Published online Aug 28, 2009. doi: 10.3748/wjg.15.4016
Revised: July 19, 2009
Accepted: July 26, 2009
Published online: August 28, 2009
AIM: To test whether the expression and activity of H,K-ATPase in parietal cells would be affected by cigarette smoke extract.
METHODS: Extracts of cigarette smoke were administered into mice by gastric gavage (5 mg/kg body weight/day) for 3 d or in drinking water for 7 or 14 d. For the latter, each day a mouse consumed 5 mL water containing extracts of two cigarettes, on average. Control littermate mice received only vehicle. To compare the amount of H,K-ATPase in control and smoke-treated mice, the stomach was processed for Western blotting and immunohistochemical analysis using monoclonal antibodies specific for α- or β-subunits of H,K-ATPase. The p-nitrophenylphospatase activity assay was used as a measurement for K-dependent H,K-ATPase activity.
RESULTS: Probed transblots showed an increase in the amount of H,K-ATPase in smoke-treated mice which was confirmed by immunohistochemistry and was found to be due to increased amounts of protein per parietal cell rather than an increased parietal cell number. The increase in the amount of H,K-ATPase was associated with an enhancement of its enzymatic activity. K-dependent activity in control and smoke-treated mice was significantly different (respectively, 0.12 μmol/mg vs 0.27 μmol/mg per minute, P < 0.05).
CONCLUSION: Administration of cigarette smoke extract is associated with an increase in the amount and activity of H,K-ATPase and hence, smokers are susceptible to development of peptic ulcer.
- Citation: Hammadi M, Adi M, John R, Khoder GA, Karam SM. Dysregulation of gastric H,K-ATPase by cigarette smoke extract. World J Gastroenterol 2009; 15(32): 4016-4022
- URL: https://www.wjgnet.com/1007-9327/full/v15/i32/4016.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4016
Cigarette smoking is a major worldwide health problem. According to the World Health Organization, smoking is the largest preventable cause of premature death worldwide. It is a common habit among teenagers, adults, and even health professionals[1]. In addition to the well known adverse effects of smoking on cardiovascular and respiratory systems, some studies have shown that smoking is a major risk factor for some gastrointestinal diseases[2–4]. Several clinical and epidemiological studies have provided evidence suggesting that smokers are more susceptible to peptic ulcer disease and respond to anti-ulcer drugs less efficiently than non-smokers[4–9]. In experimental animals, some studies have shown that cigarette smoking potentiates the damaging effects of ethanol or corticosteroids on the gastric mucosa[1011].
The mechanisms by which cigarette smoking adversely affects the gastric mucosa have not been fully elucidated. It has been shown that free radical production, infiltration of neutrophils, stimulation of angiotensin II production, down-regulation of epidermal growth factor and reduction of gastric blood flow play an important role in the harmful effects of smoking[712–14]. However, it is not known whether cigarette smoking or nicotine adversely affects some other factors that may influence the integrity of the gastric mucosa. The hydrochloric acid secreted by parietal cells is one of the main aggressive factors that play an important role in gastric mucosal damage and the pathogenesis of peptic ulcer disease[1516]. The major protein involved in this process of acid secretion is the proton pump or H,K-ATPase. It is not known whether the H,K-ATPase of gastric parietal cells is altered by cigarette smoking.
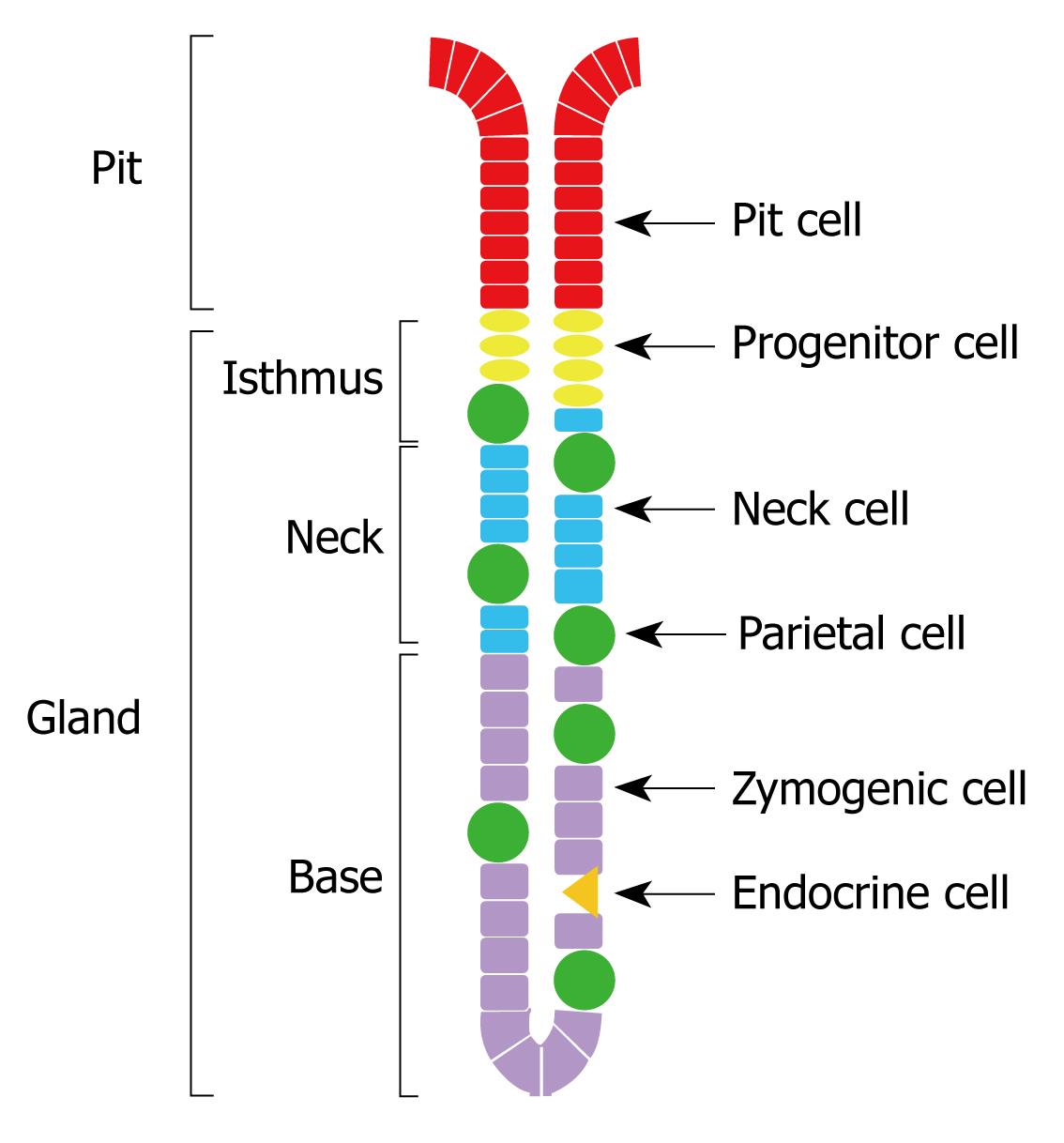
In the stomachs of rodents and humans, parietal cells are scattered throughout the gastric glands, made of pit, isthmus, neck and base regions (Figure 1). They develop from epithelial progenitors which are anchored in the isthmus region[17–19]. During their development, parietal cells concomitantly synthesize the catalytic α- and regulatory β-subunits of H,K-ATPase[20]. Following their maturation in the isthmus, parietal cells bidirectionally migrate to become scattered throughout the glandular regions of the gastric epithelium. In mice, the turnover time of parietal cells averages 54 d. Old parietal cells undergo progressive physiological deterioration and eventually die and are eliminated at the luminal surface by extrusion into the gastric lumen or deep at the gland bottom by phagocytosis via a neighboring healthier glandular cell or an invasive connective tissue macrophage[21].
In the cytoplasm of parietal cells, both α- and β-subunits of H,K-ATPase are targeted to the membranes of tubulovesicles. Upon stimulation by histamine, acetylcholine, or gastrin, the tubulovesicles translocate from the cytoplasm to the apical and canalicular membranes of the parietal cell. Therefore, expansion of the canalicular system and elongation of the microvilli are features of a stimulated parietal cell. H,K-ATPase of the apical and canalicular membranes of stimulated parietal cells utilizes ATP generated by the numerous large mitochondria to pump protons into the lumina of canaliculi and gastric glands in exchange for potassium[22]. While much research is directed to discover new inhibitors of gastric H,K-ATPase, little is known about the extrinsic factors involved in its dysregulation.
Since smokers are more susceptible than nonsmokers to developing peptic ulcer disease and more likely to experience delays in ulcer healing, and since parietal cells are considered a key player during the pathogenesis and healing of this disease, we hypothesized that smoking alters H,K-ATPase, the major protein of the parietal cell which is responsible for acid secretion. Therefore, the aim of this study was to test whether the expression and activity of H,K-ATPase of parietal cells would be affected in animals administered with cigarette smoke extract.
The method of Shin et al[23] was slightly modified to prepare ethanol and aqueous extracts of cigarette smoke. The smoke of burning red Marlboro cigarettes (Philip Morris, Inc., Richmond, VA, USA) was bubbled into ethanol or water by using a vacuum system. The ethanol extract was allowed to evaporate and the precipitate was dissolved in 0.1% dimethyl sulfoxide (DMSO).
The procedures follow experiment in this study were in accordance with the guidelines for the care and use of laboratory animals and were approved by the Animal Research Ethics Committee of the Faculty of Medicine and Health Sciences, UAE University. In this study, C57BL mice of both sexes were used at two different age groups. (1) Young adult 8-wk-old mice (n = 12) received the ethanol smoke extract, 5 mg/kg body weight/day, via orogastric gavage needle on 3 consecutive days. (2) Weaning-age mice (3-wk-old, n = 32) received the aqueous smoke extract in their drinking bottles which were made freely accessible for 7 or 14 continuous days. Fresh extract-containing water was used daily. It was estimated that every day, on average, each weaned mouse consumed 5 mL of water containing smoke extract of two cigarettes. For both age groups of smoke-treated mice, weight- and sex-matched littermate (8- or 3-wk-old) mice were used as controls and received only vehicle (0.1% DMSO or water, respectively). One day after treatment, each pair of smoke-treated and control littermate mice was killed by an overdose of ether. The stomachs were immediately removed and processed for biochemical and immunohistochemical analyses.
Gastric mucosal homogenates were prepared as previously described[2024]. Part of the oxyntic mucosa of the stomach was scraped, minced and then homogenized on ice-cold hypotonic buffer (pH 6.7) containing 113 mmol/L mannitol, 37 mmol/L sucrose, 0.4 mmol/L EDTA, 5 mmol/L piperazine-N,N’-bis(2-ethanesulfonic acid)-tris (hydroxymethyl) aminomethane. The crude homogenate was centrifuged at a low speed (35 g) for 5 min to remove unbroken cells and tissues. To obtain pellets enriched in H,K-ATPase, some solubilized crude homogenates of control and smoke-treated mice were centrifuged at a higher speed (25 000 g) for 2 h. Portions of the low-speed supernatants and the re-suspended high-speed pellets of control and smoke-treated mice were processed for measurement of protein concentration using Bradford’s method and then quantification of H,K-ATPase using Western blotting.
Portions of the homogenates or re-suspended pellets of control and smoke-treated mice were solubilized in buffer containing 1% SDS, 0.5 mol/L urea, 5% 2-mercaptoethanol, 0.25 mmol/L EDTA, 10% glycerol, 0.0025% bromophenol blue, and 30 mmol/L Tris-HCl, pH 6.8 and run through 8% or 10% acrylamide[20]. Proteins were subsequently electro-transferred onto nitrocellulose membranes (Schleicher & Schuell Bioscience, Dassel, Germany) and probed with mouse monoclonal antibodies specific for the α- (97 kDa) or β- (60-80 kDa) subunits of H,K-ATPase (Medical & Biological Laboratories Co., Woburn, MA, USA) at a dilution of 1:2000. After washing, blots were incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin (Ig) G (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) at 1:10 000 dilution. To control equal loading of proteins in both smoke-treated and control mice, anti-β-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used at 1:2000 dilution. Bands of protein-antibody complexes were visualized using SuperSignal West Pico chemiluminescence detection kit (Thermo Fisher Scientific, Rockford, IL, USA) and their relative intensities were quantified by densitometry using the Scion Imaging program for Windows (version β 4.0.3.2; developed by Scion, Frederick, MD, USA). Data were expressed as means in arbitrary units and the level of significance of differences between control and smoke-treated groups were determined by using the Student’s t test. P < 0.05 was taken as significant.
Pieces of the oxyntic regions of the stomachs of control and smoke-treated mice were fixed in Bouin’s solution and embedded in paraffin. For general histology, some sections (5 μm) were stained with hematoxylin-eosin or periodic acid Schiff technique. Adjacent sections were used for immunohistochemical analysis using antibodies specific for the α- or β-subunits of the gastric H,K-ATPase. Antigen-antibody binding sites were visualized by using fluorescine isothiocyanate (FITC)-labeled donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA).
To measure the intensity of H,K-ATPase immunolabeling of the cells, image analysis was performed on probed gastric mucosal tissue sections of some control (n = 4) and smoke-treated littermate mice (n = 4) using the Scion Imaging program as previously described[20]. Probed sections were examined at 40 × magnification with an Olympus microscope and photographed with a DP-70 digital camera with the option of fixed manual exposure to ensure equal exposure of control and smoke-treated tissue sections. Digitalized TIFF images of immunolabeled parietal cells were stored at a resolution of 300 dpi. Labeled parietal cells cut through their nuclei were only considered for measurement by using the freehand tool and drawing a line around the periphery of the cell. The number of cells examined per animal ranged from 20 to 35. Following measurement of the immunostaining intensity of various cells in one section, the background intensity was subtracted. Quantitative results of the optical density were reported in arbitrary units corresponding to immunostaining intensity which is indicative of the amount of H,K-ATPase in the sectioned cells. For each stained cell examined, the area was also measured and expressed in arbitrary units. Data are presented as mean ± SE.
In some experiments, the gastric mucosa of control and smoke-treated mice were homogenized and briefly centrifuged at low speed as mentioned above. Some of the supernatant was processed by the Bradford method for measurement of protein concentration and the remainder for K+-dependent p-nitrophenylphosphatase (pNPPase) activity assay which was used as an index of H,K-ATPase activity[20]. The pNPPase activity was measured at 37°C in buffer containing 7.5 mmol/L Tris-HCl (pH 7.5), 3.5 mmol/L MgSO4, 30 mmol/L sucrose, and 0.02 mmol/L EDTA. To eliminate the contribution of Na,K-ATPase, 0.1 mmol/L ouabain was included in the incubation buffer. The K-dependent and Na-dependent pNPPase activity was assayed with 20 mmol/L KCl and NaCl in the buffer, respectively. The reaction was initiated by the addition of 5 mmol/L sodium p-nitrophenyl phosphate and terminated by 1.5 mL of 0.5 mol/L NaOH. Liberated p-nitrophenol was read at 410 nm by a Beckman Du 70 spectrophotometer. The K-dependent pNPPase activity was obtained from the difference of the values obtained with and without 20 mmol/L KCl. The enzymatic activity was expressed in micromoles per milligram protein per minute. Student’s t test was used to compare values in control vs smoke-treated mice.
In the present study, extracts of cigarette smoke were administered to adult and weaned mice in two different modes: (1) aqueous extract in drinking water, and (2) ethanol extract via orogastric gavage needle. These two different modes of administration showed more or less similar effects on gastric H,K-ATPase. However, when the smoke extract was introduced with the drinking water, a duration-dependent effect was noted. The 7-d exposure of the gastric mucosa to smoke extract in the drinking water produced no significant difference between control and smoke-treated mice. When the duration of administration of smoke-containing drinking water was doubled, a significant difference was noted in the H,K-ATPase of smoke-treated vs control mice.
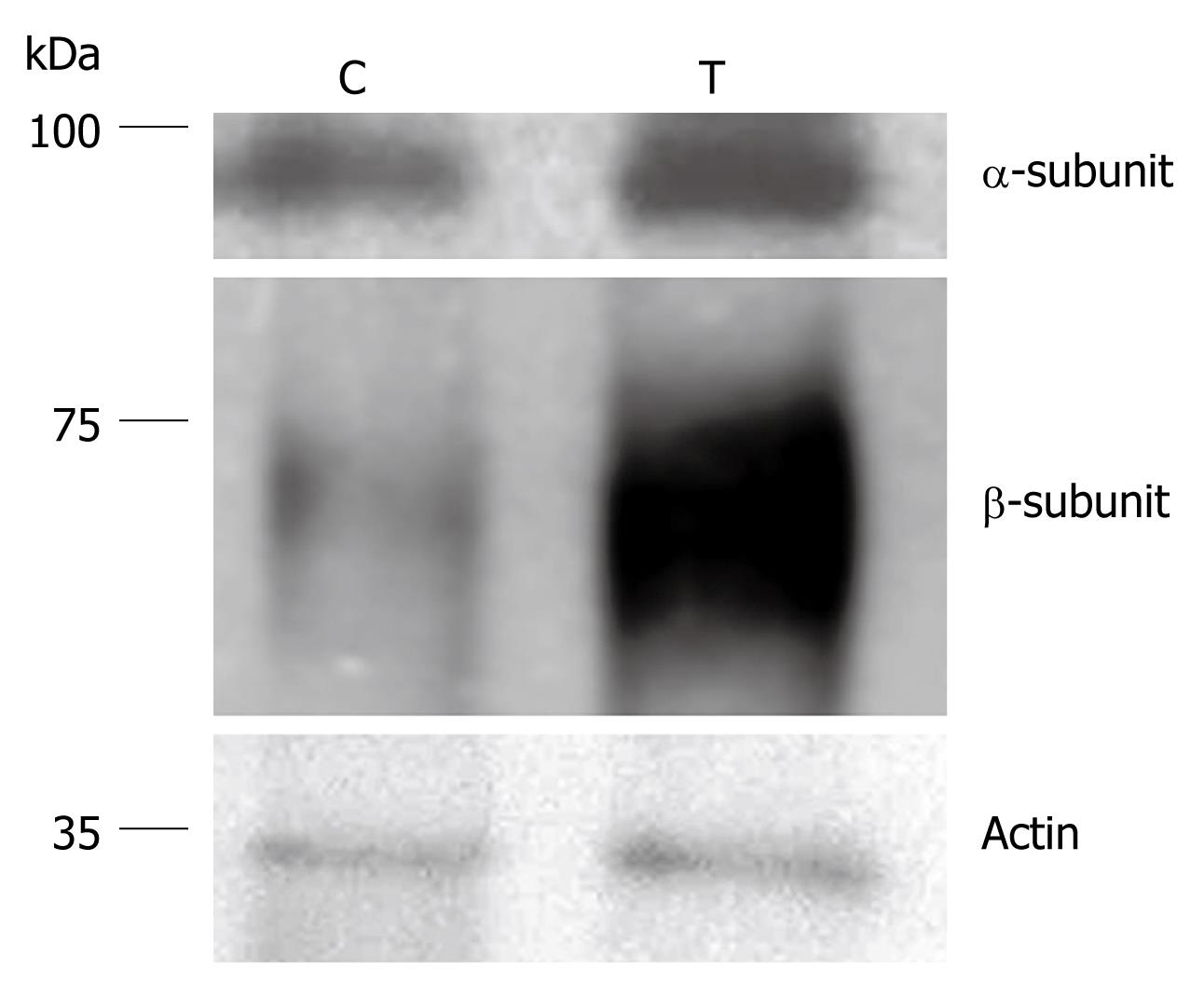
Proteins of the mucosal homogenates of mice treated with ethanol or aqueous extracts of cigarette smoke and their control littermates were separated on polyacrylamide gels and transblotted on nitrocellulose membranes. Probing of the membranes with antibodies specific for the α- or the β-subunits of H,K-ATPase revealed an increase in the amount of this protein in smoke-treated mice (Figure 2). Densitometric analysis of the protein bands of H,K-ATPase showed that the amounts of both the α- and β-subunits were significantly increased. It was estimated that the percent increase of the α subunit averaged 220% and the percent increase of the β subunit averaged 350%. However, it should be noted that in the case of aqueous extract-treated mice, there was a dose/duration-dependent effect on H,K-ATPase expression. While the 7-d-treatment showed no significant difference as compared with control littermate mice (data not shown), 14 d of treatment showed a significant increase in the amount of H,K-ATPase (P < 0.05).
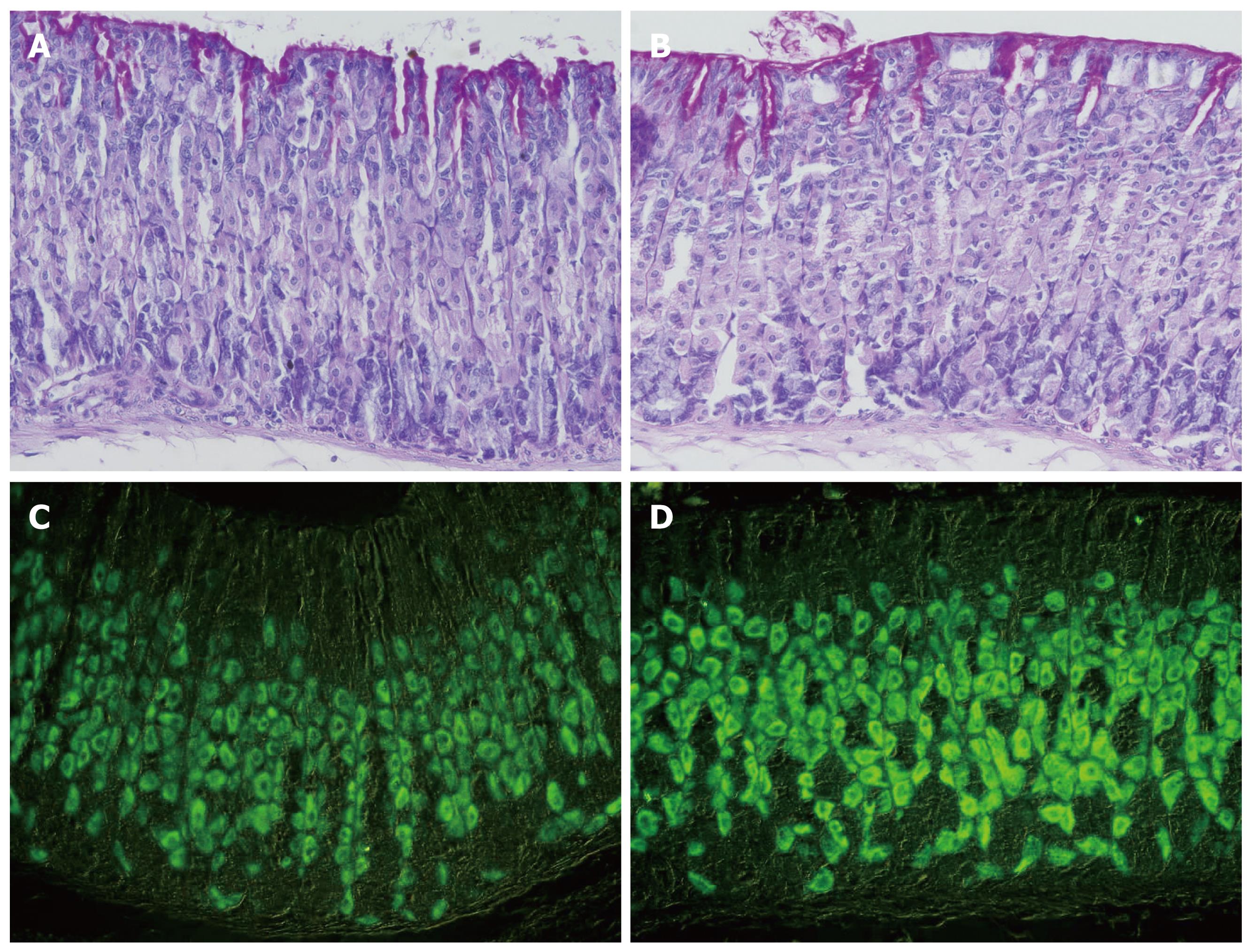
To test whether the increase in the amount of H,K-ATPase is due to an increase in parietal cell number or due to an increase in the amount of protein per cell, oxyntic mucosal tissue sections of control and smoke-treated mice were probed with antibodies specific for the α- or β-subunit of H,K-ATPase. To eliminate the possible variations in immunostaining conditions, stomach tissues obtained from smoke-treated and their littermate sex and weight-matched control mice were processed simultaneously and embedded in the same paraffin blocks. Tissue sections of control and treated mice were de-waxed and immuno-probed together on the same slides.
Microscopic examination demonstrated the usual pattern of distribution of labeled parietal cells in both control and smoke-treated tissues (Figure 3). Parietal cells were scattered throughout the mucosa of all tissues examined. Counts of parietal cells in control and smoke-treated tissues showed no significant difference. The number of H,K-ATPase-labeled parietal cells averaged 12.3 cells per gland in control mice and 11.7 cells in the gland of smoke-treated mice. However, when the intensity of the immuno-labeling was compared in control and smoke-treated tissues, an apparent difference was noted. In general, immuno-stained cells of smoke-treated tissues appeared darker than in control tissues (Figure 3). This difference reflected an increase in the amount of H,K-ATPase per cell after treatment with cigarette smoke extract. Quantification of the intensity of H,K-ATPase immunostaining confirmed this difference. Measurements of parietal cell density in each pair of control and smoke-treated mice showed that the percentages of increase in staining intensity after smoke treatment are highly significant (P < 0.001) and varied from 150% to 200%. In all mice examined, a similar staining pattern was obtained with antibodies specific for the α- and β-subunits.
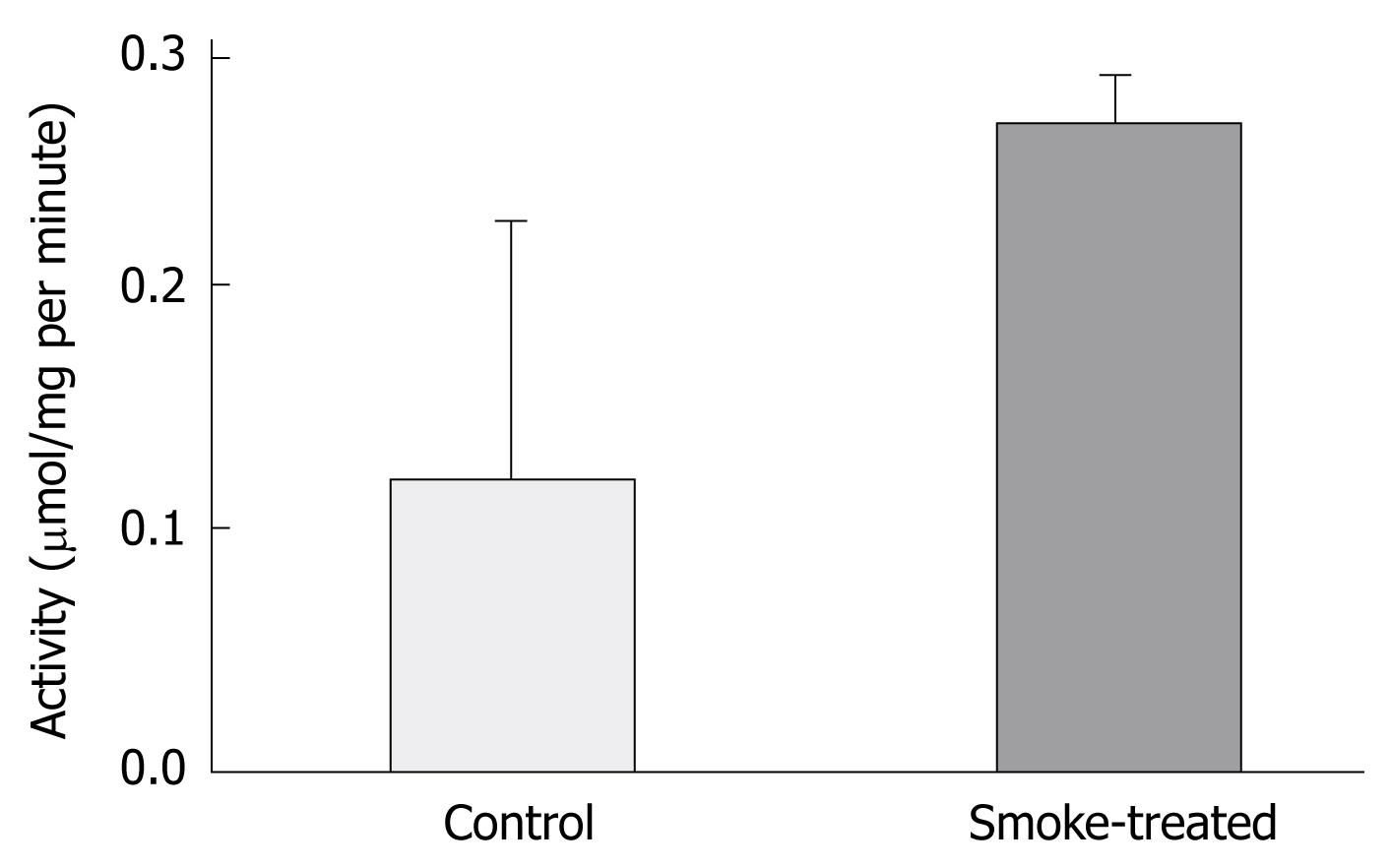
To test whether the increase in the expression of H,K-ATPase protein is associated with a change in its enzymatic activity, some of the mucosal homogenates were processed for pNPPase activity assay. The results indicated that while the enzymatic activity in control tissues averaged 0.12 μmol/mg per minute, smoke-treated tissues showed more than 2-fold increased activity, 0.27 μmol/mg per minute (P < 0.05, Figure 4).
The present study demonstrates that cigarette smoke extract enhances the expression and activity of H,K-ATPase in the oxyntic mucosa of the mouse stomach which may explain the susceptibility of smokers to development of peptic ulcer disease.
The pathogenesis of peptic ulcer disease involves several factors including enhanced acid secretion, which is regarded as one of the major ulcerogenic factors. Acid secretion is also considered the primary target of contemporary drug therapy for peptic ulcer disease.
The available data concerning the effects of cigarette smoking on gastric acid secretion are controversial. Clinical studies showed that smoking may stimulate[25–28], inhibit[2930] or have no effect[3132] on gastric acid secretion. Such disparity between studies may be explained, in part, by the differences in nicotine content and number of cigarettes used, and by the lack of adequate controls due to marked individual variability of basal gastric secretion. In the current study, we did not intend to measure acid secretion, but the plan was to dissect the event down stream and examine the major enzyme of the parietal cell, H,K-ATPase, which is responsible for acid secretion.
Nicotine is one of the main constituents of cigarette smoke responsible for the adverse effects of smoking. The effect of nicotine on gastric acid secretion is also controversial. Several lines of evidence have suggested that it has a stimulating effect. Nicotine administered to rats for 10 d caused an increase in gastric secretory volume and acid output. This finding was attributed to increased muscarinic receptor sensitivity, and consequently, basal acid secretion[33]. Lindell et al[34] also noted that nicotine administration enhances gastric acidity and impairs postprandial gastric neutralization in humans. Likewise, nicotine treatment abolishes in a dose-dependent manner the depressing effect of ethanol on acid secretion in rats[35] and significantly stimulates basal gastric acid output in cats[36]. In vitro study demonstrated that nicotine could exert direct stimulatory effects on parietal cells and potentiate the histamine-mediated response in the isolated cell model[36].
Some other studies showed contrasting findings. In one study, the gastric acid secretion stimulated by intravenous pentagastrin was completely inhibited by nicotine[37]. Another study also showed that acid output together with gastric secretory volume one hour after vagal stimulation induced by modified sham feeding was lower in human subjects on smoking than non-smoking days[38]. Based on the contrasting nature of evidence, the effect of nicotine or smoking on gastric acid secretion remained elusive.
The present study supports the view that smoking enhances gastric acid secretion by up-regulating the expression and activity of H,K-ATPase. Based on previous studies, the mechanism by which the smoke extract affected the acid secreting parietal cells could be a combination of receptor mediated and direct effects on the parietal cell.
Therefore, the present study provides an answer to some questions which were raised by some epidemiological studies. Why are cigarette smokers more susceptible to development of peptic ulcer disease? Why is recurrence of peptic ulcer more common in smokers than non-smokers? Why is the healing of peptic ulcer disease delayed in smokers? As an answer to these questions, we hypothesized that H,K-ATPase in the parietal cells is more aggressive in smokers than non-smokers. Then we tested this hypothesis by using an ethanol or aqueous extract of cigarette smoke, which were previously found to be rich in nicotine by various chromatography techniques[39]. The extract was administered to mice orally and three methods were carried out to characterize the anticipated alteration of the gastric H,K-ATPase. First, Western blotting analysis of homogenates obtained from the gastric mucosae of control and smoke-treated mice showed an increase in the amount of H,K-ATPase in the 3-d-treated adult mice and 14-d-treated weaning-age mice. However, when the weaned mice were treated for only 7 d with half the dose, there was no significant change in the amount of H,K-ATPase as compared to their control littermates. Therefore, there is a duration- and dose-dependent effect of cigarette smoke extract in weaning-age mice. During this age, the developing gastric glands undergo compartmentalization into isthmus, neck and base regions[40]. In these developing glands, parietal cells might not have acquired all the machinery to respond to an extrinsic stimulus and therefore a longer duration of treatment with cigarette smoke extract is needed. Second, immunohistochemical analysis of gastric parietal cells using antibodies specific for their H,K-ATPase revealed an enhancement in the immunostaining of parietal cells of the smoke-treated mice. Since tissues of these treated mice and their weight- and sex-matched littermate control mice were processed together, embedded in the same tissue blocks and immunoprobed simultaneously on the same slides, the differences in immunostaining intensity were taken to represent an increase in the amount of H,K-ATPase per parietal cell. Third, the proton pump activity, measured by pNPPase assay was enhanced in the gastric mucosal homogenates of smoke-treated mice compared to control mice.
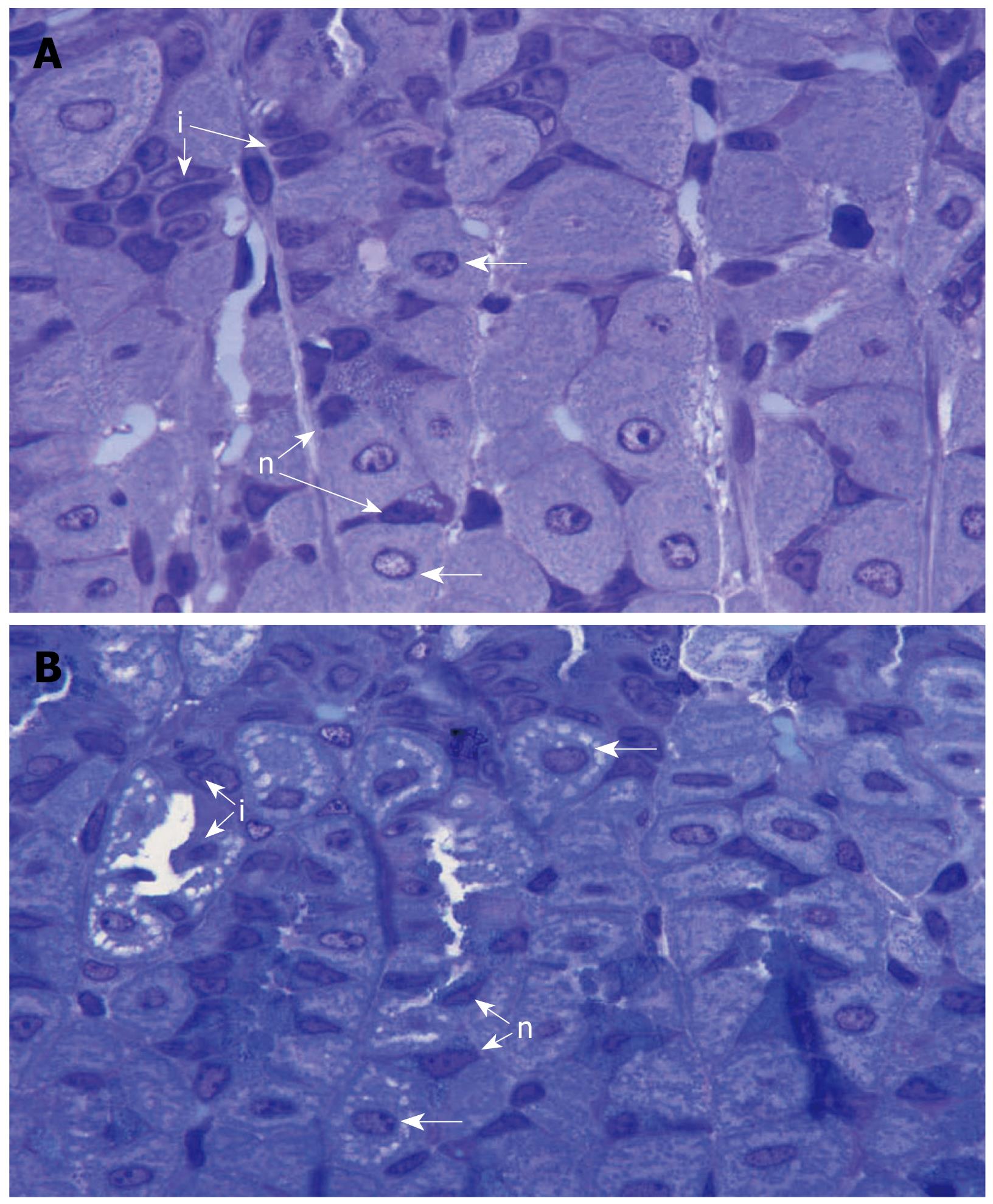
The question was raised whether the increased amount and activity of H,K-ATPase was associated with the translocation of tubulovesicles into the canalicular membranes. Some gastric mucosal tissues obtained from three pairs of smoke-treated and control mice were fixed in a Karnovesky’s solution and processed for Araldite embedding and semithin sectioning. Microscopic examination of parietal cells located in the isthmus and neck regions of the gastric glands (known to be involved in acid secretion[41]) revealed that those of the smoke treated mice tend to acquire stimulated morphology with expanded canalicular system as compared to those of control mice (Figure 5).
In conclusion, the present study demonstrates a possible explanation for the susceptibility of smokers to develop peptic ulcer disease. Therefore, we propose the following scenario. It seems that smokers develop parietal cells with an increased amount and activity of H,K-ATPase and tendency to acquire an extended canalicular system. Accordingly, parietal cells of smokers are enhanced to produce much acid upon stimulation and their gastric and duodenal mucosae become more vulnerable to development of peptic ulcer disease.
Clinical, epidemiological and experimental studies have shown that smokers are more susceptible to peptic ulcer disease and respond to anti-ulcer drugs less efficiently than non-smokers. Parietal cells are targets for anti-ulcer drugs and their inhibition is an important modality for peptic ulcer treatment.
Parietal cell activation may be associated with gastric mucosal injury and peptic ulcer disease. Since smoking is a predisposing factor to peptic ulcer, it is hypothesized that cigarette smoke extract may cause activation of gastric parietal cells. In this study, the authors demonstrated that over-expression of the gastric proton pump could be a possible mechanism for the susceptibility of smokers to peptic ulcer disease.
Previous reports have demonstrated the role of epidermal growth factors, blood flow, neutrophils, angiotensin II, and free radicals in the adverse effects of smoking on the gastrointestinal mucosa. In this study, the authors have demonstrated an additional possible effect of smoking on the expression and activity of the gastric proton pump.
By understanding how smoking may alter the biological features of the gastric mucosa, this study may help in improving the current preventive and therapeutic modalities of peptic ulcer disease.
The proton pump or H,K-ATPase is the major protein of parietal cells responsible for acid secretion. It is the main target for anti-ulcer drugs. Better understanding of the biological features of these cells and defining factors responsible for the regulation or dysregulation of their proton pump is important for designing new modalities for prevention and treatment of peptic ulcer disease.
The authors used a smoking extract to study parietal cell H,K-ATPase expression and activity. It is a very interesting study.
| 1. | Musaigera AO, Abdulraoof N. Social factors associated with smoking among men in the United Arab Emirates. Public Health. 2004;118:450-452. |
| 2. | Kamholz SL. Pulmonary and cardiovascular consequences of smoking. Med Clin North Am. 2004;88:1415-1430, ix-x. |
| 3. | Smith CJ, Perfetti TA, King JA. Perspectives on pulmonary inflammation and lung cancer risk in cigarette smokers. Inhal Toxicol. 2006;18:667-677. |
| 4. | Doll R, Jones FA, Pygott F. Effect of smoking on the production and maintenance of gastric and duodenal ulcers. Lancet. 1958;1:657-662. |
| 5. | Friedman GD, Siegelaub AB, Seltzer CC. Cigarettes, alcohol, coffee and peptic ulcer. N Engl J Med. 1974;290:469-473. |
| 6. | Korman MG, Hansky J, Eaves ER, Schmidt GT. Influence of cigarette smoking on healing and relapse in duodenal ulcer disease. Gastroenterology. 1983;85:871-874. |
| 7. | Endoh K, Leung FW. Effects of smoking and nicotine on the gastric mucosa: a review of clinical and experimental evidence. Gastroenterology. 1994;107:864-878. |
| 8. | Eastwood GL. Is smoking still important in the pathogenesis of peptic ulcer disease? J Clin Gastroenterol. 1997;25 Suppl 1:S1-S7. |
| 9. | Parasher G, Eastwood GL. Smoking and peptic ulcer in the Helicobacter pylori era. Eur J Gastroenterol Hepatol. 2000;12:843-853. |
| 10. | Chow JY, Ma L, Zhu M, Cho CH. The potentiating actions of cigarette smoking on ethanol-induced gastric mucosal damage in rats. Gastroenterology. 1997;113:1188-1197. |
| 11. | Takeuchi Y, Takahashi M, Fuchikami J. Vulnerability of gastric mucosa to prednisolone in rats chronically exposed to cigarette smoke. J Pharmacol Sci. 2008;106:585-592. |
| 12. | Seno K, Zhu JH, Barrett JD, Eggena P, Scremin OU, Lam K, Leung JW, Leung FW. Cigarette smoke increases gastric ulcer size in part by an angiotensin II-mediated mechanism in rats. Dig Dis Sci. 1997;42:74-78. |
| 13. | Chow JY, Ma L, Cho CH. Effect of cigarette smoke on ethanol-induced gastric mucosal lesions: the role of nitric oxide and neutrophils. Eur J Pharmacol. 1998;342:253-260. |
| 14. | Ma L, Chow JY, Cho CH. Mechanistic study of adverse actions of cigarette smoke exposure on acetic acid-induced gastric ulceration in rats. Life Sci. 1998;62:257-266. |
| 15. | Konturek PC, Konturek JW, Konturek SJ. Gastric secretion and the pathogenesis of peptic ulcer in the Helicobacter pylori infection. J Physiol Pharmacol. 1996;47:5-19. |
| 16. | Dickerson BA, Ott DJ, Chen MY, Gelfand DW. Peptic ulcer disease: pathogenesis, radiologic features, and complications. Acad Radiol. 2000;7:355-364. |
| 17. | Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259-279. |
| 18. | Karam SM, Alexander G, Farook V, Wagdi A. Characterization of the rabbit gastric epithelial lineage progenitors in short-term culture. Cell Tissue Res. 2001;306:65-74. |
| 19. | Karam SM, Straiton T, Hassan WM, Leblond CP. Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells. 2003;21:322-336. |
| 20. | Karam SM, Ansari HR, Al-Dhaheri WS, Alexander G. Retinol enhances differentiation of the gastric parietal cell lineage in developing rabbits. Cell Physiol Biochem. 2004;14:333-342. |
| 21. | Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec. 1993;236:314-332. |
| 22. | Yao X, Forte JG. Cell biology of acid secretion by the parietal cell. Annu Rev Physiol. 2003;65:103-131. |
| 23. | Shin VY, Liu ES, Koo MW, Wang JY, Matsui H, Cho CH. Cigarette smoke extracts delay wound healing in the stomach: involvement of polyamine synthesis. Exp Biol Med (Maywood). 2002;227:114-124. |
| 24. | Reenstra WW, Forte JG. Isolation of H+,K(+)-ATPase-containing membranes from the gastric oxyntic cell. Methods Enzymol. 1990;192:151-165. |
| 25. | Steigmann F, Dolehide RH, Kaminski L. Effects of smoking tobacco on gastric acidity and motility of hospital controls and patients with peptic ulcer. Am J Gastroenterol. 1954;22:399-409. |
| 27. | Murthy SN, Dinoso VP Jr, Clearfield HR, Chey WY. Simultaneous measurement of basal pancreatic, gastric acid secretion, plasma gastrin, and secretin during smoking. Gastroenterology. 1977;73:758-761. |
| 28. | Parente F, Lazzaroni M, Sangaletti O, Baroni S, Bianchi Porro G. Cigarette smoking, gastric acid secretion, and serum pepsinogen I concentrations in duodenal ulcer patients. Gut. 1985;26:1327-1332. |
| 29. | Wilkinson AR, Johnston D. Inhibitory effect of cigarette smoking on gastric secretion stimulated by pentagastrin in man. Lancet. 1971;2:628-632. |
| 30. | Sonnenberg A, Hüsmert N. Effect of nicotine on gastric mucosal blood flow and acid secretion. Gut. 1982;23:532-535. |
| 31. | Fung WP, Tye CY. Effect of smoking on gastric acid secretion. Aust N Z J Med. 1973;3:251-254. |
| 32. | Whitecross DP, Clarke AD, Piper DW. The effect of cigarette smoking on human gastric secretion. Scand J Gastroenterol. 1974;9:399-403. |
| 33. | Qiu BS, Cho CH, Hui SC, Ogle CW. Chronic nicotine intake increases the responses to muscarinic receptor stimulation. Pharmacology. 1992;44:41-47. |
| 34. | Lindell G, Brudin L, Ohlin P, Graffner H. Does nicotine administration influence intragastric acidity? Scand J Gastroenterol. 1992;27:143-146. |
| 35. | Cho CH, Chen BW, Hui WM, Lam SK. The influence of acute or chronic nicotine treatment on ethanol-induced gastric mucosal damage in rats. Dig Dis Sci. 1990;35:106-112. |
| 36. | Albinus M, Frisch G, Klein S. The effects of nicotine on basal and stimulated gastric secretions in the conscious cat and in isolated guinea pig gastric mucosal cells. Agents Actions. 1988;23:289-292. |
| 37. | Leung FW. Dissociated effect of nicotine on pentagastrin-stimulated acid secretion and blood flow in the rat stomach. Scand J Gastroenterol. 1994;29:782-785. |
| 38. | Lindell G, Farnebo LO, Chen D, Nexø E, Rask Madsen J, Bukhave K, Graffner H. Acute effects of smoking during modified sham feeding in duodenal ulcer patients. An analysis of nicotine, acid secretion, gastrin, catecholamines, epidermal growth factor, prostaglandin E2, and bile acids. Scand J Gastroenterol. 1993;28:487-494. |
| 39. | Shin VY, Wu WK, Ye YN, So WH, Koo MW, Liu ES, Luo JC, Cho CH. Nicotine promotes gastric tumor growth and neovascularization by activating extracellular signal-regulated kinase and cyclooxygenase-2. Carcinogenesis. 2004;25:2487-2495. |
| 40. | Karam SM, Li Q, Gordon JI. Gastric epithelial morphogenesis in normal and transgenic mice. Am J Physiol. 1997;272:G1209-G1220. |
| 41. | Karam SM, Yao X, Forte JG. Functional heterogeneity of parietal cells along the pit-gland axis. Am J Physiol. 1997;272:G161-G171. |