Published online Sep 21, 2009. doi: 10.3748/wjg.15.4410
Revised: July 28, 2009
Accepted: August 4, 2009
Published online: September 21, 2009
AIM: To investigate the effect of paeonol on controlling the proliferation of colorectal cancer cell line HT-29 and to discuss its possible mechanism.
METHODS: The inhibitory effect of paeonol on proliferation of HT-29 cells was detected by MTT assay. The results of apoptosis were measured by flow cytometry. Immunocytochemical staining was performed to detect the expression of cyclooxygenase-2 (COX-2) and protein p27 in HT-29 cells treated with paeonol at different concentrations. Reverse transcription-polymerase chain reaction (RT-PCR) was used for mRNA analysis.
RESULTS: From the data of both MTT and flow cytometry, we observed that cell proliferation was inhibited by different concentrations of paeonol. By immunocytochemical staining, we found that HT-29 cells treated with paeonol (0.024-1.504 mmol/L) reflected reduced expression of COX-2 and increased expression of p27 in a dose-dependent manner. RT-PCR showed that paeonol down-regulated COX-2 and up-regulated p27 in a dose- and time-dependent manner in HT-29 cells.
CONCLUSION: One of the apoptotic mechanisms of paeonol is down-regulation of COX-2. p27 is up-regulated simultaneously and plays an important part in controlling cell proliferation and is a crucial factor in the Fas/FasL apoptosis pathway.
- Citation: Ye JM, Deng T, Zhang JB. Influence of paeonol on expression of COX-2 and p27 in HT-29 cells. World J Gastroenterol 2009; 15(35): 4410-4414
- URL: https://www.wjgnet.com/1007-9327/full/v15/i35/4410.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4410
It has been confirmed that inhibition of expression of cyclooxygenase-2 (COX-2) has antitumor activity against gastrointestinal carcinoma[1]. Selective COX-2 inhibitor celecoxib can prevent and reduce the incidence of colorectal cancer to some extent[2]. However, a recent study has shown that cardiovascular toxicity is caused when selective COX-2 inhibitors are used in the long term[3]. Consequently, it is considerable importance that a more effective and less toxic selective COX-2 inhibitor, which can be used for the treatment of colorectal carcinoma, is found.
Paeonol is a herb that is the main active component of peony bark. It has been shown to have many pharmacological activities, such as antifebrile, analgesic, antipyrotic, anti-atherosclerotic, anti-platelet and antioxidative activity[4,5]. It has also been demonstratedthat paeonol has antitumor effects in animal experiments[6]. Our recent study on apoptosis after addition of paeonol in human colorectal carcinoma cell line HT-29 shows that paeonol has antitumor activity, by regulating the expression of Fas, Bcl-2, Bax and p53 and finally causing apoptosis[7,8]. However, the pathway of paeonol-induced apoptosis is still confusing, which emphasizes the necessity of identifying its antitumor mechanism.
In the present study, the expression of COX-2 and p27 was detected and compared, and one of the antitumor mechanisms of paeonol was explored.
Human colorectal carcinoma cell line HT-29 was purchased from the Cancer Research Institute of the Wuhan University in China. Paeonol (Tianzhen Pharmaceutical Company of Ningbo, China), 5 mg/mL, RPMI 1640 (Hyclone Company, USA), calf serum, DABC immunoassay kit (Beijing Zhongshan Company, China), mouse anti-p27, rabbit anti-COX-2, GAPDH, anti-mouse and anti-rabbit secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were purchased as indicated.
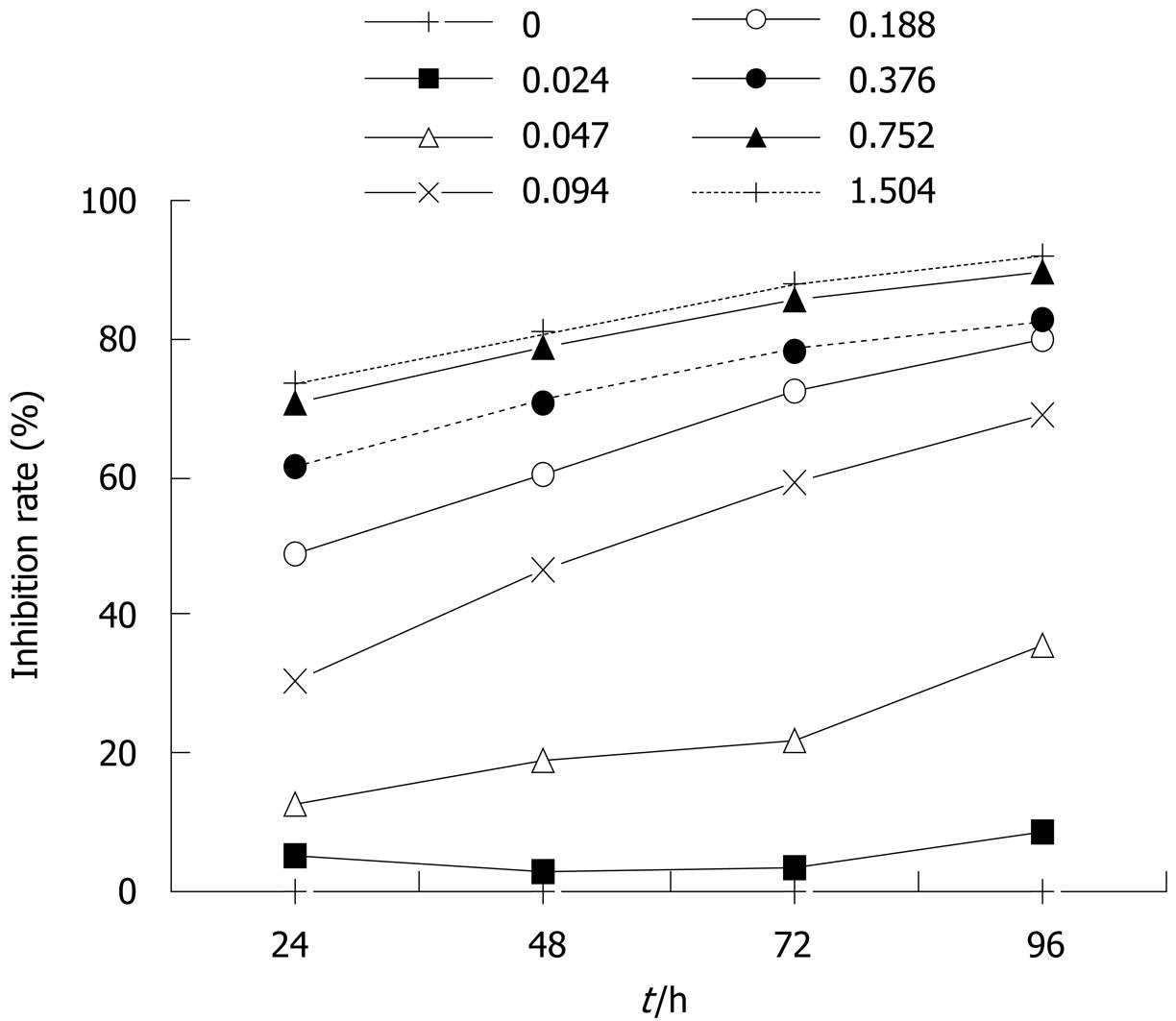
MTT assay[9]: Cell proliferation was measured using the MTT assay. Cells were plated in triplicate at 5 × 103 cells/well in 96-well plates, and treated with increasing concentrations of paeonol for culture of 24, 48, 72 and 96 h. Twenty microliters of 5 mg/mL MTT (Amresco, OH, USA) was added to each well and the cells were cultured at 37°C for an additional 4 h. The resulting formazan crystals were solubilized by the addition of 150 μL DMSO to each well. The optical density at 570 nm was measured and the percentage cell viability was calculated using the following formula: percentage cell viability = (absorbance of experimental well - absorbance of blank)/(absorbance of untreated control well - absorbance of blank) × 100% (Figure 1).
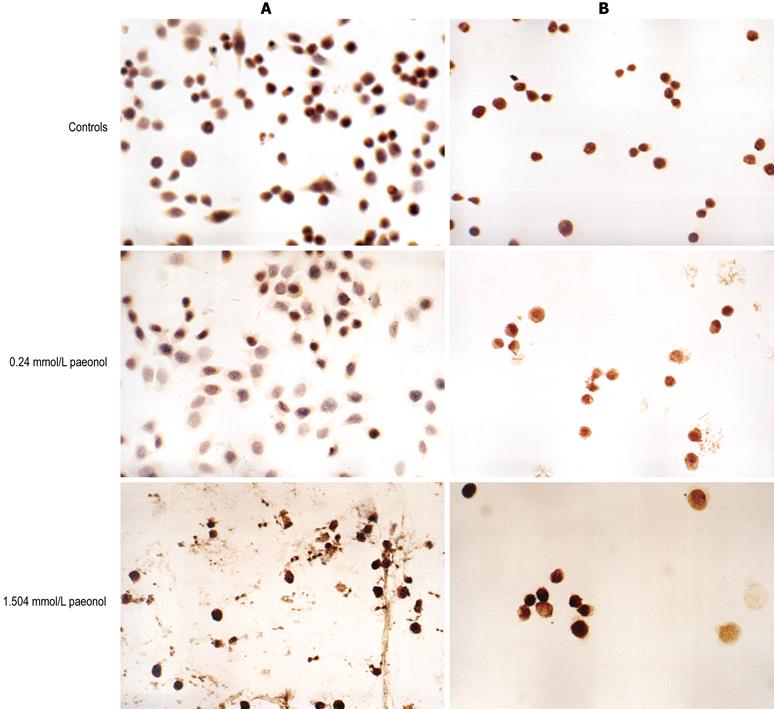
Immunocytochemistry: HT-29 cells (5 × 105-7 × 105) were seeded into 24-well plates and treated with paeonol at a concentration of 0, 0.094, 0.376 or 1.504 mmol/L for 48 h, in triplicate, and then cells were trypsinized, and rinsed with PBS. Immunocytochemical staining was carried out using the DABC immunoassay kit according to the manufacturer’s instructions. Immunostaining was observed by immunofluorescence microscopy. Positive staining for COX-2 was located mainly in the cytosol, and p27 in the nucleus. Immunocytochemical staining was classified as follows[10]: number of positive cells < 10% was deemed as negative (-), 10%-25% as (+), 25%-50% as (++), and > 50% as (+++).
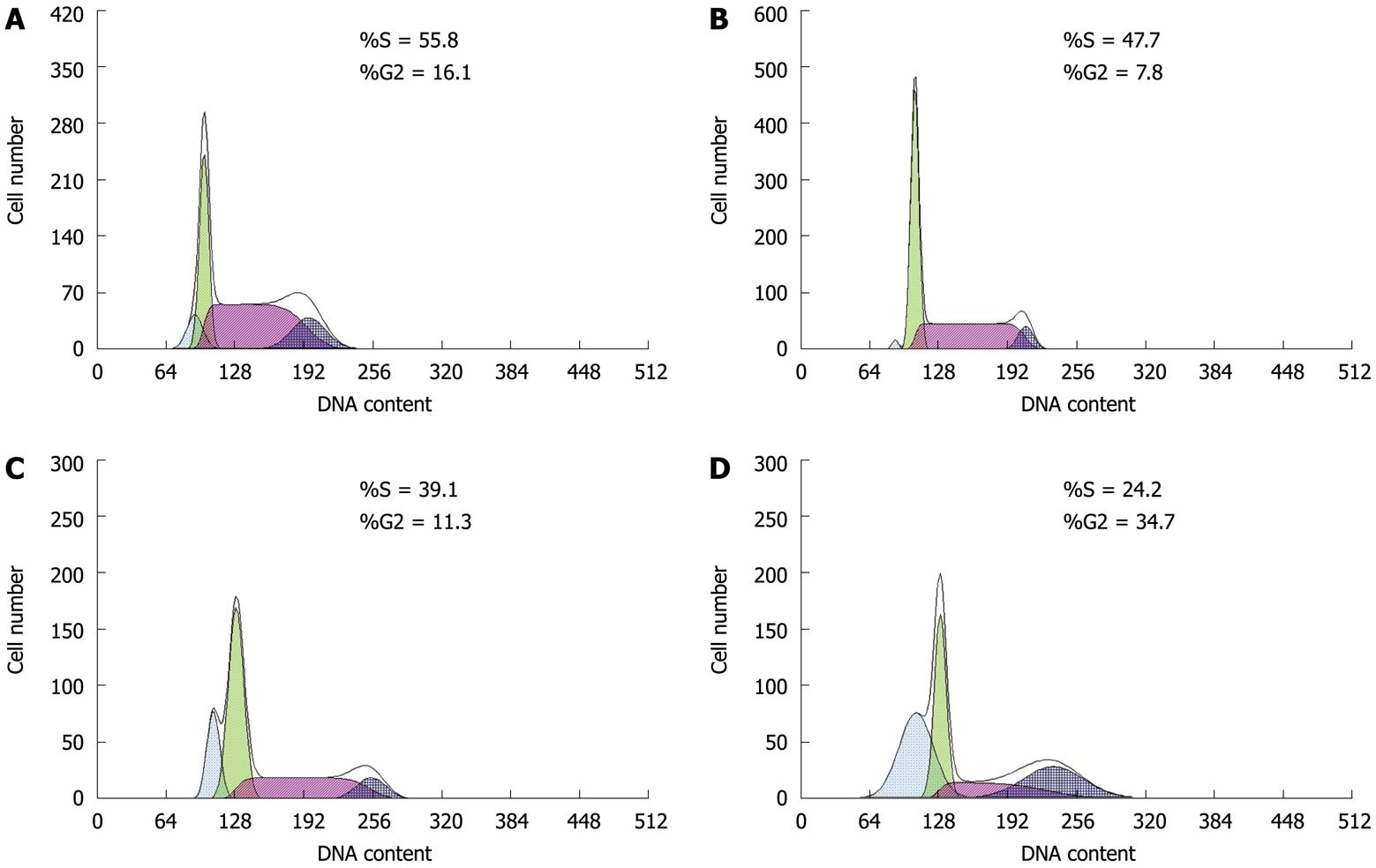
Flow cytometry: HT-29 cells (5-7 × 105) were seeded in six-well plates. Paeonol at 0, 0.094, 0.376 or 1.504 mmol/L was added to each well, in triplicate. Cells were incubated for another 48 h. Cells (5 × 106/L) were collected and detected by flow cytometry.
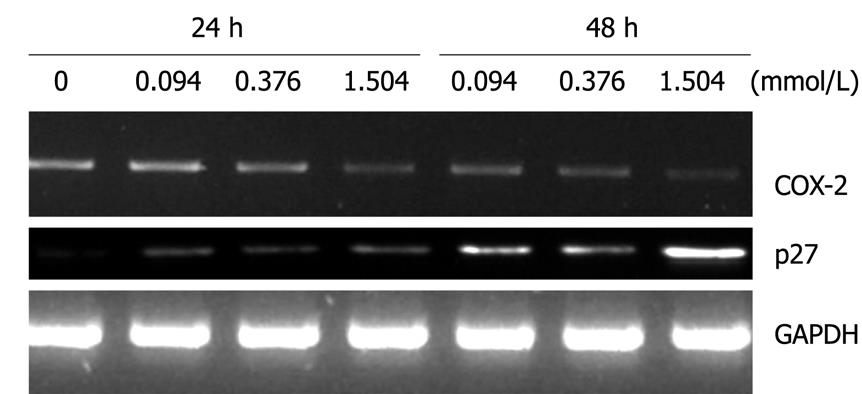
RNA preparation and reverse transcription-polymerase chain reaction (RT-PCR): Total RNA was isolated using the Trizol reagent (Invitrogen, CA, USA) and 1 μg RNA was used as a template for the synthesis of cDNA using the RversetAid First Strand cDNA Aynthesis Kit (Fermentas, ME, USA) according to the manufacturer’s instructions. PCR analysis was performed in a final volume of 25 μL using PCR Master Mix (Fermentas). COX-2-specific primer sequences (forward: 5'-TTCAAATGAGATTGTGGGAAAATTGCT-3'; reverse: 5'-AFATCATCTCTGCCTGAGTATCTT-3’); p27-specific primer sequences (forward: 5'-CGTGCGAGTGTCTAACGG-3'; reverse: 5'-CGGATCAGTCTTTGGGTC-3'); GAPDH-specific sequences (forward: 5'-ACGGATTTGGTCGTATTGGG-3'; reverse: 5'-TCCTGGAAGATGGTGATGGG-5') were designed using the Primer Premier 3 (Applied Biosystems, CA, USA). PCR products were separated in 1.5% agarose gels, stained with ethidium bromide and photographed.
Statistics were calculated with the SPSS version 11.5 (SPSS Inc., Chicago, IL, USA). All determinations were repeated in triplicate. Data are presented as means ± SD. Significance for comparison of samples was determined by Student’s t test and χ2 test. P < 0.05 were considered to be statistically significant throughout the study.
HT29 cells were treated with paeonol at different concentrations for 0, 24, 48 and 96 h, and the rate of inhibition was determined by MTT assay. We observed that growth of HT-29 cells was suppressed in a dose- and time-dependent manner (Figure 1).
In order to confirm the results obtained from the MTT assay and to elucidate the mechanisms of action of paeonol, we analyzed apoptosis using DABC immunocytochemistry (Figure 2A and B) and flow cytometry (Figure 3). HT-29 cells were treated with 0, 0.094, 0.376 or 1.504 mmol/L paeonol and examined after 48 h. Paeonol lowered the proportion of COX-2-positive cells, while increasing the level of p27, in a dose-dependent fashion (Tables 1 and 2).
Cell cycle distribution was assessed by flow cytometry. As shown in Figure 2B, the rate of apoptosis increased significantly as higher concentrations of paeonol were used, which reached 34.5% when paeonol was 1.054 mmol/L. Paeonol caused S-phase arrest and thus inhibited transition to the G0 and G2/M phases in a dose-dependent manner (P < 0.01) (Table 2).
The results suggested that apoptosis was the main mechanism by which paeonol inhibited the proliferation of HT-29 cells, and COX-2 and p27 were involved in the changes.
It has already been reported that COX-2 plays a critical role in controlling the growth as well as apoptosis in carcinogenesis. To elucidate the interaction between COX-2 and paeonol, HT-29 cells were exposed to 0, 0.094, 0.376 or 1.504 mmol/L paeonol for 48 h, and expression of COX-2 and p27 was detected, as shown in Figure 4. We observed that the level of COX-2 mRNA from cells treated with 0.094 mmol/L paeonol was lower than in the controls. Treatment with 1.504 mmol/L paeonol caused a further decrease in COX-2 expression, which indicated a dose-dependent decrease in COX-2 expression.
p27, which also plays a critical role in apoptosis, showed increased mRNA expression as the concentration of paeonol was increased (Figure 4), which indicated a dose- and time-dependent response.
COX-2 is an key inducible enzyme that regulates prostaglandin E2 (PGE2) synthesis[11]. It has a low expression level in normal tissue, while its expression in multiple malignant cancer cells is exceedingly high[12,13]. By promoting the proliferation of cancer cells and suppressing the expression of certain angiogenesis genes, PGE2 contributes to reducing apoptosis and increasing tumor invasiveness[14,15]. We found in the present study that paeonol had an inhibitory effect on the expression of COX-2 in HT-29 cells, which was dose- as well as time-dependent. The results show that one of the possible pharmacological effects of paeonol is inhibiting the expression of COX-2.
p27 is an anti-oncogene which is one of the cyclin-dependent kinase inhibitors, and it belongs to the p21 family. It inhibits cell proliferation by blocking the cell cycle[16,17]. We discovered in the present study that paeonol promoted the expression of p27 in HT-29 cells in a dose-dependent manner (P < 0.01). The cell cycle was blocked in S phase, and the percentages of G0/G1 and G2/M cells decreased significantly after paeonol treatment. From the cytometry figures, we observed that there was an obvious apoptosis peak after adding paeonol to HT-29 cells at a concentration of 1.504 mmol/L, and the rate of apoptosis was higher than that in the controls. This shows that paeonol can cause apoptosis by promoting the expression of p27, which blocks the cell cycle in S phase and interferes with DNA synthesis.
We also discovered that not only does paeonol down-regulate the expression of COX-2, but it also promotes the expression of p27 at the same time, which suggests that they are on the same apoptotic pathway. It has been reported that COX-2 is one of the upstream factors that controls Bcl-2 expression[18]. It has already been demonstrated in our previous study that paeonol can decrease the expression of Bcl-2 and increase the expression of Fas and caspase-8 in HT-29 cells, and we drew the conclusion that Fas/FasL is a central pathway that leads to paeonol-induced apoptosis[19]. In the present study, we found that COX-2 and p27 were correlated negatively after paeonol treatment, and consequently, we deduced that COX-2 and p27 may cooperate in controlling the proliferation of colorectal carcinoma cells. That is, when activated, COX-2 inhibits expression of p27 and promotes cell proliferation. Another inference we can make from this study is that p27 plays an important part in the control of Fas/FasL apoptosis and promotes apoptosis when its expression is up-regulated.
To identify the apoptotic mechanism of paeonol more clearly, further investigations are needed. We expect that paeonol may replace nonsteroidal anti-inflammatory drugs or selective COX-2 inhibitors because of their toxic effects, and form a new strategy for the therapy of colorectal carcinoma.
Paeonol has been shown to have many pharmacological activities, which include an antitumor effect in animal experiments. The authors’ previous work on the expression of apoptosis and apoptosis-related genes after treating human colorectal cell line HT-29 with paeonol has shown that paeonol has antitumor activity, by regulating the expression of apoptosis genes Fas, Bcl-2, Bax and p53, and inducing apoptosis. However, until now, the mechanism of action of paeonol has remained unclear. In the present study, the mechanism of the antitumor effect of paeonol was explored, and the expression of cyclooxygenase-2 (COX-2) and protein p27 was detected and discussed.
Colorectal carcinoma is common all around the world and has a high incidence among all the carcinomas. In research on paeonol, the hot topics are to identify the mechanism of its antitumor action, and to establish the possible pathways by which paeonol induces apoptosis in the HT-29 cell line.
The authors discovered that p27 also played some role in regulating COX-2, which indicates that pP27 may play a part in the control of the Fas/FasL apoptosis pathway and promote apoptosis when it is up-regulated.
The authors expect that paeonol may replace nonsteroidal anti-inflammatory drugs or selective COX-2 inhibitors because of their toxic effects and form a new therapeutic strategy for colorectal carcinoma.
This study determined the changes in expression pattern of COX-2 and p27 following paeonol treatment of HT-29 cells. The results showed that there was a significant decrease in COX-2 expression that was associated with an increase in p27 activity in a dose-dependent manner. The authors also conducted flow cytometry analysis to differentiate the growth phases after paeonol administration.
Peer reviewer: Jian-Ying Wang, Professor, University of Maryland School of Medicine, Baltimore VA Medical Center (112), 10N. Greene St, Baltimore, MD 21201, United States
S- Editor Li LF L- Editor Kerr C E- Editor Ma WH
| 1. | Elder DJ, Halton DE, Crew TE, Paraskeva C. Apoptosis induction and cyclooxygenase-2 regulation in human colorectal adenoma and carcinoma cell lines by the cyclooxygenase-2-selective non-steroidal anti-inflammatory drug NS-398. Int J Cancer. 2000;86:553-560. |
| 2. | Abdullah M, Sudoyo AW, Pranowo BS, Rini D, Sutrisna B, Rani AA. Expression of NF-kB and COX-2 in Young Versus Older Patients with Sporadic Colorectal Cancer. Acta Med Indones. 2009;41:70-74. |
| 3. | Lev-Ari S, Strier L, Kazanov D, Madar-Shapiro L, Dvory-Sobol H, Pinchuk I, Marian B, Lichtenberg D, Arber N. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin Cancer Res. 2005;11:6738-6744. |
| 4. | Li Q. The pharmocological research of paeonol. Zhongguo Zhongcaoyao. 1988;19:36. |
| 5. | Zhang LH, Shang PG, Huang Y. Parmocology of paeonol and development of clinical research. Chin J Integr Med. 1996;16:187-190. |
| 6. | Sun GP, Shen YX, Zhang LL. The research of paeonol on the immunological control and anti-tumor of Hepa mouse. Zhongguo Yaolixue Tongbao. 2003;19:160-162. |
| 7. | Liu CQ, Tan SY, Ji CY. The discussion of paeonol on the inhibition of the proliferation of HT-29 colorectal cell line. Zhongguo Yaolixue Tongbao. 2005;21:1251-1254. |
| 8. | Ji CY, Tan SY, Liu CQ. Inhibitory effect of paeonol on the proliferation of human colorectal cancer cell line HT-29 and its synergetic effect with chemotherapy agents. Linchuang Zhongliuxue Zazhi. 2005;32:513-515. |
| 9. | Sargent JM, Taylor CG. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br J Cancer. 1989;60:206-210. |
| 10. | Fan GK, Fujieda S, Sunaga H, Tsuzuki H, Ito N, Saito H. Expression of protein p27 is associated with progression and prognosis in laryngeal cancer. Laryngoscope. 1999;109:815-820. |
| 11. | Koehne CH, Dubois RN. COX-2 inhibition and colorectal cancer. Semin Oncol. 2004;31:12-21. |
| 12. | Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306-1311. |
| 13. | Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99:2254-2259. |
| 14. | Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362-366. |
| 15. | Sheng H, Shao J, Washington MK, DuBois RN. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075-18081. |
| 16. | Chang HL, Hung CF, Yeh CC, Chang WC, Chung JG. Paeonol promoted 2-aminofluorene and p-aminobenzoic acid acetylations by mononuclear leucocytes from Sprague-Dawley rats. Cytobios. 2000;103:149-158. |
| 17. | Pietenpol JA, Bohlander SK, Sato Y, Papadopoulos N, Liu B, Friedman C, Trask BJ, Roberts JM, Kinzler KW, Rowley JD. Assignment of the human p27Kip1 gene to 12p13 and its analysis in leukemias. Cancer Res. 1995;55:1206-1210. |
| 18. | Li GQ, Tan SY, Liu CQ. The correlation of Fas/FasL, bcl-2, caspase-8 in paeonol inducing colorectal cancer cell line HT-29 apoptosis and molecule mechanism. Weichangbing He Ganbingxue Zazhi. 2006;15:194-196. |
| 19. | Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362-366. |