Published online Aug 21, 2009. doi: 10.3748/wjg.15.3960
Revised: July 17, 2009
Accepted: July 24, 2009
Published online: August 21, 2009
We analyzed the clinical manifestations and experiences of diagnosing and treating central pontine myelinolysis following living donor liver transplantation. The clinical data of three patients with central pontine myelinolysis following living donor liver transplantation from January 2005 to November 2007 were retrospectively analyzed at the West China Hospital, Sichuan University, China. The three patients developed hyponatremia prior to surgery. Case 1 suffered locked-in syndrome following surgery, and received a large dose of gamma globulin, and subsequently recovered. Case 2 was in a coma for three days, and received hyperbaric chamber treatment. This patient remained in a mild coma for six months following surgery. Case 3 developed consciousness disturbances, gradually went into a coma following surgery, and died due to pulmonary infection. Central pontine myelinolysis is a severe complication in patients following living donor liver transplantation. Large-dose gamma globulin treatment, as well as hyperbaric oxygen, might be effective therapeutic methods.
- Citation: Zhang ZW, Kang Y, Deng LJ, Luo CX, Zhou Y, Xue XS, Wang D, Yin WH. Therapy of central pontine myelinolysis following living donor liver transplantation: Report of three cases. World J Gastroenterol 2009; 15(31): 3960-3963
- URL: https://www.wjgnet.com/1007-9327/full/v15/i31/3960.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3960
With the increased number of living donor liver transplantation (LDLT) patients, some special complications have become apparent. Central pontine myelinolysis (CPM) is a severe nervous system complication following liver transplantation[1–3]. To date, no studies have been performed addressing CPM occurrence and treatment following LDLT. This study aimed to report the clinical manifestations and treatment of three patients diagnosed with CPM following LDLT from January 2005 to November 2007. The three donors in this study were lineal relatives of these patients. No organs were used from prisoners and no subjects were prisoners at the time of data collection.
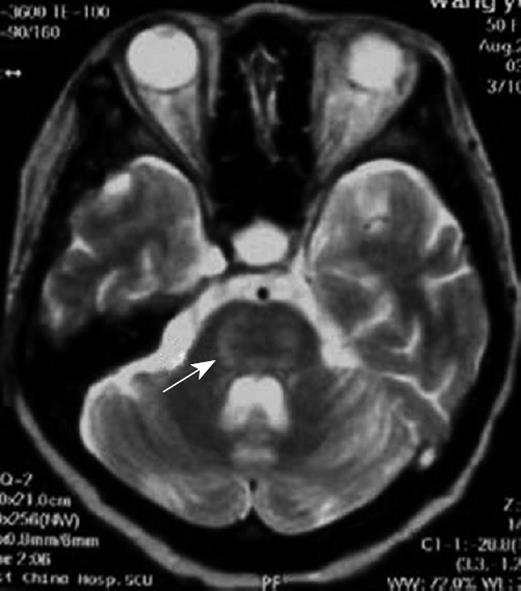
This patient was a 51-year-old female diagnosed with chronic, type B hepatitis, hepatic decompensation, and hepatic encephalopathy. Her blood type was B/Rh (-). Pre-operative examination revealed the following parameters: total bilirubin (107.9 μmol/L), direct bilirubin (53.6 μmol/L), aspartate aminotransferase (595 IU/L), glutamate-pyruvate transaminase (600 IU/L), and serum cholesterol (0.18 mmol/L). Blood electrolytes, Child-Turcotte-Pugh score, and MELD score are shown in Table 1. In November 2005, the patient underwent adult-to-adult LDLT with a right lobe graft. The donor was the patient’s son, whose blood type was B/Rh (+). Electrolytes were re-examined in the intensive care unit (Table 1). Post-operative immunosuppressive regimen was administered. The patient was treated with cyclosporine A (250 mg, twice daily), 0.75 g mycophenolic acid (oral) twice daily, and methylprednisolone (iv). Subsequent, cyclosporine A blood concentration was shown to be 180-250 mg/L. At 6 h after surgery, the patient was conscious, and the tracheal cannula was removed. On day one after surgery, the patient maintained clarity, answered questions with short sentences, exhibited locomotor activities, and drank water. On day three after surgery, the patient presented with decreased spontaneous speech, slowed actions, could not answer a simple question correctly, had indistinct pronunciation, and bucked while drinking water. On day five after surgery, the patient had stable vital body signs, clear consciousness, spontaneous eye opening, ocular movement, but did not exhibit spontaneous speech, spontaneous limb activities, or reaction to pain stimulation. Physical examination showed increased tension of limb muscles, muscle force 0°, decreased deep and superficial sensations, bilateral Babinski sign (+), and an Expanded Disability Status Scale score of 9.5. Skull nuclear magnetic resonance was subsequently performed and the findings are displayed in Figure 1. The patient was diagnosed with “locked-in syndrome” induced by CPM, and was administered citicoline (0.5 g daily via intravenous drip) and a large dose of gamma globulin (0.5 g/kg daily for ten days, iv). Oral cyclosporine A was not administered. On day twelve after surgery, the patient pronounced monosyllables, performed light limb activities with grade III muscle force, but was still not able to swallow. On day fourteen after surgery, the patient was conscious, producing longer utterances, pronounced indistinctly and slowly, demonstrated increased autonomic activities, bucked during swallowing, and presented with an Expanded Disability Status Scale score of 6. Three months after surgery, the patient presented with normal liver function, clear consciousness, normal diet, self-care, slow speech rate, and slow motor reactions.
| Case 1 | Case 2 | Case 3 | |
| Pre-operative serum sodium (mmol/L) | 124 | 119 | 119 |
| Post-operative serum sodium (mmol/L) | 151 | 153 | 141 |
| Pre-operative serum magnesium (mmol/L) | 0.52 | 0.47 | 0.64 |
| Post-operative serum magnesium (mmol/L) | 0.58 | 0.62 | 0.68 |
| Pre-operative plasma osmotic pressure (mOsm/kg H2O) | 238.71 | 232.47 | 258.45 |
| Post-operative plasma osmotic pressure (mOsm/kg H2O) | 295.36 | 288.93 | 291.92 |
| Plasma cholesterol (mmol/L) | 0.18 | 1.24 | 1.07 |
| CTP score | B | C | C |
| MELD score | 30 | 36 | 35 |
| Cyclosporine A or Fk506 concentration | Cyclosporine A 180-250 mg/L | FK506 6.5-8 ng/mL | No |
| Clinical manifestations | Locked-in syndrome | Coma | Coma |
| Special therapy | Gamma globulin (i.v.) | Hyperbaric oxygen treatment | No |
This patient was a 54-year-old male diagnosed with chronic severe hepatitis (type B), hepatic encephalopathy, and hepatorenal syndrome. His blood type was O/Rh (+). Pre-operative examination revealed the following parameters: total bilirubin (280 μmol/L), direct bilirubin (158.6 μmol/L), aspartate aminotransferase (269 IU/L), glutamate-pyruvate transaminase (188 IU/L), serum cholesterol (1.24 mmol/L), and serum creatinine (263.7 μmol/L). Blood electrolytes, Child-Turcotte-Pugh score, and MELD score are presented in Table 1. In March 2006, the patient received adult-to-adult LDLT with a right lobe graft. The donor was the patient’s brother, whose blood type was O/Rh (+). Electrolytes were re-tested in the intensive care unit (Table 1).
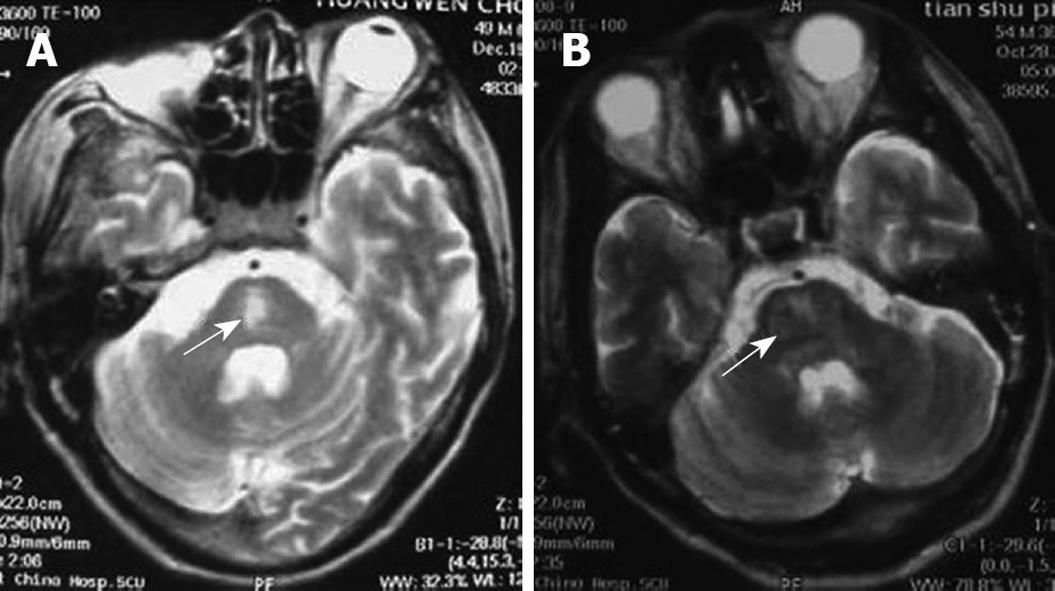
Post-operative immunosuppressive regimen was administered. The patient received tacrolimus (FK506, 2 mg twice daily, oral), 0.75 g mycophenolic acid (oral, twice daily), and methylprednisolone (iv). Subsequent tacrolimus blood concentration was found to be 6.5-8 ng/mL. At 11 h after surgery, the patient was conscious, and the tracheal cannula was removed. The patient had stable vital body signs, poor mental state, and little spontaneous speech. On day two after surgery, the patient presented with lethargy, eye-opening reaction to powerful stimulation, and indistinct pronunciation. On day three after surgery, the bilateral pupils reacted poorly to light reflex and were equal in size and shape. The patient suffered from moderate coma, with decreased muscular tension, Babinski sign of the right side (+), Babinski sign of the left side (-), and a Glasgow-Pittsburgh score of 20. On day 6 after surgery, the patient received a tracheotomy, and respiration was supported by a respirator due to pulmonary infection. CPM was diagnosed with skull magnetic resonance imaging (Figure 2A). On day ten after surgery, the patient developed moderate coma, with good recovery of liver function. Following hyperbaric chamber treatment (2 h, once per day, for 14 d), the patient presented with good light reflex in both pupils, mild coma, increased autonomic activities, improved tension of limb muscles, and a Glasgow-Pittsburgh score of 26. At six months, the patient suffered from mild coma, but exhibited good liver function.
This patient was a 47-year-old male diagnosed with chronic severe hepatitis (type B), upper gastrointestinal bleeding, and hepatorenal syndrome. His blood type was B/Rh (+). Pre-operative examination revealed the following parameters: total bilirubin (197.4 μmol/L), direct bilirubin (116.5 μmol/L), aspartate aminotransferase (141 IU/L), glutamate-pyruvate transaminase (107 IU/L), serum cholesterol (1.07 mmol/L), and serum creatinine (207.7 μmol/L). Blood electrolytes, Child-Turcotte-Pugh score, and MELD score are presented in Table 1. In August 2007, the patient received adult-to-adult LDLT with a right lobe graft. The donor was the patient’s wife, whose blood type was B/Rh (+). Electrolytes were re-measured in the intensive care unit (Table 1). Post-operative immunosuppressive regimen was performed. The patient was treated with zenapax (containing daclizumab), 0.75 g mycophenolic acid (oral, twice daily), and methylprednisolone (iv). At 9 h following surgery, the patient was conscious, and the tracheal cannula was removed. The patient received hemofiltration due to worsened renal function. At 1 wk, the patient was conscious, displayed autonomic activities, reduced speech, low speech sound, and could provide correct answers to simple questions. Both pupils exhibited good light reflexes and normal ocular fundus, and were equal in size and shape. Tendon reflexes were symmetric and active, with pathological sign (-). Muscular tension of the upper limbs was increased, with flexion deformity and grade II muscle force, but muscular tension of the lower limbs was decreased. On day 8 after surgery, the patient went into a coma, opened eyes when powerfully stimulated, could not pronounce or perform autonomic limb activities, and presented with normal liver and kidney function. CPM was diagnosed using skull magnetic resonance imaging (Figure 2B). On day 14 after surgery, the patient suffered from mild coma and received a tracheotomy, his respiration was supported by a respirator due to pulmonary infection. Subsequently, the patient developed severe sepsis and multiple organ failure, and died at twenty-three days following surgery.
CPM is a severe complication of the nervous system following liver transplantation, with high mortality and disability rates[4–6]. CPM is difficult to diagnose, due to the various clinical manifestations, and precise epidemiological data are lacking. From January 2005 to December 2007, 3/184 patients at the Center for Liver Transplantation, West China Hospital, Sichuan University, China undergoing living donor liver transplantation were diagnosed with CPM, with an occurrence rate of 1.63%.
CPM following liver transplantation is due to multiple factors. (1) Severe hyponatremia, especially during rapid correction of chronic hyponatremia: patients who develop CPM following liver transplantation suffer from severe hyponatremia prior to transplantation. However, not all hyponatremia patients develop CPM[7]. Rapid correction of hyponatremia, as well as larger alterations in plasma osmotic pressure, influences CPM occurrence[1]. Increased serum sodium (> 15 mmol/L) within 24 h has also been closely associated with CPM occurrence[1]. (2) Neurotoxicity of cyclosporine A and tacrolimus can induce or aggravate CPM. Cyclosporine A-related motor aphasia patients were shown to develop CPM, as diagnosed by skull magnetic resonance imaging[89]. (3) Severe damage to liver function prior to surgery is associated with CPM occurrence, especially in patients with hepatic encephalopathy and hypocholesterolemia. (4) Hemorrhage, infection, vascular complications, and impaired graft function following liver transplantation can result in CPM occurrence. Immune factors might participate in CPM pathogenesis, such as myelin sheath antibody formation[1011]. In the present study, these three cases developed severe hyponatremia prior to surgery. Serum sodium ion concentration increased by > 20 mmol/L, and plasma osmotic pressure was greatly altered 6-8 h after surgery. These factors were responsible for CPM occurrence. Living donor liver transplantation is an elective procedure. Thus, there should be ample opportunity to correct serum sodium before surgery. A large number of blood products and synthetic colloids should be infused into patients during the peri-operative period, such as erythrocyte suspension, fresh frozen plasma, platelets, cryoprecipitate, hetastarch, and succinylated gelatin. These liquids contain many sodium salts, which increase serum sodium within a short period of time. More attention should be paid to regulating serum sodium during the peri-operative period to avoid adverse outcomes due to rapid correction of hyponatremia. It is also important for anesthesiologists to correct serum sodium very slowly during surgery. In case 1, the post-operative immunosuppressive agent was cyclosporine A. The blood drug concentration ranged between 180-250 mg/L. In case 2, the post-operative immunosuppressive agent was tacrolimus, and the blood drug concentration ranged between 6.5-8 ng/mL. Although blood drug levels were not significantly increased, severe liver disease-induced metabolic disturbances could lead to brain glial cell damage. It seems that critically ill patients with end-stage liver disease may have an organic mental syndrome. Any insult such as calcinerin inhibitors under these circumstances may cause further damage resulting in CPM.
The clinical manifestations of CPM are multi-faceted following liver transplantation, and include mental disorder, cortical blindness, pseudobulbar palsy, aphasia, ataxia, visual hallucination, epileptic seizure, locked-in syndrome, and coma[12–14]. Case 1 was diagnosed with locked-in syndrome, meaning that the bilateral corticospinal tract and corticobulbar tract were blocked surrounding the pontine abducent nucleus. The characteristics of this syndrome include quadriplegia and anarthria, with preservation of consciousness. Patients retain vertical eye movement, and can facilitate non-verbal communication. Few patients with this syndrome have been reported, especially in LDLT. Cases 2 and 3 suffered from coma. Magnetic resonance imaging has proven to be a good diagnostic method for CPM. Positive diagnosis has not been detected in many patients using magnetic resonance imaging following the onset of clinical symptoms. This was associated with abnormal imaging manifestations following clinical symptoms.
To date, there remains a lack of effective therapeutic methods for CPM. The present methods primarily attempt to nourish brain cells, improve cerebral metabolism, and reduce immunosuppressive concentrations. In addition, gamma globulin has been injected intravenously into CPM patients, resulting in improved scores on the Expanded Disability Status Scale[1011]. In the present study, case 1 was treated with a large dose of gamma globulin and was tested according to the Expanded Disability Status Scale. Case 2 received hyperbaric oxygen treatment and underwent Glasgow-Pittsburgh Score testing. Case 1 presented with improved (> 36%) scores on the Expanded Disability Status Scale following treatment. This might be correlated to treatment with large-dose gamma globulin which destroys myelin sheath-related antibodies by decreasing toxic substances in the myelin sheath and by accelerating remyelination. Case 2 presented with improved (30%) scores on the Glasgow-Pittsburgh Score following treatment. This might be correlated to hyperbaric oxygen, which reduced myelin sheath destruction, accelerated cell regeneration, and decreased edema in the injured regions. However, we cannot thoroughly assess the outcomes of these two methods, due to the limited number of cases.
CPM, a life-threatening illness, is difficult to diagnose and treat, and presents with a poor prognosis following living donor liver transplantation. More attention should be directed to the risk factors for CPM to avoid clinical complications, and to continue the search for an effective therapeutic method.
| 1. | Abbasoglu O, Goldstein RM, Vodapally MS, Jennings LW, Levy MF, Husberg BS, Klintmalm GB. Liver transplantation in hyponatremic patients with emphasis on central pontine myelinolysis. Clin Transplant. 1998;12:263-269. |
| 2. | Yu J, Zheng SS, Liang TB, Shen Y, Wang WL, Ke QH. Possible causes of central pontine myelinolysis after liver transplantation. World J Gastroenterol. 2004;10:2540-2543. |
| 3. | Lui CC, Chen CL, Chang YF, Lee TY, Chuang YC, Hsu SP. Subclinical central pontine myelinolysis after liver transplantation. Transplant Proc. 2000;32:2215-2216. |
| 4. | Ayus JC, Krothapalli RK, Armstrong DL. Rapid correction of severe hyponatremia in the rat: histopathological changes in the brain. Am J Physiol. 1985;248:F711-F719. |
| 5. | Lee YJ, Lee SG, Kwon TW, Park KM, Kim SC, Min PC. Neurologic complications after orthotopic liver transplantation including central pontine myelinolysis. Transplant Proc. 1996;28:1674-1675. |
| 6. | Boon AP, Carey MP, Adams DH, Buckels J, McMaster P. Central pontine myelinolysis in liver transplantation. J Clin Pathol. 1991;44:909-914. |
| 7. | Lampl C, Yazdi K. Central pontine myelinolysis. Eur Neurol. 2002;47:3-10. |
| 8. | Liang TB, Ke QH, Zheng SS, Yu J, Wang WL, Shen Y. Central pontine myelinolysis after liver transplantation: report of three cases. Zhonghua Qiguan Yizhi Zazhi. 2005;26:292-293. |
| 9. | Fryer JP, Fortier MV, Metrakos P, Verran DJ, Asfar SK, Pelz DM, Wall WJ, Grant DR, Ghent CN. Central pontine myelinolysis and cyclosporine neurotoxicity following liver transplantation. Transplantation. 1996;61:658-661. |
| 10. | Deleu D, Salim K, Mesraoua B, El Siddig A, Al Hail H, Hanssens Y. "Man-in-the-barrel" syndrome as delayed manifestation of extrapontine and central pontine myelinolysis: beneficial effect of intravenous immunoglobulin. J Neurol Sci. 2005;237:103-106. |
| 11. | Finsterer J, Engelmayer E, Trnka E, Stiskal M. Immunoglobulins are effective in pontine myelinolysis. Clin Neuropharmacol. 2000;23:110-113. |
| 12. | Price BH, Mesulam MM. Behavioral manifestations of central pontine myelinolysis. Arch Neurol. 1987;44:671-673. |
| 13. | Celesia GG. Persistent vegetative state: clinical and ethical issues. Theor Med. 1997;18:221-236. |
| 14. | Bonham CA, Dominguez EA, Fukui MB, Paterson DL, Pankey GA, Wagener MM, Fung JJ, Singh N. Central nervous system lesions in liver transplant recipients: prospective assessment of indications for biopsy and implications for management. Transplantation. 1998;66:1596-1604. |