Published online Sep 21, 2009. doi: 10.3748/wjg.15.4434
Revised: August 13, 2009
Accepted: August 20, 2009
Published online: September 21, 2009
AIM: To compare the diagnostic accuracy of computed tomography (CT) and positron emission tomography (PET) for the preoperative detection of paraaortic lymph node (PAN) metastasis in patients with intra-abdominal malignancies.
METHODS: Sixty-six patients with intra-abdominal malignancies who underwent both CT and PET before lymphadenectomy were included in this study. Histopathologically, 13 patients had metastatic PAN, while 53 had non-metastatic PAN. The CT criteria for metastasis were: short diameter of > 8 mm, lobular or irregular shape, and/or combined ancillary findings, including necrosis, conglomeration, vessel encasement, and infiltration. The PET criterion was positive fluorodeoxyglucose (FDG) uptake. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of both modalities were compared with the pathologic findings, and the false positive and false negative cases with both CT and PET were analyzed.
RESULTS: The sensitivity, specificity, PPV, NPV, and accuracy of CT were 61.5%, 84.9%, 50%, 90% and 80.3%, respectively. For PET, the percentages were 46.2%, 100%, 100%, 88.3%, and 89.4%. Additionally, there were 8 false positive CT cases (8/53, 15.1%) and zero false positive PET cases. Of the 13 metastatic PANs, there were 5 false negative CT scans (38.5%) and 7 (53.9%) false negative PET scans.
CONCLUSION: For detecting PAN metastasis, CT is more sensitive than PET, while PET is more specific.
- Citation: Lee MJ, Yun MJ, Park MS, Cha SH, Kim MJ, Lee JD, Kim KW. Paraaortic lymph node metastasis in patients with intra-abdominal malignancies: CT vs PET. World J Gastroenterol 2009; 15(35): 4434-4438
- URL: https://www.wjgnet.com/1007-9327/full/v15/i35/4434.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4434
Paraaortic lymph node (PAN) metastasis is considered an important prognostic factor in several abdominal and pelvic malignancies, such as stomach cancer, colorectal cancer, cholangiocarcinoma, and cervical cancer[1-4]. While it is very important to evaluate PAN metastasis in preoperative evaluations, the correct diagnosis is not always definitively determined. Because of this, sampling and pathologic confirmation of the paraaortic nodes should be carried out before starting a radical operation, which is why many surgeons, including those in our hospital, perform paraaortic node dissection before radical surgery[5-8]. Although lymphadenectomy followed by histologic examination of the lymph nodes is still the gold standard for determining metastasis, doctors must be careful in using this technique because of the perioperative risks and postoperative complications of PAN dissection[3,9,10]. All of this makes preoperative, noninvasive imaging diagnosis of PAN metastasis very important[11,12].
For preoperative evaluation of abdominal malignancies, computed tomography (CT) has usually been used as the first-line examination. However, many investigators have reported CT to have low sensitivity and low specificity when used for lymph node (LN) diagnosis. Nowadays, positron emission tomography (PET) with fluorodeoxyglucose (FDG) has been recognized as a useful diagnostic technique in clinical oncology, not only for primary tumor evaluation, but also for detection of metastasis (including nodal metastasis)[13]. However, FDG-PET has also been reported to have low sensitivity for the detection of LN metastasis[14], and FDG-PET cannot determine the anatomical location of small lesions, such as lymph nodes[15]. To our knowledge, however, there has been limited study comparing CT with PET in evaluating PAN metastasis[16,17].
The purpose of our study is to compare the diagnostic accuracy of CT and FDG-PET in the preoperative detection of PAN metastasis in patients with an intra-abdominal malignancy. We also analyze the false positive and false negative cases of both CT and PET scans and suggest clinical guidelines for using CT and PET results.
The protocol for this study was approved by the Institutional Review Board at our institution and informed consent was not required. Our pathologic database was retrospectively searched from September 2002 to July 2006 for all patients who underwent paraaortic lymphadenectomy before or during a surgical resection operation. A total of 305 patients were selected from the database, and, among these, 113 had underlying intra-abdominal malignancies (excluding lymphoma). Finally, 66 patients (39 women and 27 men, age range 28-78 years, mean age 56 years) who underwent both CT and PET no ≤ 1 mo before a lymph node biopsy were chosen for the study. The underlying malignancies were as follows: hepatobiliary cancer (n = 11), pancreatic cancer (n = 10), colorectal cancer (n = 20), gastric cancer (n = 3), cervical cancer (n = 7) and tubo-ovarian cancer (n = 15).
For CT, all patients underwent single-section spiral CT (HiSpeed CT/I, GE Medical Systems, Milwaukee, WI) or multi-detector CT scanning (four detector row; Lightspeed Plus, GE Medical Systems, Milwaukee, WI or sixteen-detector row; Sensation 16, Siemens, Erlangen, Germany) according to an established protocol. A 60% iodinated contrast material [Iopromide (Ultravist); Schering, Berlin, diatrizoate meglumine (Hypaque) or iohexol (Omnipaque 300); Nycomed Amersham, Princeton, NJ] was administered intravenously at a rate of 2-4 mL/s by using an automatic power injector with a volume of 2 mL/kg, up to a maximum volume of 150 mL. Portal venous phase CT scans were obtained 70 s after initiating the contrast material injection, and the abdomen and pelvis, from the level of the hepatic dome to the ischial tuberosities, were scanned with a pitch of 1.0-1.5 and a reconstruction thickness of 3.0-5.0 mm. The transverse images were reconstructed with a soft-tissue algorithm.
For PET, patients fasted at least 4 h before intravenous injection of 18F-FDG, and scanning began 60 min later. Images from the neck to the proximal thigh were obtained either on an Advance PET scanner (GE advance, GE Medical Systems, Milwaukee, WI), with a spatial resolution of 5 mm in the center of the field of view, or an Allegro PET scanner (Allegro, Philips-ADAC medical systems, Cleveland, OH), with a spatial resolution of 5.3 mm in the center of the field of view. For the Advance scanner, approximately 370 MBq of 18F-FDG were intravenously injected, and the emission scan was acquired for 5 min per bed position in the 2-dimensional mode. The Allegro acquired data in the 3-dimensional mode after administration of 5.18 MBq (0.14 mCi)/kg of 18F-FDG. Transmission scans (3 min per bed position) to correct for non-uniform attenuation were obtained using point sources of 68Ge for the Advance or 137Cs for the Allegro. Transmission scans were interleaved with the multiple emission scans for the Allegro. The images were reconstructed using an iterative reconstruction algorithm: that is, either the ordered-subset expectation maximization for the Advance or the low-action maximal-likelihood algorithm for the Allegro.
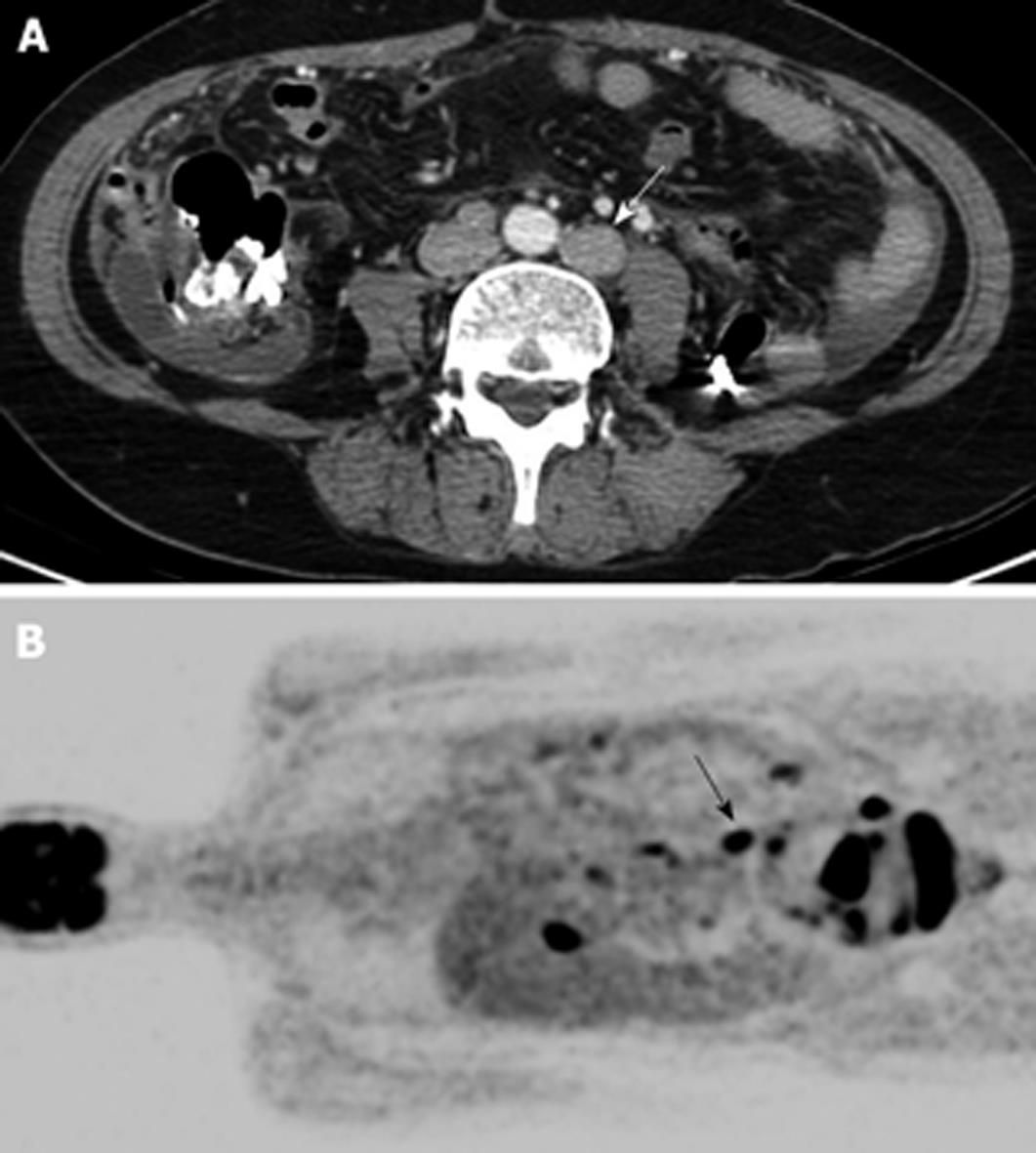
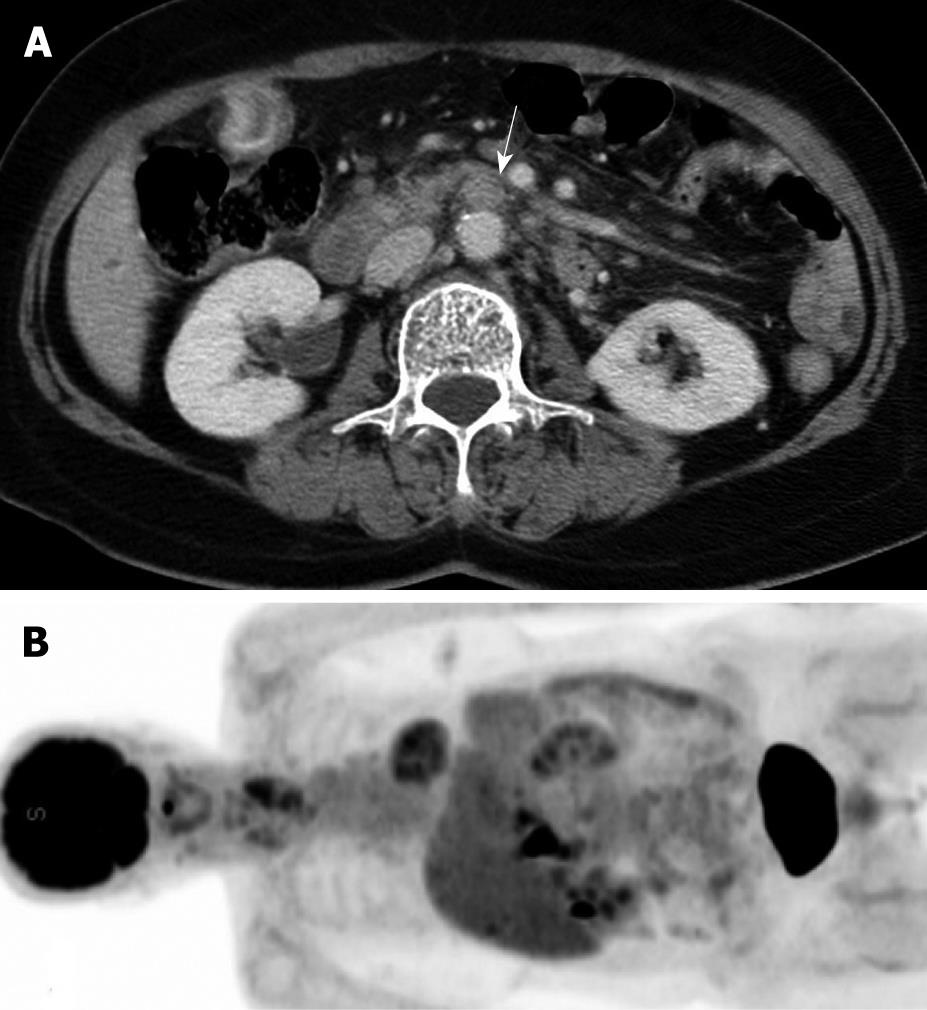
All imaging analysis was performed on a picture archiving and communication system (PACS) workstation (Centricity 1.0; GE Medical Systems). Two radiologists independently evaluated preoperative CT images in the 66 patients without knowledge of the final pathologic diagnoses. They considered the following criteria as the primary findings for a metastatic PAN: short diameter > 8 mm, lobular or irregular shape, and/or combined ancillary findings including necrosis, conglomeration, vessel encasement, and infiltration (Figure 1). Reviewers characterized the PAN as metastatic or non-metastatic. When discrepancies were detected, interpretations were achieved via consensus. Two experienced nuclear medicine physicians also interpreted the preoperative PET images, where the criterion was positive FDG uptake. When discrepancies were detected, interpretations were determined via consensus. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of CT and PET for the detection of metastatic PAN were then calculated.
As a second portion of our study, a third radiologist analyzed the cases of false diagnosis for both CT and PET scans. She also evaluated the relationship between primary tumors and PAN in terms of the FDG uptake of the primary tumors on PET.
In our 66 patients, we found 13 patients with metastatic paraaortic nodes and 53 patients with non-metastatic ones according to the pathologic results.
The sensitivity, specificity, PPV, NPV, and accuracy of CT for detecting PAN metastasis were 61.5% (8/13), 84.9% (45/53), 50% (8/16), 90% (45/50), and 80.3% (53/66), respectively. For PET, the results were 46.2% (6/13), 100% (53/53), 100% (6/6), 88.3% (53/60), and 89.4% (59/66), respectively. From these numbers, we can conclude that CT was more sensitive while PET was more specific, and the overall accuracy was slightly higher with PET.
With CT, 8 (15.1%) of the 53 non-metastatic nodes were false positively diagnosed (Figure 2). One of these cases showed central necrosis. There were no false positives using PET, as all 53 showed no FDG uptake.
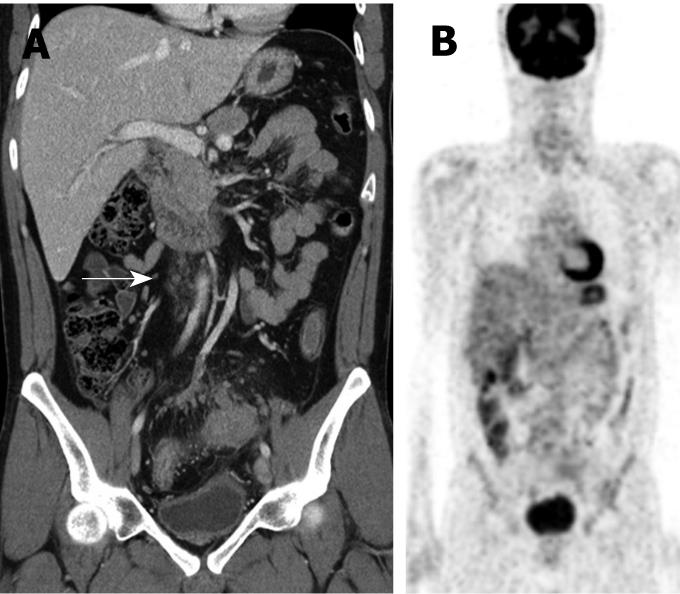
For the 13 metastatic nodes, 5 cases (38.5%) on CT, 7 cases (53.9%) on PET, and 4 cases (30.8%) on both CT and PET were false negatively diagnosed. Among the false negative CT cases, only one (1/5, 20%) showed positive FDG uptake on PET. Among the false negative PET cases, 3 (3/7, 42.9%) were diagnosed as malignant nodes on CT (Figure 3).
In terms of the relationship between a primary tumor and PAN on FDG uptake, six of the seven false negative metastatic PAN cases showed high FDG uptake on the primary tumor. Only one patient with primary stomach cancer with ovarian metastasis showed negative FDG uptake on both the metastatic PAN and the primary tumor. A total of 3 cases (3/66, 4.5%) showed no FDG uptake on the primary tumor, and one of these (1/3, 33%) showed negative FDG uptake on the metastatic PAN.
Our study shows that CT is more sensitive than PET, while PET is more specific than CT for the diagnosis of metastatic PAN in patients with abdominal malignancies.
PET has generally been considered a sensitive tool for detecting distant metastasis[13,18,19]. In daily practice, PET detects some lesions that are difficult to detect by CT, such as mesenteric or pelvic nodes between bowel loops. However, when considering only PAN, CT showed more sensitivity than PET in our study.
PET is a functional imaging modality based on the increased glucose metabolism of physiologic or pathologic conditions. Because of this, PET has a limitation associated with metabolism: the primary tumor must have a strong avidity for 18F-FDG, which may or may not be the case, depending on the histopathologic type of the tumor[14,20]. Low metabolic activity can cause a false negative PET scan[13], while a non-tumorous hypermetabolic condition, such as a systemic inflammation or an infection, can lead to false positive uptake[13]. The usefulness of PET imaging is also limited in patients with uncontrolled diabetes mellitus (> 150 mg/dL)[14].
In our study, PET (53.9%) showed higher false negative rates than CT (38.5%) in metastatic PAN. One false negative metastatic PAN case (14.3%) also showed no FDG uptake on the primary tumor. The FDG activity of the primary tumor may reflect that of the metastatic PAN, meaning more careful attention is required to diagnose PAN if the primary tumor shows no FDG uptake.
Among the 7 false negative PET cases, 3 (42.9%) were diagnosed as metastatic PAN using CT because they showed necrosis or conglomeration, even though the short diameter was < 8 mm. This means that CT may be helpful in the characterization of false negative PET cases.
CT also showed higher false positive rates than PET in our study. Many studies discuss the criteria for the detection of metastatic lymph nodes on CT, with size as the most frequently used parameter[14,19,21-24]. However, this places a major limitation on CT, as it will be unable to detect small, metastatic lymph nodes and might misrepresent large, reactive lymph nodes[13,19]. All of the false positive cases in our study showed no FDG uptake, which suggests that FDG-PET may be very useful for increasing the specificity of CT.
Integrated PET-CT has been introduced into clinical practice, providing shorter total imaging time and a reduction in the number of ambiguous lesions[25,26]. Moreover, some researchers have reported that PET-CT improves diagnostic accuracy when compared with PET alone in abdominal and pelvic cancer[27]. Recent studies report the sensitivity, specificity, and accuracy of PET-CT for lymph node metastasis as 28.6%-51.7%, 92.9%-99.8%, and 75.0%-99.8%, respectively[15,17,28], but this still shows the lower sensitivity and higher specificity of PET-CT. While the role of CT in PET-CT is usually limited to anatomic localization and attenuation correction[25], the results of our study suggest that CT is useful not only for anatomic localization, but also for a reduction in the false negative rate of PET. Previous studies have also reported that dedicated CT scans do additional work in some cases, such detecting as stomach cancer, liver cancer and mucinous primary or metastatic tumors[19,26,29]. Moreover, there are some reports about integrated PET/contrast-enhanced CT as an accurate modality for assessing colorectal cancer in recent studies[30,31]. However, the usefulness of integrated PET/contrast-enhanced CT in other malignancies is still being debating and additional study of this topic is needed.
There are some limitations to our study. First, the diagnoses were variable with intra-abdominal malignancy (including hepatobiliary cancer, gastrointestinal cancer and gynecologic cancer) except lymphoma. Tumor characteristics on PET and lymphatic drainage pattern can be different between these malignancies. The second limitation is that we evaluated the data not on a per-node basis, but on a per-case basis. This is a limitation of retrospective study, and further evaluation with prospective study is needed.
In conclusion, CT is more sensitive than PET for detecting PAN metastasis, but PET is more specific. PET is useful for ruling out enlarged reactive lymph nodes and reducing the false positive rate of CT scans alone. To reduce the false negative rate of PET, CT can be helpful by showing necrosis or other ancillary findings of PAN. These results all suggest that the two modalities are complementary to each other in the diagnosis of PAN metastasis.
Paraaortic lymph node (PAN) metastasis is considered an important prognostic factor in several abdominal and pelvic malignancies. However, the correct diagnosis is not always definitively determined. Pathologic confirmation before starting a radical operation has perioperative risks and postoperative complications. Therefore, preoperative, noninvasive imaging diagnosis of PAN metastasis is very important.
For preoperative evaluation of abdominal malignancies, computed tomography (CT) has usually been used as the first-line examination. Positron emission tomography (PET) with fluorodeoxyglucose (FDG) has been recognized as a useful diagnostic technique in clinical oncology. However, there has been limited study comparing CT with PET in evaluating PAN metastasis. The aim of our study is to compare the diagnostic accuracy of CT and FDG-PET in the preoperative detection of PAN metastasis in patients with an intra-abdominal malignancy.
CT is more sensitive than PET for detecting PAN metastasis, but PET is more specific. PET is useful for ruling out enlarged reactive lymph nodes and reducing the false positive rate of CT scans alone. To reduce the false negative rate of PET, CT can be helpful by showing necrosis or other ancillary findings of PAN.
CT and PET are complementary to each other in the diagnosis of PAN metastasis in patients with an intra-abdominal malignancy.
Preoperative evaluation of paraaortic lymph node metastasis is very important in intra-abdominal malignancy. The results of this study are interesting as a preliminary result. It may be a first step to confirm the results of a prospective study in the future.
Peer reviewer: Reiji Sugita, MD, Department of Radiology, Sendai City Medical Center, 5-22-1, Tsurugaya, Miyagino-ku, Sendai 983-0824, Japan
S- Editor Tian L L- Editor O’Neill M E- Editor Yin DH
| 1. | Min BS, Kim NK, Sohn SK, Cho CH, Lee KY, Baik SH. Isolated paraaortic lymph-node recurrence after the curative resection of colorectal carcinoma. J Surg Oncol. 2008;97:136-140. |
| 2. | Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, Williams CJ. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001;358:781-786. |
| 3. | Kosaka T, Usami K, Ueshige N, Hasegawa T, Yoshitani S, Sugaya J, Nakano Y, Takashima S. Paraaortic lymph node dissection for gastric cancer in 244 consecutive cases. Hepatogastroenterology. 2006;53:629-633. |
| 4. | Uenishi T, Yamazaki O, Horii K, Yamamoto T, Kubo S. A long-term survivor of intrahepatic cholangiocarcinoma with paraaortic lymph node metastasis. J Gastroenterol. 2006;41:391-392. |
| 5. | Miyazaki K. [Surgical strategy based on the spread mode of gallbladder carcinoma]. Nippon Geka Gakkai Zasshi. 2005;106:286-290. |
| 6. | Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Kanai M, Uesaka K, Yuasa N, Sano T. [Value of paraaortic lymphadenectomy for gallbladder carcinoma]. Nippon Geka Gakkai Zasshi. 1998;99:728-732. |
| 7. | Miyazaki I, Kayahara M, Nagakawa T. [Changes in lymph node dissection for pancreatic cancer]. Nippon Geka Gakkai Zasshi. 1997;98:610-614. |
| 8. | Kondo S, Nimura Y, Kamiya J, Nagino M, Kanai M, Uesaka K, Hayakawa N. Mode of tumor spread and surgical strategy in gallbladder carcinoma. Langenbecks Arch Surg. 2002;387:222-228. |
| 9. | Fujita K, Nagano T, Suzuki A, Sakakibara A, Takahashi S, Hirano T, Okagaki A, Ban C. Incidence of postoperative ileus after paraaortic lymph node dissection in patients with malignant gynecologic tumors. Int J Clin Oncol. 2005;10:187-190. |
| 10. | Yonemura Y, Wu CC, Fukushima N, Honda I, Bandou E, Kawamura T, Kamata T, Kim BS, Matsuki N, Sawa T. Randomized clinical trial of D2 and extended paraaortic lymphadenectomy in patients with gastric cancer. Int J Clin Oncol. 2008;13:132-137. |
| 11. | Kim YC, Park MS, Cha SW, Chung YE, Lim JS, Kim KS, Kim MJ, Kim KW. Comparison of CT and MRI for presurgical characterization of paraaortic lymph nodes in patients with pancreatico-biliary carcinoma. World J Gastroenterol. 2008;14:2208-2212. |
| 12. | Endo I, Shimada H, Tanabe M, Fujii Y, Takeda K, Morioka D, Tanaka K, Sekido H, Togo S. Prognostic significance of the number of positive lymph nodes in gallbladder cancer. J Gastrointest Surg. 2006;10:999-1007. |
| 13. | Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology. 2004;231:305-332. |
| 14. | Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, Lee JH, Ryu KW, Kim YW, Bae JM. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006;33:148-155. |
| 15. | Tsunoda Y, Ito M, Fujii H, Kuwano H, Saito N. Preoperative diagnosis of lymph node metastases of colorectal cancer by FDG-PET/CT. Jpn J Clin Oncol. 2008;38:347-353. |
| 16. | Yildirim Y, Sehirali S, Avci ME, Yilmaz C, Ertopcu K, Tinar S, Duman Y, Sayhan S. Integrated PET/CT for the evaluation of para-aortic nodal metastasis in locally advanced cervical cancer patients with negative conventional CT findings. Gynecol Oncol. 2008;108:154-159. |
| 17. | Kitajima K, Murakami K, Yamasaki E, Fukasawa I, Inaba N, Kaji Y, Sugimura K. Accuracy of 18F-FDG PET/CT in detecting pelvic and paraaortic lymph node metastasis in patients with endometrial cancer. AJR Am J Roentgenol. 2008;190:1652-1658. |
| 18. | Park JY, Kim EN, Kim DY, Suh DS, Kim JH, Kim YM, Kim YT, Nam JH. Comparison of the validity of magnetic resonance imaging and positron emission tomography/computed tomography in the preoperative evaluation of patients with uterine corpus cancer. Gynecol Oncol. 2008;108:486-492. |
| 19. | Lim JS, Yun MJ, Kim MJ, Hyung WJ, Park MS, Choi JY, Kim TS, Lee JD, Noh SH, Kim KW. CT and PET in stomach cancer: preoperative staging and monitoring of response to therapy. Radiographics. 2006;26:143-156. |
| 20. | Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, Cho A, Lee JD. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med. 2005;46:1582-1588. |
| 21. | Ba-Ssalamah A, Prokop M, Uffmann M, Pokieser P, Teleky B, Lechner G. Dedicated multidetector CT of the stomach: spectrum of diseases. Radiographics. 2003;23:625-644. |
| 22. | Fukuya T, Honda H, Hayashi T, Kaneko K, Tateshi Y, Ro T, Maehara Y, Tanaka M, Tsuneyoshi M, Masuda K. Lymph-node metastases: efficacy for detection with helical CT in patients with gastric cancer. Radiology. 1995;197:705-711. |
| 23. | Yang DM, Kim HC, Jin W, Ryu CW, Kang JH, Park CH, Kim HS, Jung DH. 64 multidetector-row computed tomography for preoperative evaluation of gastric cancer: histological correlation. J Comput Assist Tomogr. 2007;31:98-103. |
| 24. | D'Elia F, Zingarelli A, Palli D, Grani M. Hydro-dynamic CT preoperative staging of gastric cancer: correlation with pathological findings. A prospective study of 107 cases. Eur Radiol. 2000;10:1877-1885. |
| 25. | Schöder H, Erdi YE, Larson SM, Yeung HW. PET/CT: a new imaging technology in nuclear medicine. Eur J Nucl Med Mol Imaging. 2003;30:1419-1437. |
| 26. | Kamel IR, Cohade C, Neyman E, Fishman EK, Wahl RL. Incremental value of CT in PET/CT of patients with colorectal carcinoma. Abdom Imaging. 2004;29:663-668. |
| 27. | Wahl RL. Why nearly all PET of abdominal and pelvic cancers will be performed as PET/CT. J Nucl Med. 2004;45 Suppl 1:82S-95S. |
| 28. | Schiavina R, Scattoni V, Castellucci P, Picchio M, Corti B, Briganti A, Franceschelli A, Sanguedolce F, Bertaccini A, Farsad M. 11C-choline positron emission tomography/computerized tomography for preoperative lymph-node staging in intermediate-risk and high-risk prostate cancer: comparison with clinical staging nomograms. Eur Urol. 2008;54:392-401. |
| 29. | Oliva MR, Saini S. Liver cancer imaging: role of CT, MRI, US and PET. Cancer Imaging. 2004;4 Spec No A:S42-S46. |
| 30. | Kitajima K, Murakami K, Yamasaki E, Domeki Y, Tsubaki M, Sunagawa M, Kaji Y, Suganuma N, Sugimura K. Performance of integrated FDG PET/contrast-enhanced CT in the diagnosis of recurrent colorectal cancer: Comparison with integrated FDG PET/non-contrast-enhanced CT and enhanced CT. Eur J Nucl Med Mol Imaging. 2009;36:1388-1396. |
| 31. | Dirisamer A, Halpern BS, Flöry D, Wolf F, Beheshti M, Mayerhoefer ME, Langsteger W. Performance of integrated FDG-PET/contrast-enhanced CT in the staging and restaging of colorectal cancer: Comparison with PET and enhanced CT. Eur J Radiol. 2009;36:Epub ahead of print. |