Published online Sep 21, 2009. doi: 10.3748/wjg.15.4464
Revised: August 11, 2009
Accepted: August 18, 2009
Published online: September 21, 2009
A 55-year-old male patient with hepatitis B-related liver cirrhosis was found to have advanced hepatocellular carcinoma. His AFP was initially 9828 μg/L and rapidly dropped to 5597 μg/L in ten days after oral sorafenib treatment. However, he developed acute renal failure, hyperkalemia, and hyperuricemia 30 d after receiving the sorafenib treatment. Tumor lysis syndrome was suspected and intensive hemodialysis was performed. Despite intensive hemodialysis and other supportive therapy, he developed multiple organ failure (liver, renal, and respiratory failure) and metabolic acidosis. The patient expired 13 d after admission.
- Citation: Huang WS, Yang CH. Sorafenib induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol 2009; 15(35): 4464-4466
- URL: https://www.wjgnet.com/1007-9327/full/v15/i35/4464.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4464
Molecular targeted therapy is currently the new treatment modality for advanced cancer. Sorafenib is an oral multi-kinase inhibitor that blocks tumor growth and cell proliferation by targeting Raf kinase, VEGFR-2, VEGFR-3, and PDGFR-β[1]. Sorafenib was approved by the FDA in 2007 to treat hepatocellular carcinoma (HCC)[2]. An uncontrolled sorafenib phase II study in 137 patients with advanced HCCs confirmed that the higher pERK patients had a longer time to progression compared to the lower pERK patients[3,4]. Therefore, sorafenib is a specific target drug for the Raf/MEK/ERK pathway and pERK could be a useful biomarker. Sorafenib showed efficacy in prolonging patients’ survival in a previous SHARP trial[5]. The study obtained an overall 44.9% improvement and the sorafenib group had a partial response rate of 2.3% (n = 7) vs placebo of 0.7% (n = 2). Sorafenib opens a new era for advanced HCC treatment. However, that sorafenib might induce tumor lysis syndrome (TLS) had never been reported in previous studies. Here, we report sorafenib induced TLS in an advanced HCC male patient. This reported case developed multiple organ failure (liver failure, renal failure and respiratory failure) and metabolic acidosis. Despite intensive hemodialysis and other supportive therapy, he succumbed to the complication of tumor lysis syndrome.
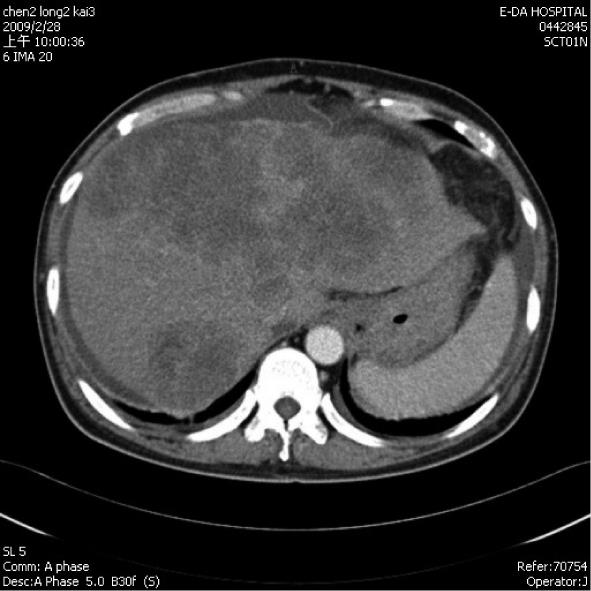
A 55-year-old male hepatitis B carrier visited our Clinic with the symptoms of general fatigue, abdominal fullness, poor appetite and body weight loss of 5 kg over 2 mo. An abdominal sonography showed multiple mixed-hypoechoic masses (larger than 12 cm in aggregated diameter or more than 50% of whole liver capacity) involving both lobes of the liver. Additionally, there was left portal vein thrombosis and decompensated cirrhosis with ascites. Laboratory data showed that HBeAg was negative, creatinine was 106 (97-133) μmol/L. AST was 435 (0-38) U/L, ALT was 79 (0-44) U/L, total bilirubin was 74 (10-24) μmol/L, prothrombin time was 12.7 s (control was 10.7 s), albumin was 37 (35-50) g/L, and α-feto protein (AFP) was 9828.63 (1.09-8.04) μg/L. The Child-Turcotte-Pugh score was eight. His abdominal triphase computed tomography (CT) confirmed the sonography findings, namely, advanced HCC in a background of liver cirrhosis (Figure 1). He was given oral sorafenib 400 mg twice every day and oral diuretics (furosemide 40 mg once, spironolactone 25 mg three times every day since February 28, 2009). Ten days later (March 9, 2009), his AFP level dropped to 5597.52 μg/L and his creatinine level was 80 μmol/L. Thirty days after he received sorafenib treatment, he was found to have profound jaundice with total bilirubin of 344 (10-24) μmol/L, decreased urine output, and general weakness. Abdominal sonography showed no ascites. The laboratory data revealed a creatinine level of 177 μmol/L, BUN at 34 (3.6-7.1) mmol/L, serum sodium at 112 (135-148) mmol/L, potassium at 7.0 (3.5-5.3) mmol/L, ammonia at 92 (16-41) μmol/L, AST at 698 (0-38) U/L, and ALT at 297 (0-44) U/L. Acute renal failure with hyperkalemia was diagnosed and he was treated intravenously with calcium gluconate, sodium bicarbonate, glucose water with insulin, corticosteroid, and oral kalimate (calcium polystyrene sulfonate). The HBV DNA (Simens, VERSAN® HBV DNA 3.0, Australia) was shown to be 48 800 IU/ml (273 288 copies/mL) and antiviral therapy with entecavir (0.5 mg on day one, followed by 0.5 mg every 3 d) for the suspicion of liver failure secondary to hepatitis B exacerbation. On the next day, his serum potassium was 7.2 (3.5-5.3) mmol/L. Emergency hemodialysis began and sorafenib treatment was discontinued. On the next day, his serum potassium dropped to 4.5 mmol/L, sodium to 128 mmol/L, uric acid to 547 (238-506) μmol/L, and his creatinine level was 248 μmol/L. Half of the previous dose of sorafenib was restarted with hydration of 2000-3000 mL saline for suspicious dehydration and diuretics were discontinued. Two days later, his potassium level had increased to 5.0 mmol/L, uric acid was 851 μmol/L, phosphorus was 5.4 mmol/L (2.7-4.5) and his creatinine level was 309 μmol/L. Recurrence of TLS was suspected and sorafenib was discontinued. Allopurinol 300 mg (po st) and 600 mg (po every day) was started. Another course of emergency hemodialysis was performed after the patient failed to respond to the medication. His general condition stabilized but multiple organ failure and metabolic acidosis persisted, despite another course of hemodialysis. He expired 13 d after admission.
Tumor lysis syndrome (TLS) is a life threatening oncological emergency. It is the result of rapid breakdown of tumor cells with release of cellular contents, such as potassium, uric acid, phosphate, purine, and proteins into the circulation[6]. Risk factors for TLS include the following: (1) hematological malignancy; (2) high proliferation rate tumors; (3) chemo- or radio-sensitive tumors; (4) large tumor burden; and (5) dehydrated state or patients with preexisting renal impairment[6,7]. In hematological malignancy, TLS may develop rapidly within 24-48 h or it may develop one week after effective chemotherapy. HCC is a solid tumor with slower growth rate than that of hematological malignancies. Therefore, the TLS developed one month after treatment with sorafenib in this case. His renal function was within normal limits before, and 10 d after, treatment. The occurrence of TLS in this patient could be the combination of effective therapy due to sorafenib chemosensitivity of the HCC, the use of diuretics with secondary dehydration, and advanced HCC with large tumor burden. Diuretics induced secondary dehydration could result in acute renal failure, but after effective hydration and supportive therapy, hyperkalemia and hyperuricemia persisted. Therefore, sorafenib induced TLS was the more likely cause of the symptoms described.
The degree of abnormities in his uric acid and potassium fulfill the criteria of TLS as defined by Cairo-Bishop[8]; however, our case had no initial calcium data due to hyperkalemia treated with calcium gluconate. The initial serum calcium was 2.32 (2.2-2.6) mmol/L and dropped to 1.65 mmol/L ten days later. The decrease of calcium level over 25% also matched the Cairo-Bishop definition of TLS[8]. Hypophosphatemia did happen in the sorafenib treatment group (11/297) vs control group (2/302) (P < 0.001) in Llovet et al’s SHARP study[5]. Phosphorus in our case was 5.4 (2.7-4.5) mmol/L and did not go over 6.5 mmol/L as Cairo-Bishop definition of TLS, which might be the result of sorafenib effect.
The prognosis of TLS is poor with a high mortality rate in solid tumor. Prevention of TLS by hydration, alkalization, or therapy to correct metabolic disturbance can be effective for some hematological malignancies[6-9]. Based on previous reports, hemodialysis can be helpful in improving the clinical course and can even cure TLS in some patients[6-10]. This case had a satisfactory initial course following the sorafenib therapy; however, his condition deteriorated after TLS developed. Llovet et al[5] reported that sorafenib could significantly extend median survival and prolong the time of radiologically evidenced progression by approximately three months compared with a placebo in individuals with advanced hepatocellular carcinoma (HCC) with well-preserved liver function, naive to systemic therapy. In comparison with the control group given the placebo, they reported that sorafenib was significantly associated with more diarrhea of any grade, weight loss, hand-foot skin reaction, alopecia, anorexia, and voice changes[5]. However, in their report, TLS was not mentioned. We believed that TLS is a rare side effect of sorafenib treatment. Once it occurred, however, TLS can induce multiple organ failure with a catastrophic outcome.
In conclusion, in the therapy of HCC with sorafenib, especially in patients with high tumor burden and showing a good initial response, the possible occurrence of TLS should be kept in mind and appropriate laboratory data should be monitored. Once TLS developed, the prognosis was poor even when intensive hemodialysis was given.
Peer reviewers: Hartmut Jaeschke, Professor, Liver Research Institute, University of Arizona, College of Medicine, 1501 N Campbell Ave, Room 6309, Tucson, Arizona 85724, United States; Dr. Yogesh K Chawla, Professor, Department of Hepatology, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India
S- Editor Li LF L- Editor Stewart GJ E- Editor Yin DH
| 1. | Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099-7109. |
| 2. | FDA approves Nexavar for patients with inoperable liver cancer. Available from: URL: http://www.fda.gov/bbs/topics/NEWS/2007/NEW01748.html. Retrieved December 3, 2007. |
| 3. | Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293-4300. |
| 4. | Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851-11858. |
| 5. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. |
| 6. | Davidson MB, Thakkar S, Hix JK, Bhandarkar ND, Wong A, Schreiber MJ. Pathophysiology, clinical consequences, and treatment of tumor lysis syndrome. Am J Med. 2004;116:546-554. |
| 7. | Rampello E, Fricia T, Malaguarnera M. The management of tumor lysis syndrome. Nat Clin Pract Oncol. 2006;3:438-447. |
| 8. | Gobel BH. Management of tumor lysis syndrome: prevention and treatment. Semin Oncol Nurs. 2002;18:12-16. |
| 9. | Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127:3-11. |