine and uridine, while CNT3 transports purines and pyrimidines. The expression of CNTs and their substrate specificity vary among species. CNT3 was not found in intestinal epithelial cells of human and rat[34,50,51]. CNTs belong to the solute carrier family 28 (SLC-28). CNTs are expressed in the apical membranes of intestinal epithelial cells, as well as in other cell types including hepatocytes, endothelial cells, neutrophils, lymphocytes and macrophages.
CNT2 has been cloned and characterized in humans, mice, rats and rabbits. The rat CNT2 cDNA is 2.9 Kb and encodes a 659 amino acid protein with molecular weight of 72 kDa[52]. The apparent molecular weight on western blot is usually around 60 kDa due to high hydrophobicity of membrane protein. Fourteen putative transmembrane domains were identified by hydropathy analysis. The presence of several consensus sequences for protein kinase-C (PKC) and protein kinase-A (PKA) phosphorylation sites on both N- and C-termini, and an ATP/GTP binding motif in N-terminus, suggest that CNT2 may be regulated by protein kinases and intracellular ATP and GTP. CNT2 may be a glycoprotein as there are five possible N-linked glycosylation sites. Na+-adenosine cotransport in brush-border membranes from rabbit ileum was identified and partially characterized in one previous study[53]. In vitro expressed CNT2 in Xenopus laevis oocytes exhibited Na+-dependent adenosine uptake with an apparent Km for adenosine of 6 μmol/L, with substrate selectivity to purine and uridine[54].
REGULATION OF ADENOSINE TRANSPORT AND CNT2 EXPRESSION IN GENERAL
The CNT2 expression and adenosine uptake are highly regulated processes and are species and tissue specific[55]. Sub-cellular trafficking (i.e. internalization of membrane transporters to sub-cellular storage vesicles) has been shown to be a regulatory mechanism for CNT2 in several cell lines including adrenal chromaffin cells, reticulocytes and cholangiocytes[56-58]. Although CNT1 has been shown to be up-regulated in intestine and down-regulated in hepatocytes of pyrimidine-free diet fed animals, the dietary effects of adenosine on CNT2 activity and molecular expressions are not known[59,60]. Several studies have shown that nucleoside transport functions and expressions are regulated by hormones[61-66]. Tyrosine and glucagon have been shown to stimulate adenosine transport and CNT2 expression in both in vitro and in vivo models[61-63]. Studies with insulin and glucose have yielded different results regarding adenosine transport and molecular expression. Insulin regulates adenosine transport through different signaling pathways that involve PI3K, MAPK, NO synthase, PKC and MAP kinase[64-66]. One study showed that adenosine transport is up-regulated by activation of the A1 adenosine receptor through ATP-sensitive K-channels in hepatocytes, suggesting some positive feedback regulation among adenosine receptors and the adenosine transporter[67].
The effects of proliferative (EGF and TGF-α) and differentiating (glucocorticoid) hormones were demonstrated in IEC-6 cells (a rat intestinal cryptal cell line). A four-fold increase of adenosine transport activity and CNT2 molecular expression after treatment with dexamethasone was observed while no significant impact was noted from treatment with proliferative hormones[68]. However, a more recent paper by the same research group found that TGF-β can transcriptionally up-regulate CNT2 gene expression in rat hepatocytes[69]. Adenosine transport and CNT2 expression are altered during cell growth cycle and differentiation. Hepatocarcinogenesis is accompanied by loss of CNT2 expression and increased expression of ENTs. CNT2 mRNA and protein levels were increased right before the peak of incorporation of thymidine into DNA and during liver regeneration after partial hepatectomy[70,71].
CNT2 expression was up-regulated by lipopolysaccharide (LPS), NO, INF-α and TNF-α in macrophages and B-lymphocytes in several studies[72-74]. The LPS-induced increase of adenosine transport and molecular expression is TNF-α-dependent but not iNOs-dependent. cNOs is required for maintaining the basal transport activities of adenosine in activated B-cells. The CNT2 is recognized as an important regulator of extracellular adenosine concentrations. CNT2 expression is suppressed in inflamed tissue as a mechanism to maintain high extracellular adenosine concentration. The CNT2 expression is suppressed in neutrophils and macrophages during inflammation[75]. There is controversy between the in vivo and in vitro studies about the functional status of adenosine transport and CNT2 expression during inflammation and the effects of inflammatory mediators.
MECHANISM OF REGULATION OF ADENOSINE TRANSPORT AND CNT2 EXPRESSION IN INTESTINAL EPITHELIAL CELLS
A systematic study for the mechanism of regulation of adenosine transport and CNT2 expression in intestinal epithelial cells is not available. Adenosine functions as a nutrient for nucleic acid metabolism, an energy carrier molecule for cell energy metabolism, and a second messenger in autocrine and paracrine hormone regulation. Adenosine transport is regulated differently from other nutrient transporters such as glucose and amino acid transporters. Luminal adenosine and ATP have been reported to regulate glucose and bile acid transport in intestine, proximal renal tubule cells and cholangiocytes[42,76,77]. The role of the activation of purinergic receptors on adenosine uptake was also studied in vascular endothelial cells and chromaffin cells. ATP has been shown to up-regulate adenosine transport and protein expression in vascular endothelial cells and chromaffin cells[78-80]. However, the effect of ATP on adenosine transport in normal and inflamed intestine remains to be elucidated.
MECHANISM OF REGULATION OF NA+-CO-TRANSPORT IN INTESTINAL EPITHELIAL CELLS DURING CHRONIC INTESTINAL INFLAMMATION
The regulation of intestinal epithelial transporters during chronic inflammation is a very complex process, involving many cell types and immune-inflammatory mediators. The cells involved in chronic enteritis include all inflammatory cells (neutrophils, basophils, eosinophils, macrophages, lymphocytes), fibroblasts, vascular endothelial cells, nerve cells and epithelial cells. These cells produce numerous cytokines and immune-inflammatory mediators such as prostaglandins, leukotrienes, reactive oxidative metabolites (ROMs) and nitric oxide[1-6]. So far, there are no perfect animal models for IBD though several different models exist with chemically-induced and genetically-induced enteritis. It is very difficult to fully understand the regulation of nutrient and electrolyte transport in the chronically inflamed intestine.
The mast cell has been implicated as an important player in chronic intestinal inflammation. It releases multiple inflammatory mediators including histamine, serotonin, cytokines, prostaglandins, leukotrienes and ROMs. The released mediators are very important regulators of transporters in epithelial cells. It was consistently demonstrated that blocking mast cell degranulation prevented down-regulation of a number of transporters including Na+-glucose and Na+-amino acid cotransport as well as electrolyte transporters such as Cl-/HCO3- exchange[81].
Nitric oxide has been identified as an important regulating factor in the intestinal tract. Constitutive nitric oxide synthase (cNOs) plays an important role in the regulation of transporters in normal intestine. It is essential for some transporters to function properly under physiologic conditions. Inducible nitric oxide synthase (iNOS) is activated during chronic inflammation, ischemia and tissue injury. iNOS produces large amounts of nitric oxide (NO) during chronic inflammation, which is generally considered as detrimental to tissue and cells because of formation of peroxynitrous acid, though controversy continues about its role in inflammation. The mechanism of cNOS and iNOS effects on nutrient co-transporters in epithelial cells is possibly through direct and indirect effects, by stimulating the production and/or release of other inflammatory mediators such as arachidonic acid metabolites (e.g. prostaglandins and leukotrienes)[82-84].
Prostaglandins have been shown to be very important inflammatory mediators in chronic intestinal inflammation. A large amount of prostaglandins are produced in the intestinal tissue during IBD. Prostaglandins inhibit electrolyte absorption and Na-nutrient co-transporter functions[85-89]. In addition, they also promote mucous secretion and cytoprotection in the gastrointestinal tract during IBD[85,88]. Prostaglandin E2 (PGE2) was also reported to suppress glucose transport in the ovine intestine[90]. Leukotrienes have been demonstrated to inhibit electrolyte transport in a similar pattern to prostaglandins[91-98].
Corticosteroids are the most frequently used broad spectrum immunomodulators in IBD for blocking the production of most major inflammatory mediators. Corticosteroids prevent mast cell degranulation and block the phospholipase A2 (PLA2) enzyme pathway. They also down-regulate arachidonic acid release and production of prostaglandins and leukotrienes. In addition, corticosteroids suppress the production of iNOS and inducible cyclooxygenase (COX-2) during chronic inflammation[99].
It is possible that mast cells, nitric oxide, arachidonic acid metabolites and steroids are all involved in the regulation of adenosine transport and CNT2 expression during chronic enteritis. It should be noted that adenosine itself is an inflammatory modulator. Extracellular adenosine can suppress all these above-mentioned inflammatory cells and mediators. The interplay among adenosine, inflammatory cells and mediators can be very complex and is also very interesting for further study.
CONCLUSION
The pathways of adenosine metabolism and transport are fully illustrated in Figure 1. Luminal adenosine is absorbed by intestinal epithelial cells through CNT and ENT transporters and can also be synthesized de novo. The increase in intracellular levels of adenosine leads to extracellular transport along with the release of adenosine from damaged cells during inflammation. Using various therapies during IBD, it may be possible to eventually increase the extracellular levels of adenosine so that the appropriate receptors can be activated to inhibit and reduce the effects of chronic inflammation on the gut. IBD is a common and lifelong disabling gastrointestinal disease. At present, there is no cure for IBD. Emerging treatments are being developed to target cytokines that perpetuate the chronic inflammatory response. Adenosine is an important modulator of inflammation and its anti-inflammatory effects have been well established in humans as well as in animal models. Therapeutic targeting of receptors such as the A2a receptor could reduce cytokine levels and thus reduce the effects of chronic inflammation during IBD.
Peer reviewers: Akira Andoh, MD, Department of Internal Medicine, Shiga University of Medical Science, Seta Tukinowa, Otsu 520-2192, Japan; Elke Cario, MD, Division of Gastroenterology and Hepatology, University Hospital of Essen, Instituts group I, Virchowstr. 171, Essen D-45147, Germany
S- Editor Tian L L- Editor Logan S E- Editor Yin DH