Published online Sep 21, 2009. doi: 10.3748/wjg.15.4402
Revised: July 21, 2009
Accepted: July 28, 2009
Published online: September 21, 2009
AIM: To assess each layer of the optical coherence tomography (OCT) image of the esophageal wall with reference to the histological structure.
METHODS: Resected specimens of fresh pig esophagus was used as a model for the esophageal wall. We injected cyanoacrylate adhesive into the specimens to create a marker, and scanned them using a miniature OCT probe. The localization of these markers was assessed in the OCT images. Then we compared the OCT-imaged morphology with the corresponding histological section, guided by the cyanoacrylate adhesive markers. We prepared a second set of experiments using nylon sutures as markers.
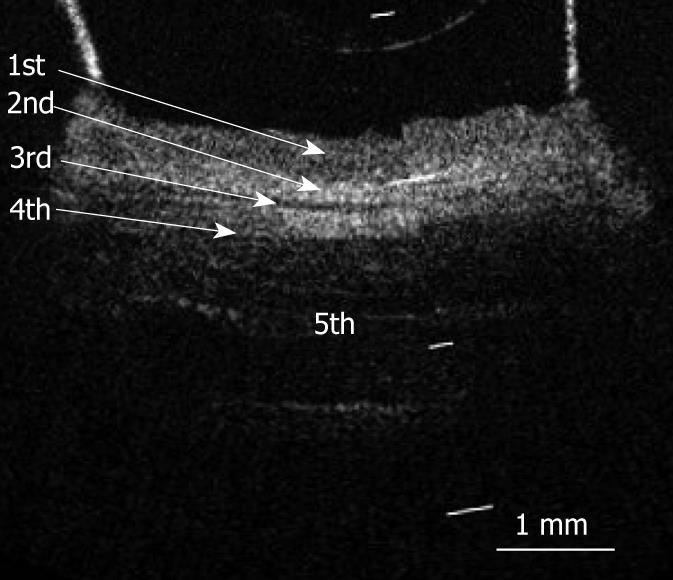
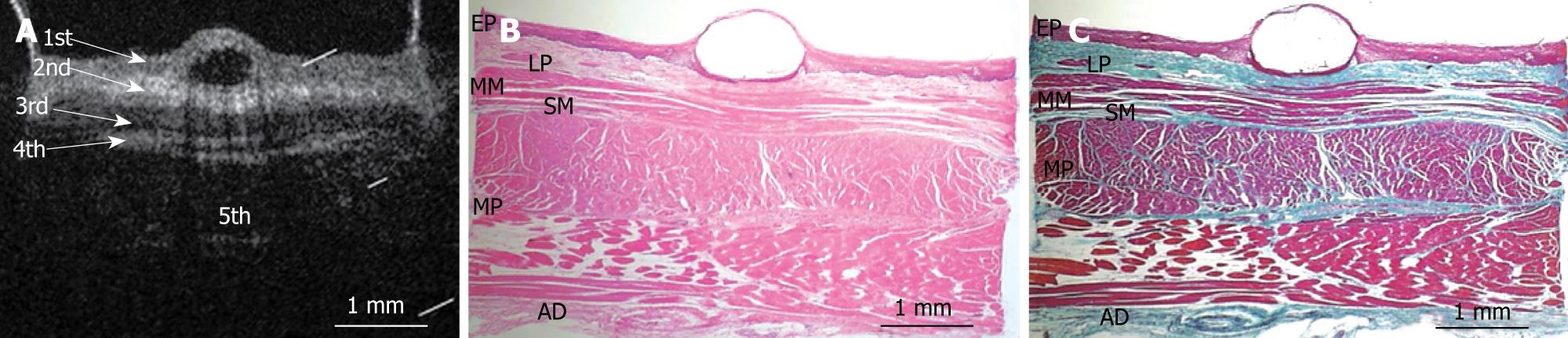
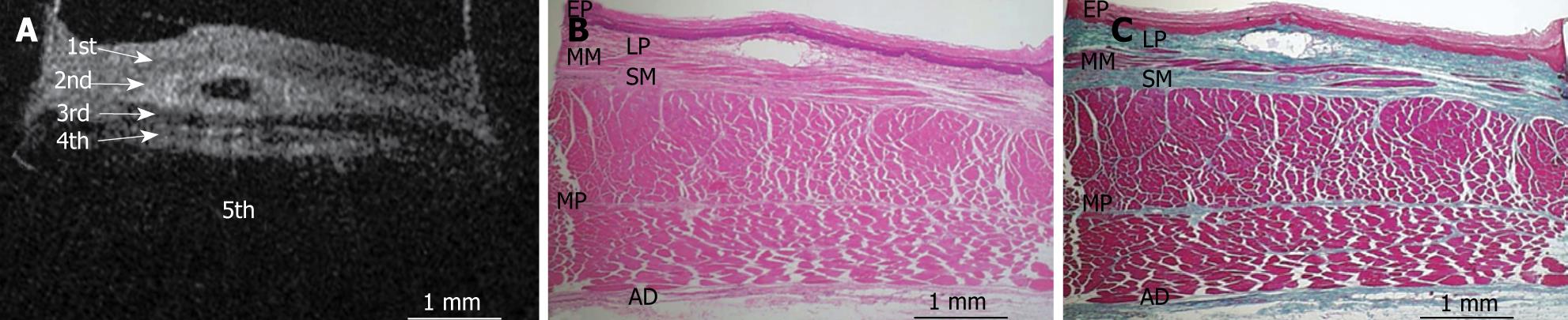
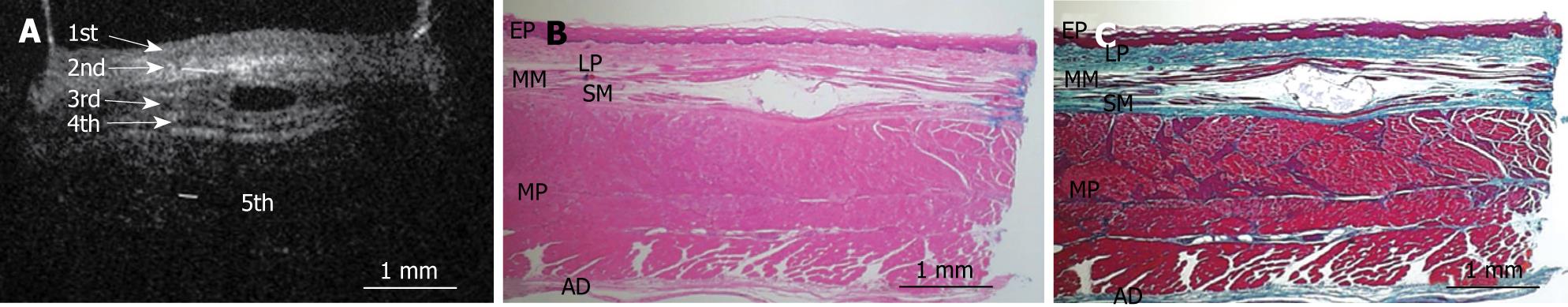
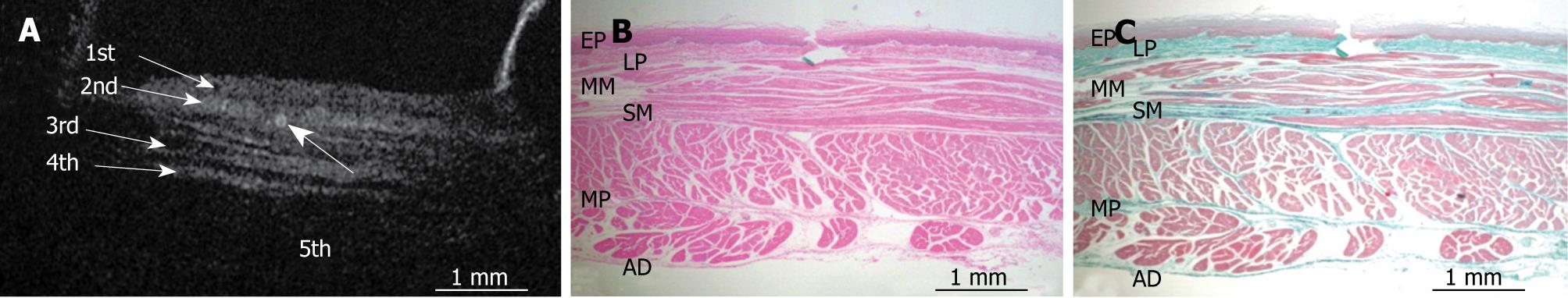
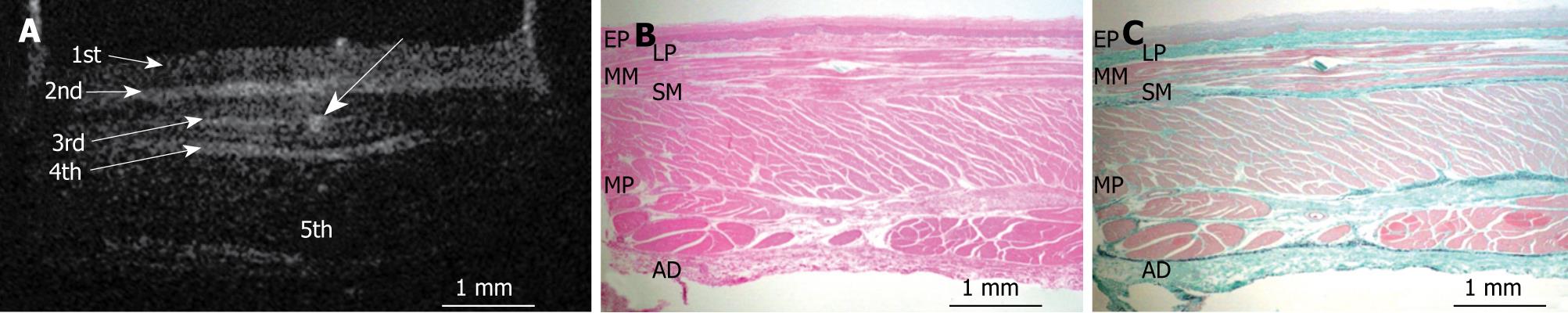
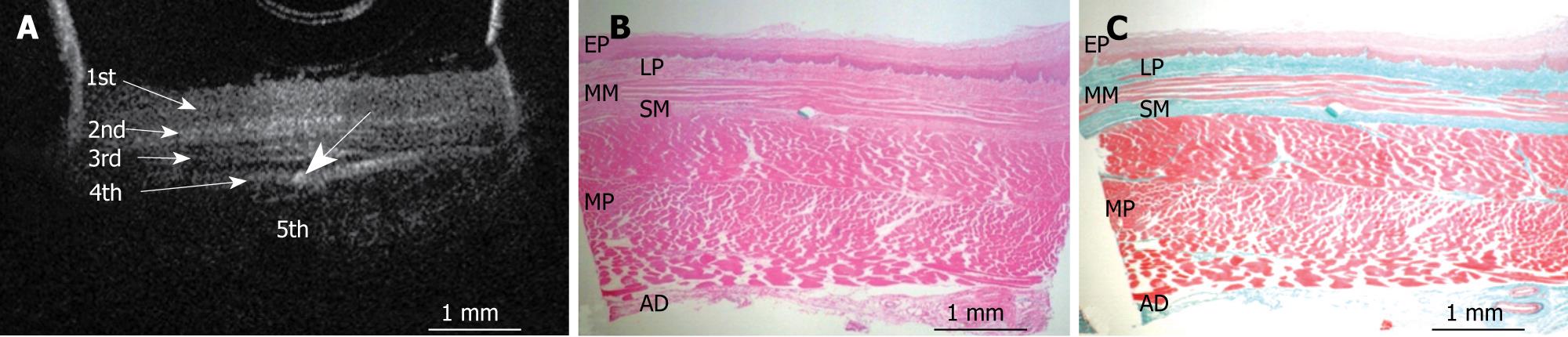
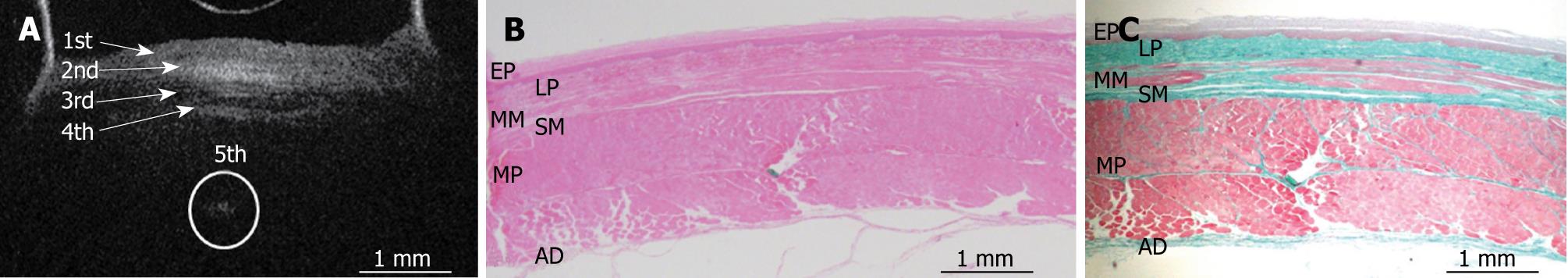
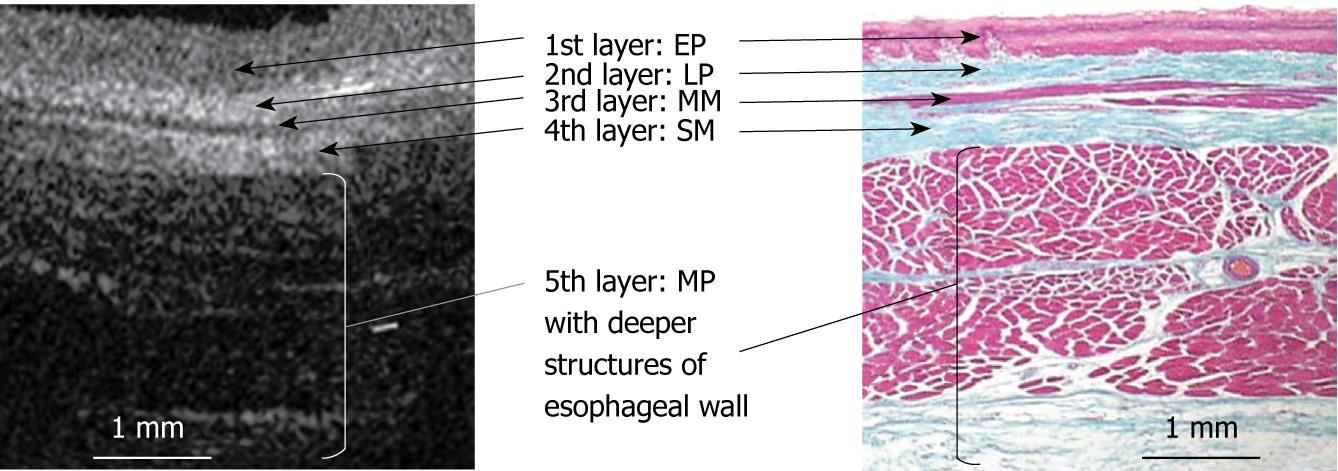
RESULTS: The OCT image of the esophageal specimen has a clear five-layered morphology. First, it consisted of a relatively less reflective layer; second, a more reflective layer; third, a less reflective layer; fourth, a more reflective layer; and fifth, a less reflective layer. Comparing the OCT images with marked histological sections showed that the first layer corresponded to stratified squamous epithelium; the second to lamina propria; the third to muscularis mucosa; fourth, submucosa; and fifth, muscularis propria with deeper structures of the esophageal wall.
CONCLUSION: We demonstrated that the OCT image of the normal esophageal wall showed a five-layered morphology, which corresponds to histological esophageal wall components.
- Citation: Yokosawa S, Koike T, Kitagawa Y, Hatta W, Uno K, Abe Y, Iijima K, Imatani A, Ohara S, Shimosegawa T. Identification of the layered morphology of the esophageal wall by optical coherence tomography. World J Gastroenterol 2009; 15(35): 4402-4409
- URL: https://www.wjgnet.com/1007-9327/full/v15/i35/4402.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4402
The recent widespread use of endoscopy has enabled us to detect early stage superficial esophageal squamous cell carcinoma (SESCC). Endoscopic resection can be performed for these SESCC with the advantage of it being a minimally invasive treatment for patients[1-5]. According to a draft of subclassifications of SESCC that was formulated by the Japanese Society for Esophageal Disease[6], the depth of invasion of SESCC is defined as follows: epithelial layer (T1a-EP), proper mucosal layer (T1a-LPM), muscularis mucosa (T1a-MM), upper third of the submucosal layer (SM1), middle third of the submucosal layer (SM2), lower third of the submucosal layer (SM3). Based on this subclassification, endoscopic resection is indicated only for “T1a-EP” or “T1a-LPM” cancers because their frequency of lymph node metastasis is extremely rare. On the other hand, it was reported that lymph node metastasis was observed in 8% of “T1a-MM” cancers and 13% of “SM1” cancers[7], and that endoscopic resection may be insufficient as a radical therapy for these “T1a-MM” or “SM1” cancers[6-10]. Therefore, an accurate diagnosis of depth of cancer invasion is important in the decision for an appropriate treatment for the SESCC patients, especially for the patients with “T1a-MM” or “SM1” cancers. Until now, endoscopic ultrasonography (EUS) has been the standard examination for the assessment of depth of cancer invasion in SESCC. However, its accuracy for diagnosing “T1a-MM” and “SM1” cancers is inadequate[11].
A new imaging modality, optical coherence tomography (OCT)[12] is a non-invasive optical imaging technology that provides high resolution, cross-sectional images of biological tissue in real time. OCT is analogous to B-scan ultrasonography (US); however, it measures reflected infrared light rather than acoustical waves. The resolution of OCT imaging (10-20 μm) is 5 to 25 times higher than that of high frequency US. Therefore, OCT is a currently available clinical device with the highest resolution for intraluminal tomographic imaging[13].
Several in vivo and in vitro studies have reported the feasibility of OCT imaging in the GI tract[14-28]. For the normal esophageal wall, it was reported that the OCT image was delineated as a layered morphology. However, there are few studies concerning the histological interpretation with regard to the layered morphology of the OCT image and these studies differ in their interpretation.
The aim of this study was to ascertain which layer of the OCT image corresponded to each component of the esophageal wall.
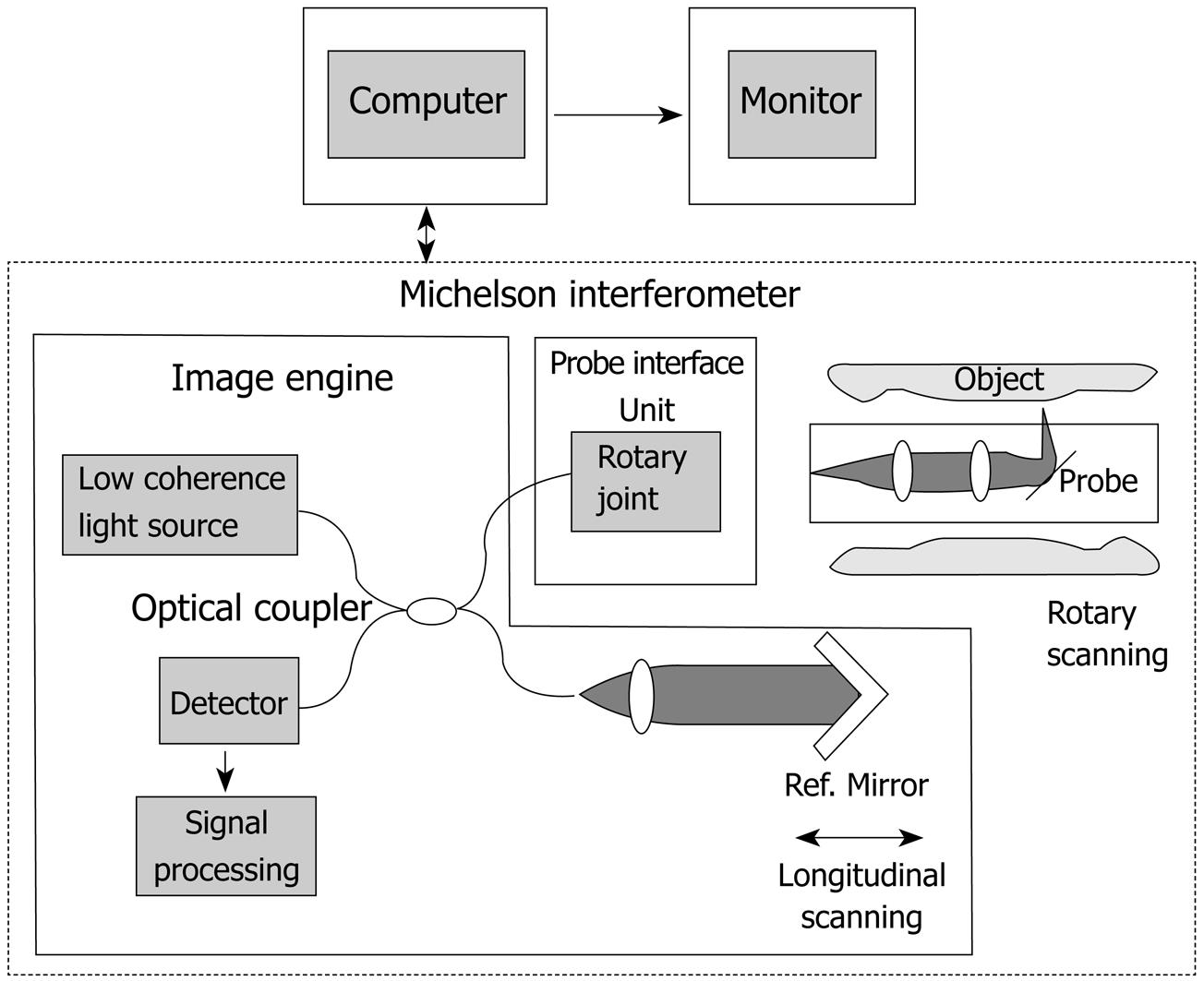
We employed an OCT system developed by Light Lab Imaging (Boston, USA) and HOYA (Tokyo, Japan) (Figure 1). The OCT images were obtained using a superluminescent diode light source with a center wavelength of 1300 nm, a bandwidth of 50 nm, and power output 10 mW, resulting in a 10-20 μm axial image resolution. The lateral or transverse resolution was determined by the diffraction limit of the OCT endoscopic catheter. The spot diameter that resulted from the diffraction of the light was selected to be comparable to the axial OCT resolution while maintaining an appropriate depth of focus. By scanning the interrogating beam across the tissue surface, a series of tomograms were obtained and constructed into a two-dimensional image.
We used a 1.5 mm diameter prototype OCT probe which could be inserted through the accessory port of an endoscope and provide a 360-degree radial scan. Since the OCT beam is invisible, the position of the beam on samples was monitored using a coincident, visible-light, guiding beam (670 nm). OCT images were typically displayed in gray-scale. That is, the image could be configured to represent highly reflective signals as white, and low reflective signals as black.
We assessed the layered morphology of OCT images of the esophageal wall, referring to previous studies for the identification of the layered morphology of the GI tract wall with EUS[29,30]. We employed fresh pig esophageal wall as tissue specimens, because the histological structure of the pig esophageal wall is similar to that of humans. The tissue specimens were used within two hours, because the inherent optical property of the specimens may change with time. Excess blood and mucus were carefully removed by washing with saline. Specimens were then stretched and pinned onto a rubber plate with the luminal surfaces exposed. The position for the OCT imaging was marked on the specimen using two needles pinned through the specimen about 2 mm apart. To create a marker for identifying the layered morphology of the OCT image, we injected a small amount of cyanoacrylate adhesive with a needle (24 G, diameter 400 μm) into the tissue specimen between the two needles. After the injection, we scanned the position between the two needles with the OCT probe.
The specimens were subjected to routine histological processing. Briefly, the specimens were immersed in 10% buffered formalin for 48 h and processed for standard paraffin embedding. Five-micron-thick sections were cut at the marked position and stained with Hematoxylin-Eosin (HE) and Elastica-Masson (EM). Finally, the layered morphology of OCT images was compared with that of each marked histological section.
We performed another experiment using nylon sutures as a marker instead of injecting cyanoacrylate adhesive. Thin nylon sutures (surgical suture with a needle: diameter 70 μm approximately) were passed through each layer of the specimens to create a marker on each layer imaged by OCT.
Figure 2 shows an OCT image of the normal pig esophagus specimen. In the OCT image, the five-layered morphology of the esophageal wall is clearly delineated. The layers were arranged outward as follows: a relatively less reflective layer (the first layer), a more reflective layer (the second layer), a less reflective layer (the third layer), a more reflective layer (the fourth layer), and a less reflective layer (the fifth layer).
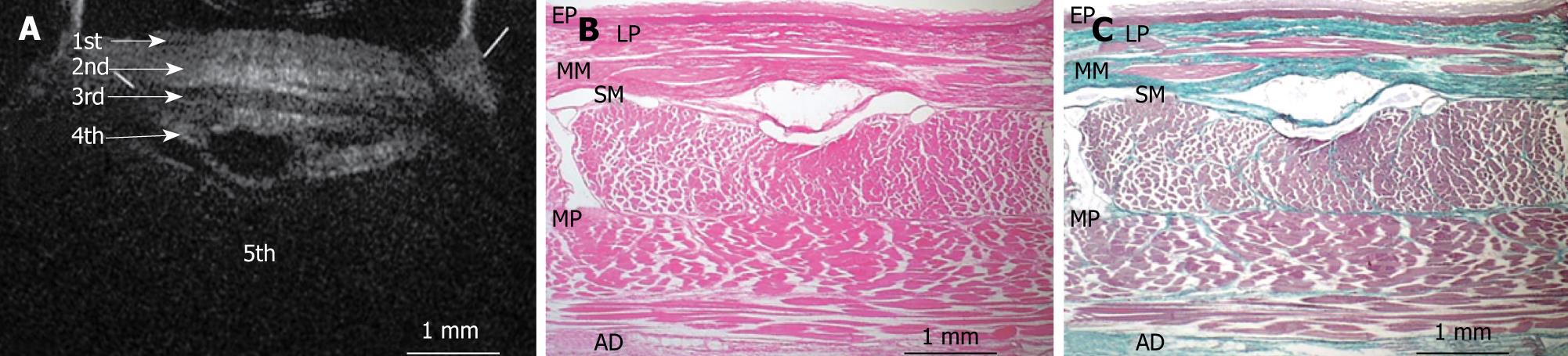
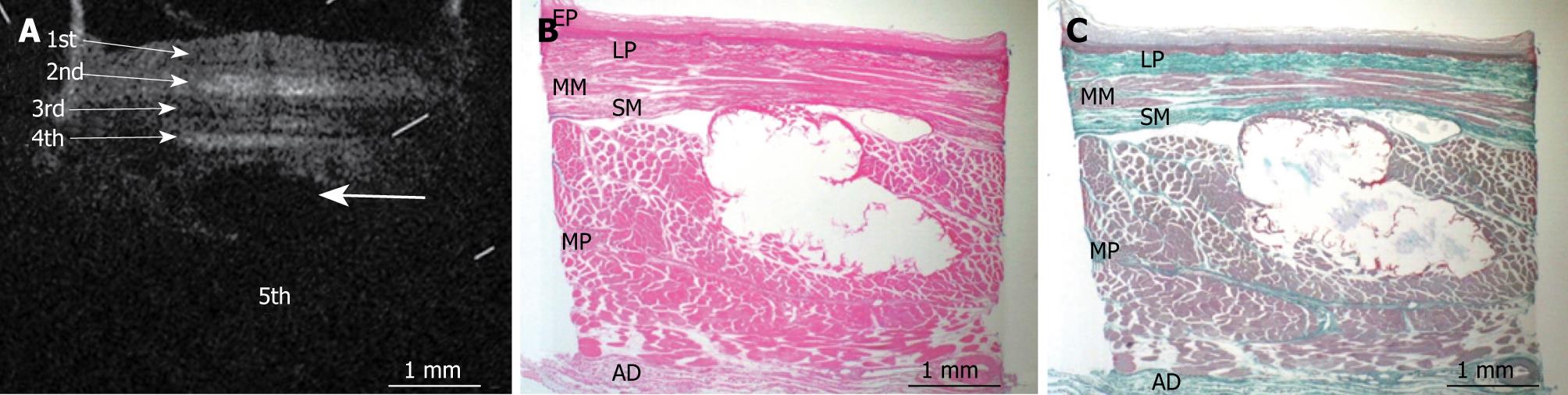
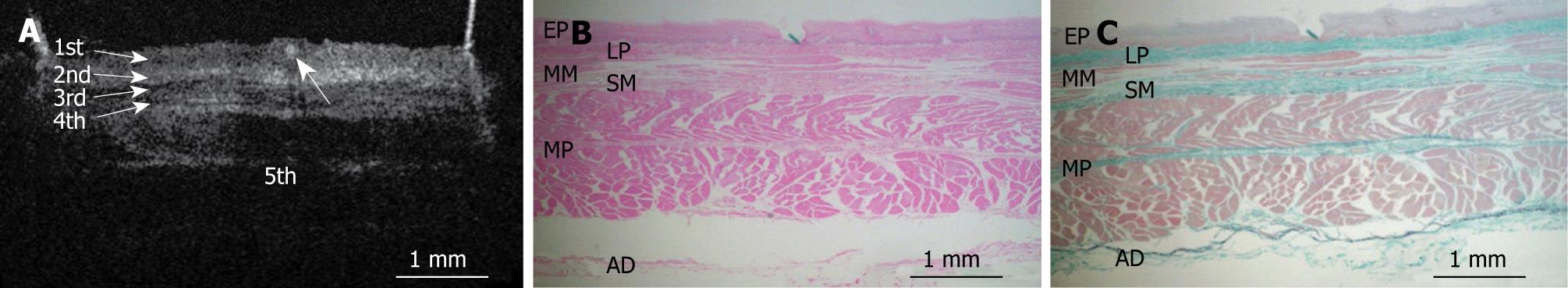
Figures 3, 4, 5, 6, and 7 show OCT images and corresponding histology of the normal pig esophageal wall injected with cyanoacrylate adhesive. The OCT image after injection with cyanoacrylate adhesive into the first layer and corresponding histology are shown in Figure 3. The position injected with cyanoacrylate adhesive was recognized as a non-reflective spot. The non-reflective spot was observed in the first layer of the OCT image. In the corresponding histological section, the cyanoacrylate adhesive was observed in stratified squamous epithelium. Therefore, we confirmed that the first (relatively less reflective) layer of the OCT image corresponded to stratified squamous epithelium. The second (more reflective) layer corresponded to lamina propria (Figure 4), the third (less reflective) layer corresponded to muscularis mucosa (Figure 5), the fourth (more reflective) layer corresponded to submucosa (Figure 6), and the fifth (less reflective) layer corresponded to muscularis propria with deeper structures of the esophageal wall (Figure 7).
Figures 8, 9, 10, 11, and 12 show OCT images and corresponding histology using thin nylon sutures as markers. In Figure 8, the thin nylon suture was recognized as a more reflective dot in the first layer of the OCT image. In the corresponding histological section, the fragment of thin nylon suture was observed in stratified squamous epithelium. Therefore, we confirmed that the first (less reflective) layer of the OCT image corresponded to stratified squamous epithelium. The second (more reflective) layer corresponded to the lamina propria (Figure 9), the third (less reflective) layer corresponded to the muscularis mucosa (Figure 10), the fourth (more reflective) layer corresponded to the submucosa (Figure 11), and the fifth (less reflective) layer corresponded to the muscularis propria with deeper structures of the esophageal wall (Figure 12).
Figure 13 presents a schema of the OCT image of the normal esophageal wall with a delineated five-layered morphology, consisting of relatively less reflective stratified squamous epithelium, more reflective lamina propria, less reflective muscularis mucosa, more reflective submucosa, and less reflective muscularis propria with deeper structures of the esophageal wall.
We have shown that OCT can clearly delineate the five-layered morphology of the normal pig esophageal wall in vitro. In addition, we confirmed that the layered morphology imaged by OCT matches the histological structure of the esophageal wall.
Sergeev et al[14] were the first to report that they obtained OCT images of the human esophageal wall and stomach. Also, several studies demonstrated the utility of OCT for the diagnosis of Barrett’s esophagus (BE) because the high resolution of OCT images could distinguish BE from stratified squamous epithelium or gastric mucosa[15-22].
In the normal squamous stratified epithelium of the human esophageal wall, several studies demonstrated in vivo that the OCT image of the esophageal wall was delineated as a five-layered morphology, and that the muscularis mucosa was delineated as a less reflective layer[14-17,23]. However, Sivak et al[24] reported that the esophageal wall was observed as an eight-layered morphology, and that the muscularis mucosa appeared to be a relatively thick triple layer (two more reflective layers separated by one of less reflectivity). Das et al[25] reported that muscularis mucosa was delineated as a more reflective multilayered structure. Jäckle et al[23] attempted to interpret in vivo OCT images compared with the histological structure using vessels or glands in the biopsy or mucosectomy specimens of the esophageal wall as markers, resulting in the association of the main structures of in vitro OCT images with epithelium, lamina propria and muscularis mucosa. However, it has proved difficult to determine all the layers of the esophageal wall visualized by OCT using only biopsy and mucosectomy specimens. Therefore, correlation of the layered morphology of the in vivo OCT image with the histological structure of esophageal wall has not been sufficiently clarified.
On the other hand, in vitro studies of surgically resected specimens or autopsies can compare OCT images of the esophageal wall with full thickness sections. Cilesiz et al[26] reported that OCT images of esophageal wall were observed to closely agree with in vivo data. But, even for in vitro studies, the interpretation of OCT images of esophageal wall is still under discussion. Tearney et al[27] suggested that muscularis mucosa was more reflective than stratified squamous epithelium. Other studies reported that stratified squamous epithelium presented as a more reflective layer than lamina propria[18,28]. In these studies, the layered morphology of the OCT image was interpreted without a definitive marker for orientation of the histological structure of the esophageal wall.
Considering the above-mentioned matters, we used fresh pig esophageal wall specimens and histologically assessed the layered morphology imaged by OCT using both cyanoacrylate adhesives and nylon sutures as markers. Under these conditions, we demonstrated that the OCT image of the normal esophageal wall clearly delineated the five-layered morphology, and that the layered morphology consisted of relatively less reflective stratified squamous epithelium, more reflective lamina propria, less reflective muscularis mucosa, more reflective submucosa, and less reflective muscularis propria with deeper structures of esophageal wall.
The mechanism by which OCT images delineate five-layered morphology may be explained by the difference of reflectivity of light through the esophageal tissues, which have different optical properties. Generally, the structure of epithelium, muscularis mucosa, and muscularis propria is uniform and orderly consisting of only epithelial cells or smooth muscle fibers. In this study, OCT images delineated them as less reflective layers because the light passes through them with less obstruction. In contrast, lamina propria and submucosa consist of various mixed components such as connective tissue, vessels, lymph follicles, and glands. The OCT images of these were delineated as more reflective layers due to obstruction of the passage of light.
Concerning the diagnosis for depth of invasion of SESCC, Murata et al[11] reported that accuracy assessed by EUS was 81% for both “T1a-EP” and “T1a-LPM” cancers, but that the accuracy for both “T1a-MM” and “SM1” cancers was only 60%, suggesting that it was hard to make a diagnosis for the “T1a-MM” and “SM1” cancers by EUS. In our study, OCT clearly imaged the muscularis mucosa which is a boundary line between “T1a-MM” and “SM1” cancer. Therefore, OCT may provide us with the opportunity to select more appropriate treatment for SESCC patients than does EUS. Das et al[25] reported that the resolution of the OCT image was superior to that of high-frequency EUS; however, the depth of penetration with OCT was limited to mucosa and submucosa when compared with the high-frequency EUS. Further investigation is necessary to evaluate the feasibility of diagnosing the depth of invasion in SESCC.
In conclusion, we have demonstrated that the OCT image of the normal esophageal wall clearly delineates a five-layered structure, and we have identified the layered morphology which consists of relatively less reflective stratified squamous epithelium, more reflective lamina propria, less reflective muscularis mucosa, more reflective submucosa, and less reflective muscularis propria with deeper structures of the esophageal wall.
In the normal esophageal wall, it has been reported that the optical coherence tomography (OCT) image is delineated as a layered morphology. But there are few studies concerning the histological interpretation of the layered morphology of the OCT image and these studies differ in their interpretation.
To ascertain which layer of the OCT image corresponded to each component of the esophageal wall.
Comparing the OCT images with marked histological sections showed that the first layer corresponded to stratified squamous epithelium; the second to lamina propria; the third to muscularis mucosa; fourth, submucosa; and fifth, muscularis propria with deeper structures of the esophageal wall.
OCT images may contribute to the accurate diagnosis of the depth of cancer invasion, and thus help when choosing therapeutic options for superficial esophageal squamous cell carcinoma (SESCC).
The authors demonstrated that the OCT image of the normal esophageal wall clearly delineates a five-layered structure, and identified the layered morphology consisting of relatively less reflective stratified squamous epithelium, more reflective lamina propria, less reflective muscularis mucosa, more reflective submucosa, and less reflective muscularis propria with deeper structures of the esophageal wall. Therefore, OCT images may contribute to the accurate diagnosis for the depth of cancer invasion, when choosing therapeutic options for SESCC.
Peer reviewers: Anders E Lehmann, PhD, Associate Professor, Senior Principal Scientist, Bioscience, AstraZeneca R&D Mölndal, Mölndal, Sweden; Michael F Vaezi, Professor, Department of Gastroenterology and Hepatology, Vanderbilt University Medical Center, 1501 TVC, Nashville, TN 37232-5280, United States
S- Editor Tian L L- Editor Logan S E- Editor Yin DH
| 1. | Monma K, Sakaki N, Yoshida M. Endoscopic mucosectomy for precise evaluation and treatment of esophageal intraepithelial cancer. Dig Endosc. 1990;2:447-452. |
| 2. | Inoue H, Takeshita K, Hori H, Muraoka Y, Yoneshima H, Endo M. Endoscopic mucosal resection with a cap-fitted panendoscope for esophagus, stomach, and colon mucosal lesions. Gastrointest Endosc. 1993;39:58-62. |
| 3. | Maku-uchi H. Endoscopic mucosal resection for early esophageal cancer. Dig Endosc. 1996;8:175-179. |
| 4. | Pech O, Gossner L, May A, Vieth M, Stolte M, Ell C. Endoscopic resection of superficial esophageal squamous-cell carcinomas: Western experience. Am J Gastroenterol. 2004;99:1226-1232. |
| 5. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Shimizu Y, Oka M. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688-694. |
| 6. | Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery. 1998;123:432-439. |
| 7. | Endo M, Yoshino K, Kawano T, Nagai K, Inoue H. Clinicopathologic analysis of lymph node metastasis in surgically resected superficial cancer of the thoracic esophagus. Dis Esophagus. 2000;13:125-129. |
| 8. | Noguchi H, Naomoto Y, Kondo H, Haisa M, Yamatsuji T, Shigemitsu K, Aoki H, Isozaki H, Tanaka N. Evaluation of endoscopic mucosal resection for superficial esophageal carcinoma. Surg Laparosc Endosc Percutan Tech. 2000;10:343-350. |
| 9. | Shimizu Y, Tsukagoshi H, Fujita M, Hosokawa M, Kato M, Asaka M. Long-term outcome after endoscopic mucosal resection in patients with esophageal squamous cell carcinoma invading the muscularis mucosae or deeper. Gastrointest Endosc. 2002;56:387-390. |
| 10. | Yoshida M, Momma K. [Endoscopic evaluation of the depth of invasion in cases of superficial esophageal cancer in determining indications for endoscopic mucosal resection]. Nippon Geka Gakkai Zasshi. 2002;103:337-342. |
| 11. | Murata Y, Napoleon B, Odegaard S. High-frequency endoscopic ultrasonography in the evaluation of superficial esophageal cancer. Endoscopy. 2003;35:429-435; discussion 436. |
| 12. | Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA. Optical coherence tomography. Science. 1991;254:1178-1181. |
| 13. | Tearney GJ, Brezinski ME, Bouma BE, Boppart SA, Pitris C, Southern JF, Fujimoto JG. In vivo endoscopic optical biopsy with optical coherence tomography. Science. 1997;276:2037-2039. |
| 14. | Sergeev A, Gelikonov V, Gelikonov G, Feldchtein F, Kuranov R, Gladkova N, Shakhova N, Snopova L, Shakhov A, Kuznetzova I. In vivo endoscopic OCT imaging of precancer and cancer states of human mucosa. Opt Express. 1997;1:432-440. |
| 15. | Bouma BE, Tearney GJ, Compton CC, Nishioka NS. High-resolution imaging of the human esophagus and stomach in vivo using optical coherence tomography. Gastrointest Endosc. 2000;51:467-474. |
| 16. | Li XD, Boppart SA, Van Dam J, Mashimo H, Mutinga M, Drexler W, Klein M, Pitris C, Krinsky ML, Brezinski ME. Optical coherence tomography: advanced technology for the endoscopic imaging of Barrett's esophagus. Endoscopy. 2000;32:921-930. |
| 17. | Zuccaro G, Gladkova N, Vargo J, Feldchtein F, Zagaynova E, Conwell D, Falk G, Goldblum J, Dumot J, Ponsky J. Optical coherence tomography of the esophagus and proximal stomach in health and disease. Am J Gastroenterol. 2001;96:2633-2639. |
| 18. | Pitris C, Jesser C, Boppart SA, Stamper D, Brezinski ME, Fujimoto JG. Feasibility of optical coherence tomography for high-resolution imaging of human gastrointestinal tract malignancies. J Gastroenterol. 2000;35:87-92. |
| 19. | Jäckle S, Gladkova N, Feldchtein F, Terentieva A, Brand B, Gelikonov G, Gelikonov V, Sergeev A, Fritscher-Ravens A, Freund J. In vivo endoscopic optical coherence tomography of esophagitis, Barrett's esophagus, and adenocarcinoma of the esophagus. Endoscopy. 2000;32:750-755. |
| 20. | Poneros JM, Brand S, Bouma BE, Tearney GJ, Compton CC, Nishioka NS. Diagnosis of specialized intestinal metaplasia by optical coherence tomography. Gastroenterology. 2001;120:7-12. |
| 21. | Isenberg G, Sivak MV Jr, Chak A, Wong RC, Willis JE, Wolf B, Rowland DY, Das A, Rollins A. Accuracy of endoscopic optical coherence tomography in the detection of dysplasia in Barrett's esophagus: a prospective, double-blinded study. Gastrointest Endosc. 2005;62:825-831. |
| 22. | Evans JA, Bouma BE, Bressner J, Shishkov M, Lauwers GY, Mino-Kenudson M, Nishioka NS, Tearney GJ. Identifying intestinal metaplasia at the squamocolumnar junction by using optical coherence tomography. Gastrointest Endosc. 2007;65:50-56. |
| 23. | Jäckle S, Gladkova N, Feldchtein F, Terentieva A, Brand B, Gelikonov G, Gelikonov V, Sergeev A, Fritscher-Ravens A, Freund J. In vivo endoscopic optical coherence tomography of the human gastrointestinal tract--toward optical biopsy. Endoscopy. 2000;32:743-749. |
| 24. | Sivak MV Jr, Kobayashi K, Izatt JA, Rollins AM, Ung-Runyawee R, Chak A, Wong RC, Isenberg GA, Willis J. High-resolution endoscopic imaging of the GI tract using optical coherence tomography. Gastrointest Endosc. 2000;51:474-479. |
| 25. | Das A, Sivak MV Jr, Chak A, Wong RC, Westphal V, Rollins AM, Willis J, Isenberg G, Izatt JA. High-resolution endoscopic imaging of the GI tract: a comparative study of optical coherence tomography versus high-frequency catheter probe EUS. Gastrointest Endosc. 2001;54:219-224. |
| 26. | Cilesiz I, Fockens P, Kerindongo R, Faber D, Tytgat G, Ten Kate F, Van Leeuwen T. Comparative optical coherence tomography imaging of human esophagus: how accurate is localization of the muscularis mucosae? Gastrointest Endosc. 2002;56:852-857. |
| 27. | Tearney GJ, Brezinski ME, Southern JF, Bouma BE, Boppart SA, Fujimoto JG. Optical biopsy in human gastrointestinal tissue using optical coherence tomography. Am J Gastroenterol. 1997;92:1800-1804. |
| 28. | Kobayashi K, Izatt JA, Kulkarni MD, Willis J, Sivak MV Jr. High-resolution cross-sectional imaging of the gastrointestinal tract using optical coherence tomography: preliminary results. Gastrointest Endosc. 1998;47:515-523. |
| 29. | Yoshino J, Nakazawa S, Inui K, Katoh Y, Wakabayashi T, Okushima K, Kobayashi T, Nakamura Y, Watanabe S, Asakura N. Gastric wall structure using a 30 MHz endoscopic ultrasound probe, focusing upon delineation of the muscularis mucosae. Dig Endosc. 2000;12:233-236. |
| 30. | Kawano T. Endoscopic esophageal ultrasonography using a sonoprobe system with transparent overtube and 20 Mhz ultrasonic images. Gastroenterol Endosc. 1992;34:1237-1251. |