Published online Sep 21, 2009. doi: 10.3748/wjg.15.4444
Revised: August 13, 2009
Accepted: August 20, 2009
Published online: September 21, 2009
Radiotherapy for locally advanced pancreatic cancer is technically difficult and frequently associated with high-grade digestive toxicity. Helical tomotherapy (HT) is a new irradiation modality that combines megavoltage computed tomography imaging for patient positioning with intensity-modulated fan-beam radiotherapy. Its recent availability opens new fields of exploration for pancreatic radiotherapy as a result of its ability to tailor very well-defined dose distributions around the target volumes. Here, we report the use of HT in two patients with locally advanced pancreatic cancer. Doses to the bowel, kidneys and liver were reduced significantly, which allowed for excellent treatment tolerance without any high-grade adverse effects in either patient.
- Citation: Chargari C, Campana F, Beuzeboc P, Zefkili S, Kirova YM. Preliminary experience of helical tomotherapy for locally advanced pancreatic cancer. World J Gastroenterol 2009; 15(35): 4444-4445
- URL: https://www.wjgnet.com/1007-9327/full/v15/i35/4444.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4444
Radiotherapy for locally advanced pancreatic cancer is technically difficult and potentially deleterious, because of the proximity of adjacent vital organs. Although intensity-modulated radiotherapy recently has proven its efficacy in sparing vital organs from irradiation, digestive toxicity remains an important limiting factor[1]. Helical tomotherapy (HT) is a new irradiation modality that combines intensity-modulated fan-beam radiotherapy with megavoltage computed tomography imaging for patient positioning[2]. Its availability recently has opened new fields of exploration for pancreatic radiotherapy as a result of its ability to tailor very well-defined dose distributions around the target areas.
To the best of our knowledge, this report is the first to explore the use of HT and its potential for sparing vital organs in two patients with locally advanced pancreatic cancer.
Patient 1 was a 59-year-old man whose pancreatic cancer was discovered fortuitously in September 2007 at a follow-up examination for previous prostatic cancer. Clinical examination was normal. Computed tomography (CT) showed a mass in the head of the pancreas, which measured 33 mm in the greatest dimension, with portal vein thrombosis. Endosonography demonstrated infiltration of the superior mesenteric vein, which reached the superior mesenteric artery. Histological examination confirmed an adenocarcinoma. Patient 2 was a 62-year-old woman who presented in April 2008 with abdominal pain and weight loss. There was no abnormality at clinical examination. The CT scan showed diffuse dilatation of the duct of Wirsung, with an irregular mass in the pancreatic isthmus. Endosonography revealed a mass in the isthmus, which measured 25 mm in the greatest dimension, with tumor extension to posterior peripancreatic fat tissue and massive splenic artery encasement. Histological examination confirmed a papillary mucinous adenocarcinoma. For both patients, laboratory tests revealed no biological abnormality and no elevation of tumor markers. No lymph node or distant metastases were observed at initial staging.
The patients were treated with induction chemotherapy of gemcitabine (1000 mg/m² on day 1 every week) and oxaliplatin (100 mg/m² on day 1 every 2 wk). In patient 1, assessment after six cycles showed no local or metastatic evolution. In patient 2, assessment after five cycles showed partial regression of the pancreatic mass, which measured 18 mm in the greatest dimension, and no metastatic extent. Patient 1 and 2 were referred for preoperative and exclusive chemoradiation, respectively.
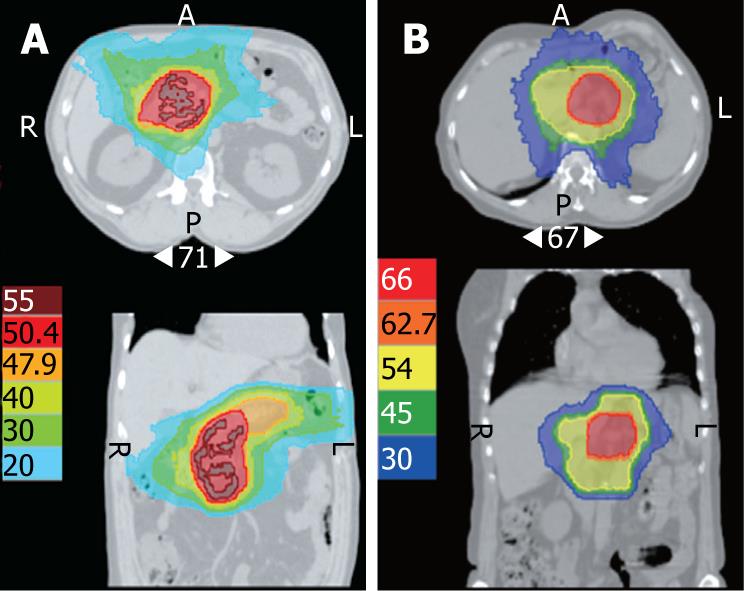
In patient 1, HT delivered 45 Gy to the whole pancreas, and 50.4 Gy to the tumoral mass, using 6-MV photons, at 1.8 Gy per daily fraction, for a total duration of 39 d. In patient 2, HT delivered 45 Gy to the whole pancreas, and 66 Gy to the tumoral mass, using 6-MV photons, at 2 Gy per daily fraction, for a total duration of 64 d. For both patients, capecitabine was given at 3000 mg/m² per day in two divided doses, 5 d/wk, concurrently with radiotherapy. Chemoradiation was marked by grade 1 nausea in both patients. No other acute toxicity was observed. For patient 1, abdominal pain disappeared after completion of chemoradiation. However, he developed early hepatic metastases and was given palliative chemotherapy with gemcitabine and oxaliplatin, followed by erlotinib for progressive disease. Patient 1 maintained a complete local response that was still present at 18 mo follow-up. Patient 2 remains disease-free at 6 mo follow-up.
Conventional radiotherapy for pancreatic cancer can involve some parts of the duodenum, stomach, small intestine, and kidneys, which results in frequent severe acute gastrointestinal toxicity when chemotherapeutic agents are administered concurrently with radiotherapy. This can lead to frequent treatment disruption and limits the delivery of a sufficient radiation dose. Moreover, high-grade toxicity compromises quality of life, which is an important end-point in patients with poor prognosis[3]. Since gemcitabine and oxaliplatin have improved the outcome of patients with locally advanced or metastatic disease, by improving survival with clinical benefits, every effort should be made to avoid additional radiation-induced digestive toxicity that might potentially compromise the delivery of subsequent chemotherapy[4].
For both of our patients who were at risk for high-grade toxicity, HT provided a potential tool to decrease the risk of gastrointestinal toxicity[5]. As a result of isodose conformation, radiation doses to the bowel, right kidney and liver were reduced significantly, which allowed for excellent treatment tolerance without any high-grade adverse effects in either patient. It is reasonable to assume that HT may have spared structures that normally would not have been spared with more conventional 3D conformal radiotherapy (Figure 1). However, very low doses are distributed to larger volumes. While the problem of radiation-induced malignancy in such patients with poor prognosis should not be considered to be of primary importance, further assessment of HT remains necessary.
The present report highlights the potential of HT to reduce the dose of radiation delivered to vital organs, thus improving tolerance to irradiation.
Peer reviewer: Ian C Roberts-Thomson, Professor, Department of Gastroenterology and Hepatology, The Queen Elizabeth Hospital, 28 Woodville Road, Woodville South 5011, Australia
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
| 1. | Ben-Josef E, Shields AF, Vaishampayan U, Vaitkevicius V, El-Rayes BF, McDermott P, Burmeister J, Bossenberger T, Philip PA. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;59:454-459. |
| 2. | Mackie TR, Holmes T, Swerdloff S, Reckwerdt P, Deasy JO, Yang J, Paliwal B, Kinsella T. Tomotherapy: a new concept for the delivery of dynamic conformal radiotherapy. Med Phys. 1993;20:1709-1719. |
| 4. | Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, Zaniboni A, Ducreux M, Aitini E, Taïeb J. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509-3516. |
| 5. | Chargari C, Zefkili S, Kirova YM. Potential of helical tomotherapy for sparing critical organs in a patient with AIDS who was treated for Hodgkin lymphoma. Clin Infect Dis. 2009;48:687-689. |