Published online Aug 28, 2009. doi: 10.3748/wjg.15.4075
Revised: July 20, 2009
Accepted: July 27, 2009
Published online: August 28, 2009
Celiac disease can be triggered by upper abdominal surgery, such as vagotomy, oesophagectomy, pancreaticoduodenectomy, and gastrojejunal anastomosis. Here we report a case of a 24 year-old woman who developed celiac disease after an ileal resection for perforated Meckel’s diverticula. This is the first reported celiac case that has been triggered, not by upper abdominal surgery, but after ileal resection for Meckel’s diverticula.
- Citation: Topal F, Akbulut S, Topcu IC, Dolek Y, Yonem O. An adult case of celiac sprue triggered after an ileal resection for perforated Meckel’s diverticulum. World J Gastroenterol 2009; 15(32): 4075-4076
- URL: https://www.wjgnet.com/1007-9327/full/v15/i32/4075.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4075
Celiac disease is an autoimmune enteropathy often seen in gluten sensitive patients[1]. It has two presentations in adults, namely the classical (diarrhea-predominant) type and the silent type[2]. The silent group includes atypical presentations. Some initiating factors, such as gluten overload, surgery, giving up smoking, and infections can trigger the disease, which can become apparent in an abrupt manner[34].
Meckel’s diverticulum is a common congenital anomaly of the small bowel. Ulcer, hemorrhage, intussusception, intestinal obstruction, perforation, and, very rarely, vesicodiverticular fistulae and tumors are complications of these diverticula[5]. We present a case of Meckel’s diverticula that was diagnosed as celiac disease after surgery. This is the first reported case of Celiac disease that has been diagnosed after an ileal resection rather than upper abdominal surgery.
A 24 year-old woman applied to the emergency service with abdominal pain, nausea, and vomiting. She did not have any bowel movements or flatus and her abdominal pain worsened after her hospitalization. There was tenderness and guarding on abdominal palpation. Her initial laboratory tests revealed a leukocytosis score of 14 000/mm3.
Due to her worsening abdominal pain and a white blood cell count that progressively increased to 16.000/mm3, urgent surgery was performed for an acute abdomen. Perforated Meckel’s diverticula, located 80 cm proximal to the ileocecal valve, were observed during the operation. Ten centimeters of small bowel segment including the Meckel’s diverticulum was resected and an end-to-end anastomosis was performed. Pathological investigation of the surgical specimen revealed perforated Meckel’s diverticula and segmental ileal resection. The patient was discharged 4 d after the surgery without any complications.
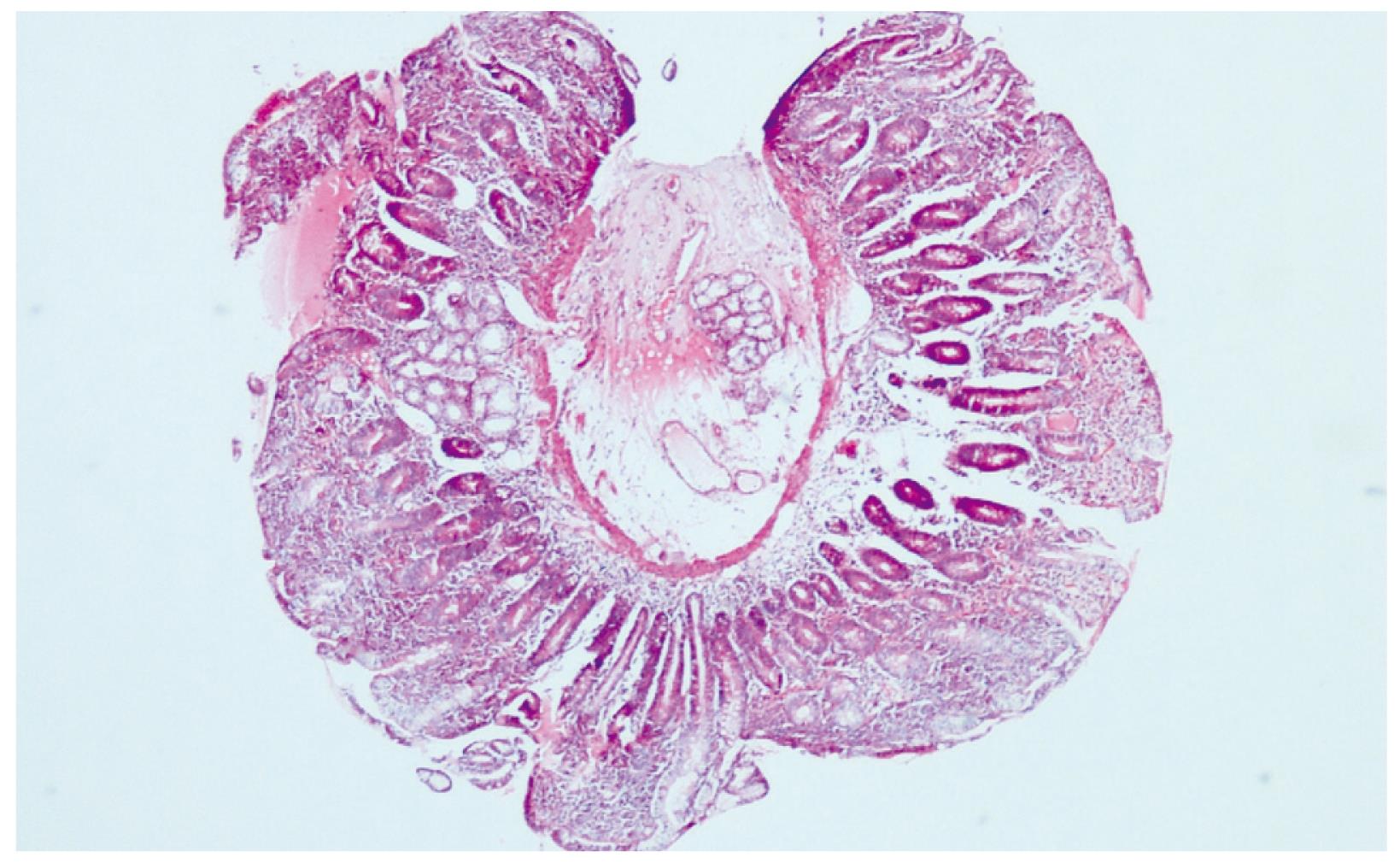
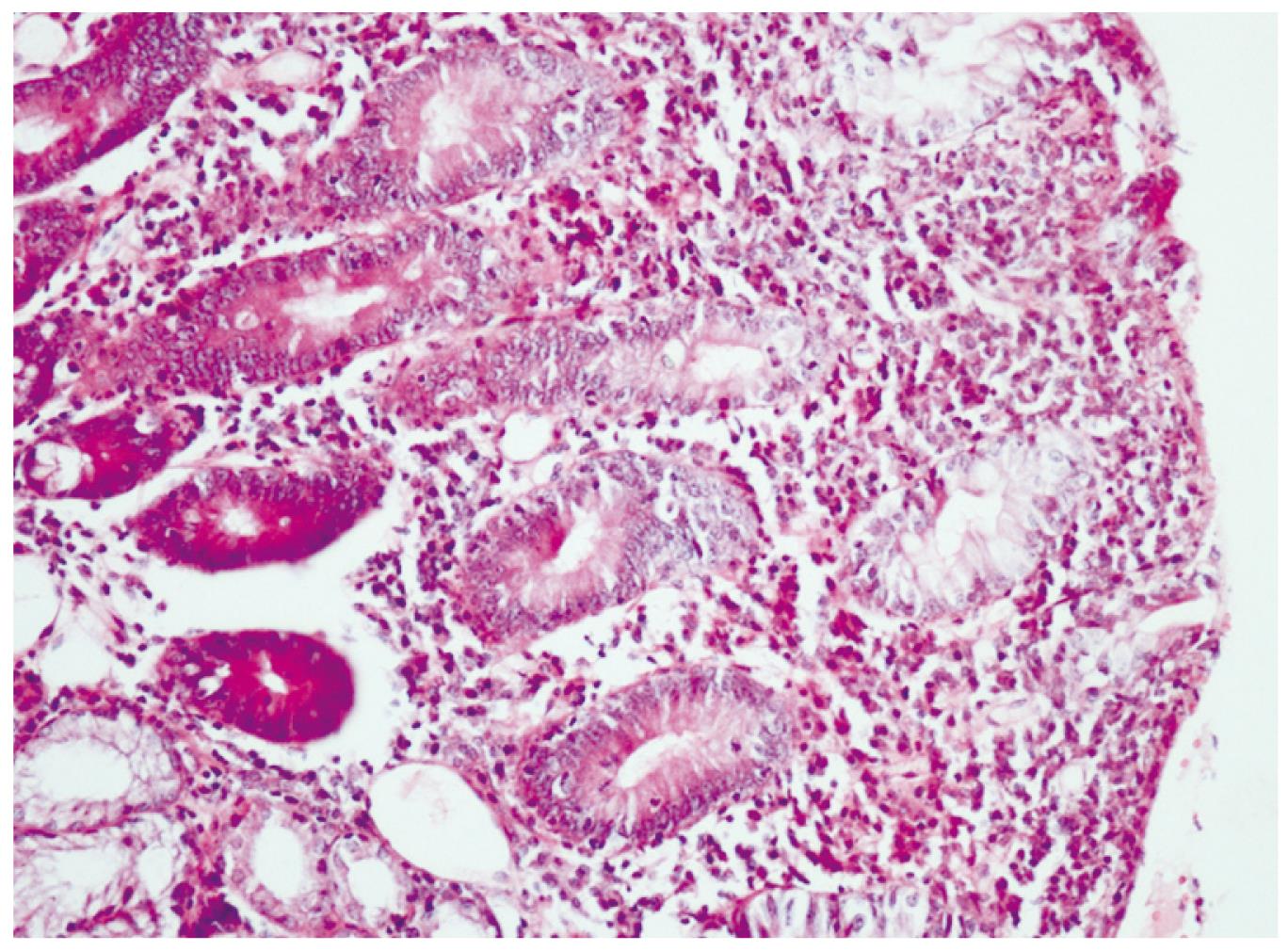
Twenty days after the discharge, the patient applied to the gastroenterology clinic with complaints of abdominal pain, flatulence and a loose stool 4-times/d. Her laboratory examination revealed Hb:10 g/dL, WBC. 5400/mm3, Plt. 458.000/mm3, vitamin B12: 119 ng/mL (180-914), AST: 14 U/L (5-45), ALT: 14 U/L (5-45), and ALP: 260 U/L (80-270). An upper gastrointestinal endoscopy was performed for her anemia, which showed antral gastritis and scalloping of duodenal mucosal folds. Endoscopic duodenal biopsy revealed diffuse atrophic villi with an increase in intraepithelial lymphocytes suggesting celiac disease (Figures 1 and 2). For confirmation of Celiac disease, gluten antibodies were determined as follows: anti-gliadin IgA, 88.2 U/mL (0-12); anti-gliadin IgG > 100 U/mL (0-12); anti-endomysial IgA antibody, (+++); anti tissue transglutaminase IgG, 51.2 U/mL (0-10); and anti tissue transglutaminase IgA, > 200 U/mL (0-10). Gluten was removed from the diet and thereafter her complaints of abdominal pain, flatulence, and diarrhea resolved. Her laboratory tests after a 2-mo gluten free diet were; Hb 12.2 g/dL and vitamin B12: 461 ng/mL (180-914).
Celiac disease is an autoimmune enteropathy seen in gluten sensitive patients. It is a common genetic disorder with a prevalence of 1%-2%[6]. The disease can manifest itself by different clinical presentations. There are gastrointestinal symptoms, diarrhea and weight loss in the classical type, while extra intestinal findings are most common in the atypical or subclinical form[2–4].
Our patient could have been in the silent form of the disease that became overt after the triggering effect of surgery. There are celiac disease patients in the literature that were triggered by upper gastrointestinal surgery, such as vagotomy, oesophagectomy, pancreaticoduodenectomy, and gastrojejunal anastomosis[7–10]. Our case is the first report of celiac disease being triggered by ileal surgery.
The autoimmune activation mechanism triggered by the surgery is not yet known. However, it has been postulated that raised intestinal permeability might be involved in the pathogenesis of celiac disease[11]. Andersen et al[12] have shown by a triple sugar test that bowel permeability is increased in ileostomy patients. Perhaps this hyperpermeability could be the triggering factor in our patient. Another possible mechanism for the emergence of post-operative Celiac disease in our patient could be antigenic overload secondary to postoperative changes[9].
Early diagnosis of Celiac disease in the postoperative period is important to prevent complications. A clinician should be aware of Celiac disease when the patient has refractory diarrhea, anemia, weight loss, and hypoalbuminemia after ileal surgery, not just after upper gastrointestinal surgery.
| 1. | Lima VM, Gandolfi L, Pires JA, Pratesi R. Prevalence of celiac disease in dyspeptic patients. Arq Gastroenterol. 2005;42:153-156. |
| 2. | Green PH. The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology. 2005;128:S74-S78. |
| 3. | Farrell RJ, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180-188. |
| 4. | Snook JA, Dwyer L, Lee-Elliott C, Khan S, Wheeler DW, Nicholas DS. Adult coeliac disease and cigarette smoking. Gut. 1996;39:60-62. |
| 5. | Sagar J, Kumar V, Shah DK. Meckel‘s diverticulum: a systematic review. J R Soc Med. 2006;99:501-505. |
| 6. | Torres MI, Lopez Casado MA, Rios A. New aspects in celiac disease. World J Gastroenterol. 2007;13:1156-1161. |
| 7. | Bai J, Moran C, Martinez C, Niveloni S, Crosetti E, Sambuelli A, Boerr L. Celiac sprue after surgery of the upper gastrointestinal tract. Report of 10 patients with special attention to diagnosis, clinical behavior, and follow-up. J Clin Gastroenterol. 1991;13:521-524. |
| 8. | Hedberg CA, Melnyk CS, Johnson CF. Gluten enteropathy appearing after gastric surgery. Gastroenterology. 1966;50:796-804. |
| 9. | Maple JT, Pearson RK, Murray JA, Kelly DG, Lara LF, Fan AC. Silent celiac disease activated by pancreaticoduodenectomy. Dig Dis Sci. 2007;52:2140-2144. |
| 10. | ten Bokkel Huinink D, de Meijer PH, Meinders AE. Coeliac disease clinically manifest after vagotomy and oesophagectomy. Neth J Med. 1996;49:235-238. |
| 11. | Johnston SD, Smye M, Watson RP. Intestinal permeability tests in coeliac disease. Clin Lab. 2001;47:143-150. |
| 12. | Anderson AD, Jain PK, Fleming S, Poon P, Mitchell CJ, MacFie J. Evaluation of a triple sugar test of colonic permeability in humans. Acta Physiol Scand. 2004;182:171-177. |