Published online Sep 21, 2009. doi: 10.3748/wjg.15.4446
Revised: July 16, 2009
Accepted: July 23, 2009
Published online: September 21, 2009
A 67-year-old man with celiac disease developed recurrent diarrhea, profound weakness and weight loss, with evidence of marked protein depletion. His clinical course was refractory to a strict gluten-free diet and steroid therapy. Postmortem studies led to definition of unrecognized collagenous sprue that caused ulceration and small intestinal perforation. Although PCR showed identical monoclonal T-cell populations in antemortem duodenal biopsies and postmortem jejunum, careful pathological evaluation demonstrated no frank lymphoma. Rarely, overt or even cryptic T-cell lymphoma may complicate collagenous sprue, however, small intestinal ulcers and perforation may also develop independently. The dramatic findings here may reflect an underlying or early molecular event in the eventual clinical appearance of overt T-cell lymphoma.
- Citation: Freeman HJ, Webber DL. Free perforation of the small intestine in collagenous sprue. World J Gastroenterol 2009; 15(35): 4446-4448
- URL: https://www.wjgnet.com/1007-9327/full/v15/i35/4446.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4446
Collagenous sprue was initially observed to complicate the clinical course of celiac disease[1]. The disorder is characterized by a pathologically distinctive lesion with the appearance of a patchy or diffusely thickened sub-epithelial collagen band in flattened small intestine. Most patients with extensive collagenous sprue have a progressive and deteriorating clinical disorder marked by severe pan-malabsorption of multiple nutrients, profound weight loss, and evidence of marked protein loss that fails to respond to a gluten-free diet[2]. Etiology and pathogenesis of collagenous sprue remain obscure, although inherited and other factors may play a role[2]. A number of treatment regimens, including steroids, have been reported anecdotally to show benefit[3,4].
Although only limited information on the natural history, pathogenesis and clinical outcome of collagenous mucosal disorders of the small and large intestine are available, occasionally, lymphoma may complicate collagenous sprue[5-8] and collagenous colitis[9]. In some of these, the lymphoma was already extensive and advanced[5-7], but in another, synchronous development of collagenous sprue and lymphoma occurred, which suggested to the authors that collagenous sprue may represent a noninvasive component of the lymphomatous process[8]. Collagenous enterocolitis has also been noted in a patient with localized colon cancer, with complete reversion to normal following resection of the malignant lesion[10]. It was hypothesized that intestinal sub-epithelial collagen deposition with concomitant malignancy represents a paraneoplastic morphologic marker that may be completely reversible[10].
In November 2006, a 67-year-old man was evaluated initially in his community hospital for progressive fatigue, diarrhea and weight loss of 15 kg over 6-8 mo. Physical examination revealed bilateral lower limb edema. Initial laboratory studies were normal except for hypoalbuminemia (serum albumin, 13 g/L; normal, 35-50) and elevated tissue transglutaminase (tTG) antibody of 35 U (normal, < 20). Endoscopy of his upper and lower gastrointestinal tract appeared normal, but duodenal biopsies showed typical changes of untreated celiac disease, with crypt hyperplastic villous atrophy. A gluten-free diet was started but his clinical course became complicated by progressive dyspnea that required hospital re-admission. Computed tomography (CT) scanning showed bilateral pulmonary emboli and pleural effusions with prominent mesenteric lymph nodes. He was started on anticoagulation and was maintained on coumadin for the next year. By February 2007, his fatigue resolved and exercise tolerance improved. His diarrhea resolved completely and his weight increased and normalized. Peripheral edema resolved and his serum albumin increased to 35 g/L. A repeat CT scan showed resolution of chest findings. Mesenteric lymph nodes were not enlarged.
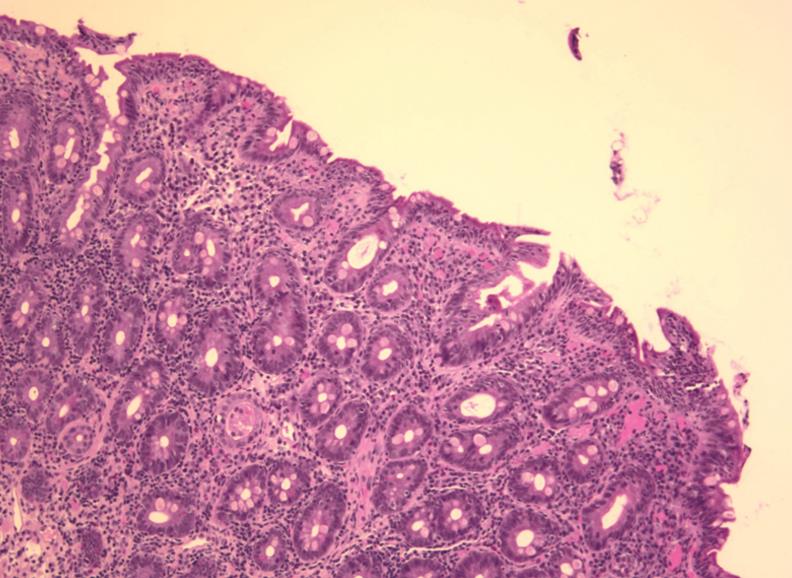
In November 2007, diarrhea and weight loss recurred in spite of strict adherence to a gluten-free diet. Over the next month, recurrent edema also developed in his lower extremities up to his waist. Physical examination now revealed generalized wasting, brawny induration with hyperpigmentation of his lower extremities, bilateral ichthyosis, especially over the anterior tibial surfaces, and pitting edema. Laboratory investigations, including hemogram, were normal except for hypoproteinemia (35 g/L; normal, 62-82 g/L) and hypoalbuminemia (11 g/L; normal, 34-50 g/L). Serum carotene was reduced to < 0.5 μmol/L (normal, 1.1-3.7 μmol/L), while serum tTG assay was normal (9.1 U), with a normal serum IgA (1.12 g/L; normal, 0.70-4.00 g/L). Endoscopy was normal except for scalloping of proximal duodenal mucosal folds. Biopsies showed crypt hyperplastic villous atrophy, despite a gluten-free diet (Figure 1). Fresh biopsy material was submitted for PCR, but analysis failed. Endoscopic biopsies of stomach and colon were normal with no intra-epithelial lymphocytosis. A barium study of the small intestine showed dilation of small bowel loops but no discrete lesion, abnormal loop separation or stricture. Gluten-free diet was continued but his weight fell further by 5 kg. A contrast CT scan of the chest, abdomen and pelvis showed no new findings, although a left common femoral vein thrombus was seen. Mesenteric lymph nodes were normal. Oral budesonide 3 mg/d was prescribed[11].
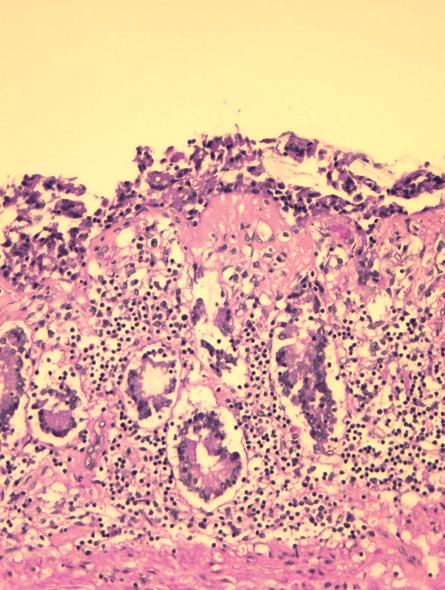
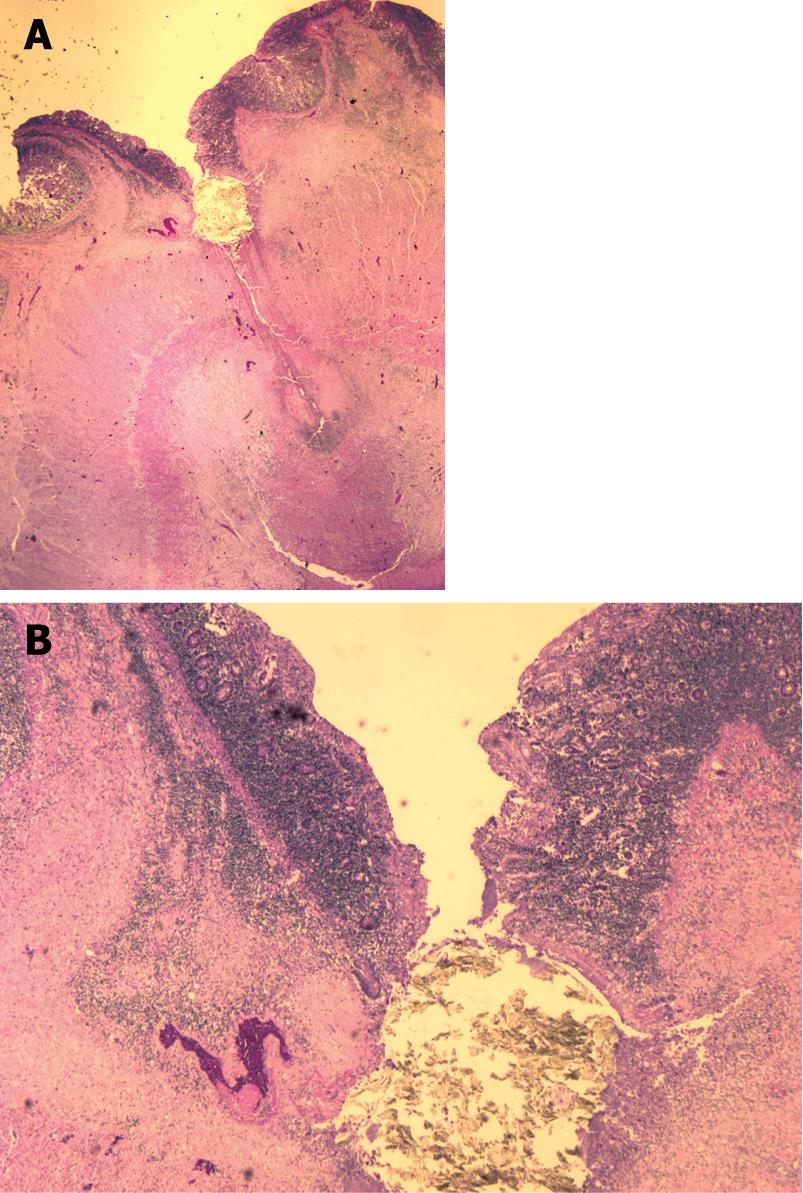
He deteriorated suddenly over 2 d with abdominal pain, worsening weakness and continued weight loss. He was readmitted to his community hospital and parenteral nutritional support was initiated. In hospital, he deteriorated rapidly with fluctuations in consciousness and died 3 d later. Autopsy showed extensive collagenous sprue (Figure 2) in the jejunum and ileum with a focal jejunal ulcer and perforation (Figure 3). Careful review failed to show lymphoma. Immunohistochemical studies (BCCA Lymphoma Referral Laboratory, Vancouver, BC, Canada) showed CD3-positive T-cells that expressed CD2 and CD7, but failed to express CD4, CD8 and CD5. CD3-positive T cells showed intracytoplasmic rather than membrane labeling. Immunostaining for CD56 was negative and cells were negative for Epstein-Barr virus by an EBER ISH stain. Stains for cytotoxic markers TIA-1, perforin and granzyme B showed that the majority of cells were negative. PCR of the previous antemortem duodenal biopsies, along with a postmortem section of jejunum, showed an identical monoclonal T-cell band.
This report documents collagenous sprue in a patient with recurrent diarrhea and profound weight loss, with severe protein depletion despite a gluten-free diet and steroid therapy. Collagenous sprue appeared to complicate preexistent celiac disease that initially had responded clinically to administration of a gluten-free diet, similar to the original description of this disorder[1]. Here, however, symptoms redeveloped despite a strict gluten-free diet and oral budesonide[11]. This appeared to be related to collagenous sprue complicated by ulceration and perforation of the distal small bowel. Even though an identical monoclonal T-cell population was defined molecularly by PCR in antemortem duodenal biopsies and postmortem jejunal sections, careful pathological review failed to reveal any neoplastic lymphoid cells. It is likely that this very unusual complication of ulceration and perforation of the small intestine reflects a stage of development of cryptic T-cell lymphoma[5], which may precede the appearance of frank pathologically defined lymphoma. Rarely, ulceration and free perforation of the small intestine may complicate celiac disease as the initial manifestation[12]. However, the sequence of events here in collagenous sprue with ulceration and perforation has not been described previously and provides an additional dimension to our understanding of the natural history and clinical outcome of this very unusual small intestinal disorder.
While development of small intestinal ulceration and perforation clearly led to our patient’s demise, it may not be an entirely surprising event in the clinical course of collagenous sprue. A number of factors may play a role. First, mucosal thickness in collagenous sprue appears to be reduced markedly so that complication of a focal ulcer with free perforation might more readily occur. Second, lymphoma has been noted previously to be responsible for free perforation of the small intestine in celiac disease[12], and a superimposed lymphoma in collagenous sprue could occur that might present with free perforation. Finally, the collagenized layer in the small intestine may be more susceptible or more prone to perforation. Indeed, in collagenous colitis, free and spontaneous perforation of the colon has been described[13], along with a propensity for instrument-induced colon fractures and perforation[14]. Since there is evidence that the collagen deposited in the small and large bowel in these disorders is similar[2], and has been hypothesized to affect the integrity of the intestinal wall[14], this outcome with ulceration and perforation of the small bowel during the clinical course of collagenous sprue may occur more frequently than is appreciated at present.
Peer reviewer: Dr. Daniel R Gaya, Gastrointestinal Unit, Molecular Medicine Centre, School of Molecular and Clinical Medicine, University of Edinburgh, Western General Hospital, Crewe Road, Edinburgh EH4 2XU, United Kingdom
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
| 1. | Weinstein WM, Saunders DR, Tytgat GN, Rubin CE. Collagenous sprue--an unrecognized type of malabsorption. N Engl J Med. 1970;283:1297-1301. |
| 2. | Freeman HJ. Collagenous mucosal inflammatory diseases of the gastrointestinal tract. Gastroenterology. 2005;129:338-350. |
| 3. | Holdstock DJ, Oleesky S. Successful treatment of collagenous sprue with combination of prednisolone and gluten-free diet. Postgrad Med J. 1973;49:664-667. |
| 4. | Freeman HJ, Davis JE, Myers DM. Complete histological resolution of collagenous sprue. Can J Gastroenterol. 2004;18:333-336. |
| 5. | Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, Macintyre E, Cerf-Bensussan N, Brousse N. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356:203-208. |
| 6. | Robert ME, Ament ME, Weinstein WM. The histologic spectrum and clinical outcome of refractory and unclassified sprue. Am J Surg Pathol. 2000;24:676-687. |
| 7. | Freeman HJ. Collagenous sprue associated with an extensive T-cell lymphoma. J Clin Gastroenterol. 2003;36:144-146. |
| 8. | Medlicott SA, Beck PL, Loken S, Crabtree T. Synchronous collagenous sprue and enteropathy-type T cell lymphoma: variants of the same disease. Can J Gastroenterol. 2004;18:329-332. |
| 9. | Freeman HJ. Lymphoproliferative disorders in collagenous colitis. Inflamm Bowel Dis. 2005;11:781-782. |
| 10. | Freeman HJ, Berean KW. Resolution of paraneoplastic collagenous enterocolitis after resection of colon cancer. Can J Gastroenterol. 2006;20:357-360. |
| 11. | Daum S, Ipczynski R, Heine B, Schulzke JD, Zeitz M, Ullrich R. Therapy with budesonide in patients with refractory sprue. Digestion. 2006;73:60-68. |
| 12. | Freeman HJ. Free perforation due to intestinal lymphoma in biopsy-defined or suspected celiac disease. J Clin Gastroenterol. 2003;37:299-302. |
| 13. | Freeman HJ, James D, Mahoney CJ. Spontaneous peritonitis from perforation of the colon in collagenous colitis. Can J Gastroenterol. 2001;15:265-267. |
| 14. | Sherman A, Ackert JJ, Rajapaksa R, West AB, Oweity T. Fractured colon: an endoscopically distinctive lesion associated with colonic perforation following colonoscopy in patients with collagenous colitis. J Clin Gastroenterol. 2004;38:341-345. |