Published online Aug 21, 2009. doi: 10.3748/wjg.15.3931
Revised: July 20, 2009
Accepted: July 27, 2009
Published online: August 21, 2009
AIM: To investigate the tacrolimus dosage requirements and blood concentrations in adult-to-adult right lobe living donor liver transplantation (AALDLT) recipients with small-for-size (SFS) grafts.
METHODS: During January 2007 and October 2008, a total of 54 cases of AALDLT with an observation period of 6 mo were enrolled in this study. The 54 patients were divided into two groups according to graft-recipient body weight ratio (GRBW): SFS grafts group (Group S, GRBW < 0.8%, n = 8) and non-SFS grafts group (Group N, GRBW ≥ 0.8%, n = 46). Tacrolimus 12-hour blood levels and doses were recorded during weeks 1, 2, 3 and 4 and months 2, 3, 4, 5 and 6 in group S and group N. Meanwhile, acute rejection rates, liver and renal function test results, and the number of potentially interacting medications were determined at each interval in the two groups. A comparison of tacrolimus dosage requirements and blood levels were made weekly in the first month post-surgery, and monthly from months 2 to 6.
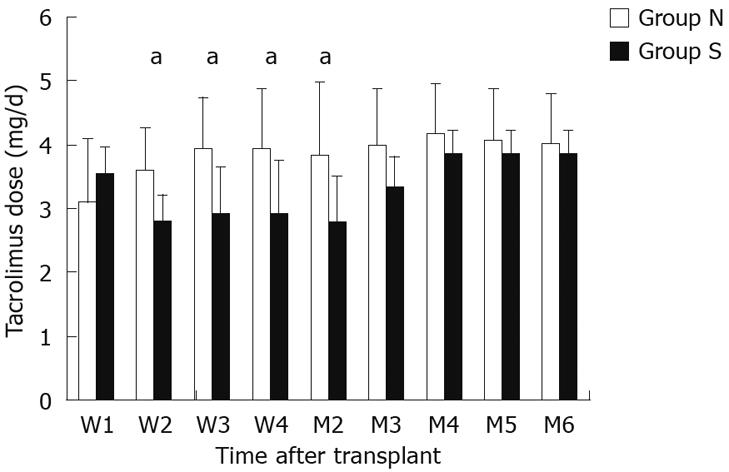
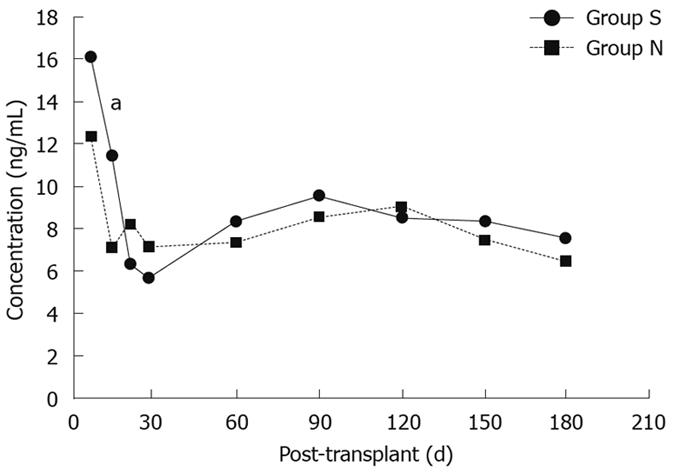
RESULTS: There were no differences in the demographic characteristics, acute rejection rates, liver and renal function test results, or the number of potentially interacting medications administered between the two groups. The tacrolimus dosage requirements in group S were significantly lower than group N at 2 wk (2.8 ± 0.4 mg/d vs 3.6 ± 0.7 mg/d, P = 0.006), 3 wk (2.9 ± 0.7 mg/d vs 3.9 ± 0.8 mg/d, P = 0.008), 4 wk (2.9 ± 0.8 mg/d vs 3.9 ± 1.0 mg/d, P = 0.023) and 2 mo (2.8 ± 0.7 mg/d vs 3.8 ± 1.1 mg/d, P = 0.033). Tacrolimus 12-h trough concentrations were similar between the two groups at all times except for 2 wk post-transplantation, when the concentrations were significantly greater in group S recipients than in group N recipients (11.3 ± 4.8 ng/mL vs 7.0 ± 3.8 ng/mL, P = 0.026).
CONCLUSION: SFS grafts recipients have significantly decreased tacrolimus dosage requirements compared with non-SFS grafts recipients in AALDLT during the first 2 mo post-surgery.
- Citation: Liu F, Li Y, Lan X, Wei YG, Li B, Yan LN, Wen TF, Zhao JC, Xu MQ, Wang WT, Yang JY. Tacrolimus dosage requirements in living donor liver transplant recipients with small-for-size grafts. World J Gastroenterol 2009; 15(31): 3931-3936
- URL: https://www.wjgnet.com/1007-9327/full/v15/i31/3931.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3931
With the shortage of cadaveric donor organs, living donor liver transplantation (LDLT) is generally accepted for end-stage liver disease. In LDLT, the graft size is inevitably small and requires regeneration, especially in small-for-size (SFS) grafts. Various studies have indicated that liver regeneration and function return rapidly in both donor and recipient after transplantation[1–3]. However, several studies have shown that living donor liver transplant recipients required smaller doses of tacrolimus compared with deceased donor liver transplant patients[45], which indicated that liver regeneration could affect the metabolism of tacrolimus in LDLT. The liver is a large metabolic pool which metabolizes many drugs including immunosuppressant drugs in humans. Tacrolimus, a calcineurin inhibitor, is predominantly metabolized in the liver by cytochrome P450 3A4 (CYP3A4)[6]. Kishino et al[7] reported that inter- and intra-individual variations in CYP3A4 activity were caused by differences in the actual ratio of graft volume (GV) to standard liver volume (SV) and donor age. Furthermore, Fukatsu et al[8] reported that graft hepatic weight was significantly correlated with clearance of tacrolimus. One possible explanation for the high variability of the optimal tacrolimus dose and its pharmacokinetics is the difference in graft size. In recent years, LDLT in adult patients with SFS grafts has become increasingly accepted[9]. However, questions related to this technique have arisen: What are the tacrolimus dosage requirements in SFS grafts which require adequate liver regeneration? Does liver regeneration of SFS grafts have any impact on tacrolimus metabolism? Are there any differences in tacrolimus dosage requirements between SFS grafts and non-SFS grafts in LDLT? The answers to these questions are unknown because there are few studies on tacrolimus dosage requirements in LDLT with SFS grafts in the existing literature. Hence, the purpose of this study was to determine the tacrolimus dosage requirements and blood concentrations in adult-to-adult right lobe living donor liver transplantation (AALDLT) recipients with SFS grafts.
Patients were included in this analysis if they were 18 years or older, had their transplantation performed at West China Hospital, received tacrolimus as basic immunosuppression and were followed up for at least 6 mo post-surgery. Exclusion criteria: (1) Patients receiving dual liver grafts. (2) Patients who had undergone combined liver and kidney transplantation. (3) Patients who were followed up for less than 6 mo and who were lost to follow up. (4) Patients who underwent retransplantation. And (5) patients who received cyclosporine as basic immunosuppression.
According to the inclusion and exclusion criteria, the study enrolled 54 AALDLT recipients and 54 donors who underwent right lobe hepatectomy at West China Hospital of Sichuan University during January 2007 and October 2008.
The 54 donors consisted of 34 males and 20 females. Donor age ranged from 19 to 53 years (mean, 30.9 ± 8.5 years). The relationships of the donors to the respective patients were: four fathers, three mothers, 11 offspring, 16 brothers, eight sisters, five wives, two husbands and five friends.
All the recipients underwent LDLT using right lobe graft without a middle hepatic vein. Of the 54 recipients, 45 (83.3%) were male and 9 (16.7%) were female. The average age of the recipients was 43 (27-64) years. Indications for transplantation were: chronic hepatitis B with liver cirrhosis (18 cases); fulminant hepatic failure (six cases); hepatocellular carcinoma (28 cases) and other causes (two cases). The pre-transplantation MELD scores of the recipients were 1-13 in 30 patients, 14-24 in 16 patients, and ≥ 25 in eight patients.
According to the GRBW, the 54 AALDLT recipients were divided into two groups: group S (GRBW < 0.8%), eight cases and group N (GRBW ≥ 0.8%), 46 cases. Written informed consent was obtained from both donors and recipients before surgery, and all the AALDLTs were approved by the Ethics Committee of West China Hospital.
Basic postoperative immunosuppression consisted of corticosteroids and tacrolimus (Prograf; Fujisawa, Osaka, Japan). Supplemental immunosuppression consisted of mycophenolate mofetil (for benign diseases) or azathioprine (for malignant diseases). Initial steroid tapers consisted of 1000 mg of intravenous (IV) methylprednisolone intraoperatively, followed by a 7-d taper (50 mg Q6h day 1, 40 mg Q6h day 2, 30 mg Q6h day 3, 20 mg Q6h day 4, 20 mg Q8h day 5, 10 mg Q6h day 6 and 10 mg Q8h day 7) to 20 mg of oral prednisone once daily. The corticosteroid was withdrawn from all patients within 3 mo after transplantation. Initial suspected or biopsy-proven rejections were treated with two 500-mg IV methylprednisolone boluses on consecutive days, followed by a 7-d taper to 20 mg of oral prednisone once daily.
Severe or steroid-resistant rejections were treated with a 7- to 14-d course of muromonab-CD3 (Orthoclone OKT3; Orthobiotech Products, Raritan, NJ, USA). Initial tacrolimus doses were 0.05-0.1 mg/kg per day divided into twice-daily dosing. Tacrolimus doses were adjusted to achieve a target 12-h trough concentration of 10-15 ng/mL for the first 3 mo post-transplantation, followed by 5-10 ng/mL thereafter. Tacrolimus 12-h trough concentrations were analyzed by the IMx assay (Abbott Laboratories, Chicago, IL, USA).
Tacrolimus daily doses and 12-h trough blood levels were recorded in both group S and group N at the following intervals after transplantation: weeks 1, 2, 3 and 4 and months 2, 3, 4, 5 and 6. At each interval, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TB), albumin (Alb), and creatinine (Cr) levels were recorded. Tacrolimus 12-h trough concentrations, AST, ALT, TB, Alb and Cr were measured twice weekly in the first month post-surgery, and weekly from month 2 to 6. At each interval, values recorded for dose, level, and respective liver function and kidney function test results represent the mean value over the past days. Meanwhile, acute rejection rates and the number of potentially interacting medications were determined at each interval in the two groups. Body weight was also recorded for each patient at each interval.
Quantitative descriptive data were expressed as mean ± standard deviation (SD) or median (minimum to maximum). Qualitative descriptive data were expressed as percentages. Fisher’s Exact, χ2, Student’s t and rank tests were used for statistical analysis. Tacrolimus doses and 12-h trough concentrations were compared weekly for the first month post-transplantation. For months 2-6 post-transplantation, tacrolimus doses and 12-h trough concentrations were compared monthly. ALT, AST, TB, Alb, and Cr were also compared between the two groups over the 6-mo period. All statistical analyses were performed using SPSS version 16.0 for Windows statistical software (SPSS Inc., Chicago, IL, USA). Differences with a P value < 0.05 were considered significant.
The demographics of group S and group N cohorts are listed in Table 1. There were no differences between the two groups with regard to recipients’ age, donors’ age, sex, and MELD score. In addition, there were no differences between the two groups in indications for transplantation or number of treated rejection episodes. There were no differences between the two groups with regard to the number of patients administered an interacting drug, total number of courses of an interacting drug, or duration of therapy (Table 2). Mean body weight for SFS grafts recipients undergoing AALDLT was slightly higher (66.6 kg) than for non-SFS grafts recipients undergoing AALDLT (61.7 kg, P = 0.244 not significant). The proportion of group S who underwent Roux-en-Y anastomosis was greater than that in group N, however, not statistically significant (12.5% vs 7%, P = 0.484).
| Group S | Group N | P | |
| Recipients’ age (yr) | 41.9 ± 11.6 | 43.4 ± 7.3 | NS (0.695) |
| Donors’ age (yr) | 28.6 ± 4.4 | 31.8 ± 9.5 | NS (0.246) |
| Sex (Male/female) | 6/2 | 39/7 | NS (0.864) |
| MELD score | NS (0.911) | ||
| 1-13 | 5 | 25 | |
| 14-24 | 2 | 14 | |
| ≥ 25 | 1 | 7 | |
| Indications for LDLT | NS (1.000) | ||
| Cirrhosis | 3 | 15 | |
| HCC | 4 | 24 | |
| FHF | 1 | 5 | |
| Others | 0 | 2 | |
| Rejection | 1/8 | 4/46 | NS (0.567) |
| Body weight | 66.6 ± 7.6 | 61.7 ± 9.8 | NS (0.244) |
| Biliary anastomosis | NS (0.484) | ||
| Roux-en-Y | 1 (12.5) | 3 (7) | |
| Choledochocholedochostomy | 7 (87.5) | 43 (93) |
| Medication interaction factors | Group S | Group N | P |
| Patients administered interacting drugs | 2/8 | 7/46 | NS |
| Total number of treatment courses | 4 | 9 | NS |
| Interacting drugs | |||
| Fluconazole | 1 | 3 | NS |
| Felodipine | 1 | 2 | NS |
| Lansoprazole | 2 | 4 | NS |
| Average length of therapy (d) | 7 | 9.5 | NS |
| No. of treatment courses on postoperative day | NS | ||
| 1-30 | 3 | 5 | |
| 31-60 | 1 | 3 | |
| 61-180 | 0 | 1 |
Tacrolimus dosage requirements for each group over the first 6 mo post-transplantation are shown in Figure 1. There was no significant difference in doses during the first week. The tacrolimus dosage requirements in group S were significantly lower than those in group N at 2 wk (2.8 ± 0.4 mg/d vs 3.6 ± 0.7 mg/d, P = 0.006), 3 wk (2.9 ± 0.7 mg/d vs 3.9 ± 0.8 mg/d, P = 0.008), 4 wk (2.9 ± 0.8 mg/d vs 3.9 ± 1.0 mg/d, P = 0.023) and 2 mo (2.8 ± 0.7 mg/d vs 3.8 ± 1.1 mg/d, P = 0.033). At 3, 4, 5 and 6 mo post-transplantation, there was no statistically significant difference between the two groups with regard to tacrolimus dosage requirements.
Despite having lower dosage requirements at 2, 3 and 4 wk and 2 mo post-transplantation, tacrolimus 12-h blood concentrations were not significantly different between the two groups except for week 2 post-transplantation (11.3 ± 4.8 ng/mL vs 7.0 ± 3.8 ng/mL, P < 0.05, Figure 2). In addition, there were no significant differences between the two groups with regard to ALT, AST, TB, Alb, or Cr over the entire study period (Table 3).
| Month | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Group N | ||||||
| ALT (IU/L) | 115.9 | 67.9 | 43.6 | 39 | 38 | 37.7 |
| AST (IU/L) | 52.7 | 47 | 34.9 | 39.3 | 36.1 | 38.9 |
| TB (μmol/L) | 23.1 | 18.5 | 17.2 | 14.9 | 17.6 | 17.9 |
| Alb (g/L) | 35.1 | 40.7 | 42.9 | 41.2 | 43 | 42.7 |
| Cr (μmol/L) | 55.5 | 55.1 | 59 | 55.7 | 54.1 | 52.3 |
| Group S | ||||||
| ALT (IU/L) | 124.2 | 78.2 | 46.8 | 41.5 | 34.4 | 39.7 |
| AST (IU/L) | 62.6 | 53.4 | 42.5 | 39.9 | 38.8 | 39.3 |
| TB (μmol/L) | 33.2 | 22.5 | 19.6 | 18.1 | 16 | 15.6 |
| Alb (g/L) | 33.6 | 39.7 | 42.3 | 42.7 | 43.9 | 42.9 |
| Cr (μmol/L) | 56.9 | 58.3 | 61 | 61.7 | 64.5 | 63 |
LDLT has emerged as an effective alternative strategy to overcome donor organ shortage. In recent years, LDLT in adult patients with SFS grafts has become increasingly accepted[9]. With SFS grafts, it is considered that reduced functional liver mass is a necessity for adequate liver regeneration. Nevertheless, the liver is a large metabolic pool which metabolizes many drugs including immunosuppressant drugs in humans. Tacrolimus (FK506) is a potent immunosuppressive agent that is widely used in organ transplantation[10]. Because FK506 has serious side-effects (such as nephrotoxicity, hypertension and neurotoxicity) and a narrow therapeutic window (5-20 ng/mL)[11], the desired blood concentration should be achieved as soon as possible. However, tacrolimus dosage requirements and blood concentrations in LDLT with SFS grafts have not yet been reported.
Ours results indicated that tacrolimus dosage requirements were substantially reduced in AALDLT recipients with SFS grafts compared with non-SFS grafts recipients during the first 2 mo post-transplantation despite having similar 12-h trough concentrations. Beyond 2 mo post-transplantation, dosage requirements between the two groups were similar.
The reason for the relatively low tacrolimus dosage requirements in SFS grafts recipients during the first 2 mo post-transplantation is not clear. However, there are several possibilities. We analyzed pharmacokinetic variables between group S and group N (such as absorption, volume of distribution, and clearance) to explain the dosage requirement difference. Tacrolimus dosage requirements may be reduced by increased absorption, decreased volume of distribution, or decreased clearance. Neither increased absorption nor decreased volume of distribution in group S was a possible explanation. The only difference in group S and group N that could impact on absorption was the greater proportion of AALDLT recipients with a Roux-en-Y anastomosis. The proportion of group S who underwent Roux-en-Y anastomosis was greater than that in group N (12.5% vs 7%), but this was not statistically significant (P = 0.484). Moreover, a Roux-en-Y anastomosis is associated with decreased immunosuppressive absorption[12]. The volume of distribution of immunosuppressants was larger rather than smaller in group S because of their heavier body weight (SFS grafts recipients were 4.9 kg heavier than non-SFS grafts recipients).
Another explanation for the lower tacrolimus dosage requirements in group S might be reduced immunosuppressive clearance, which may be explained by two possible mechanisms. The first possible mechanism is that as the graft size in group S was smaller than in group N, it did not meet the demands of metabolism. SFS grafts have a relatively small liver mass because the GRBW is less than 0.8% and severe ischemic injuries caused by decreased hepatic arterial inflow or even hepatic artery thrombosis can occur[13–15]. Fukatsu et al[8] reported that graft hepatic weight was significantly correlated with clearance of tacrolimus in adult patients who had undergone LDLT, and Sugawara et al[16]reported that the optimal tacrolimus dose was best correlated with GV/SV ratio. Lee et al[17] reported that the clearance of tacrolimus was related to GRWR and the clearance of tacrolimus was decreased in patients with a small graft. Kishino et al[18] reported that the mean T1/2 of tacrolimus in patients with SFS grafts (GV/SV ratio: smaller than 50%) was significantly (P < 0.05) longer than that in patients with GV/SV larger than 51%. These results suggest that graft size is important and could influence the clearance of tacrolimus. The reason why graft size can influence the clearance of tacrolimus is not very clear. CYP3A4 content and activity in liver grafts may partially explain the problem. Tacrolimus is mainly metabolized by CYP3A4 in the liver and intestine[619]. Powis et al[20] reported that CYP3A4 content and activity in human liver tissue decreased rapidly after surgical removal, and Kishino et al[7] reported that inter- and intra-individual variations in CYP3A4 activity were caused by differences in GV/SV ratio and donor age. In other words, the reduced CYP3A4 content and lower CYP3A4 activity in SFS liver grafts could partially explain the difference in tacrolimus dosage requirements between group S and group N.
Another explanation for reduced immunosuppressive clearance in SFS liver grafts may be related to several cytokines and growth factors. Several studies[2122] have revealed that a SFS liver graft retained the capacity to regenerate faster by modulation of the expression pattern of HGF, IL-6 and TGF-βl immediately after LDLT and both the regeneration rates and the levels of cytokines and growth factors were higher in SFS liver grafts than normal sized liver grafts. These markers have also shown an ability to decrease cytochrome P450 3A activity in the liver in both mice and humans[23–25], which may decrease its ability to metabolize drugs. In other words, there were many more cytokines and growth factors (such as HGF, IL-6) in SFS liver grafts, which could decrease the clearance of tacrolimus.
In order to eliminate the impact of drug interactions, the potential medications which could affect tacrolimus level were determined in the two groups. It was noted in several studies that fluconazole, felodipine and lansoprazole could elevate tacrolimus blood level by inhibiting the activity of CYP3A4[26–28]. In the present study, the majority of patients in both groups were administered interacting drugs in the first month post-transplantation. However, there were no differences between the two groups with regard to the number of patients administered an interacting drug, total number of courses of an interacting drug, or duration of therapy. As a result, the impact of drug interactions was balanced in the two groups.
To make a rough determination of hepatic function and kidney function in group S and group N, we measured serum AST, ALT, TB, Alb, and Cr levels at each interval post-transplantation in both groups of patients. AST, ALT, and TB reflected the hepatic metabolic function while Alb was responsible for the synthetic function of the liver. AST, ALT, and TB levels were slightly greater at almost all follow-up intervals and Alb level was slightly lower in group S in the first 3 mo post-transplantation, although there was no statistical significance. This could not explain the difference in tacrolimus dosage requirements between group S and group N, however, more sensitive measures of hepatic function, i.e. galactose, caffeine, indocyanine green and monoethylglycinexylidide clearance test, should be performed to confirm the hypothesis.
There are several limitations to this study. Firstly, the sample size was small in group S. In addition, we did not study tacrolimus pharmacokinetics in this analysis which may have strongly affected the difference in the tacrolimus dosage requirements between the two groups.
In conclusion, AALDLT recipients with SFS grafts have significantly decreased tacrolimus dosage requirements compared with non-SFS grafts recipients during the first 2 mo post-surgery, in spite of having similar tacrolimus concentrations. We recommend that relatively low initial doses of tacrolimus should be administered to these patients.
Several studies have shown that living donor liver transplant recipients required smaller doses of tacrolimus compared with deceased donor liver transplant patients, which indicated that liver regeneration could affect the metabolism of tacrolimus in living donor liver transplantation (LDLT). However, there are few studies in the existing literature on tacrolimus dosage requirements in LDLT with small-for-size (SFS) grafts which require adequate liver regeneration.
In recent years, LDLT in adult patients with SFS grafts has become increasingly accepted. The administration of immunosuppressants for SFS grafts has not been explicitly reported due to limited studies. In this field, the research goal was to determine the optimal initial tacrolimus dose for LDLT recipients with SFS grafts.
Several studies have shown that living donor liver transplant recipients required smaller doses of tacrolimus compared with deceased donor liver transplant patients. This is the first study to compare tacrolimus dosage requirements in patients who received a graft with adequate hepatic mass and patients who received a SFS graft in LDLT.
This study revealed that relatively low initial doses of tacrolimus should be administered to patients who receive a SFS graft in LDLT, which is a good guideline to follow for the rational administration of tacrolimus in future LDLT recipients with SFS grafts.
SFS graft: SFS liver graft, this is generally considered when a graft has a graft-recipient body weight ratio (GRBW) less than 0.8% in LDLT. GRBW: Graft-recipient body weight ratio.
The current paper is well-written and informative.
| 1. | Marcos A, Fisher RA, Ham JM, Shiffman ML, Sanyal AJ, Luketic VA, Sterling RK, Fulcher AS, Posner MP. Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation. Transplantation. 2000;69:1375-1379. |
| 2. | Humar A, Kosari K, Sielaff TD, Glessing B, Gomes M, Dietz C, Rosen G, Lake J, Payne WD. Liver regeneration after adult living donor and deceased donor split-liver transplants. Liver Transpl. 2004;10:374-378. |
| 3. | Pomfret EA, Pomposelli JJ, Gordon FD, Erbay N, Lyn Price L, Lewis WD, Jenkins RL. Liver regeneration and surgical outcome in donors of right-lobe liver grafts. Transplantation. 2003;76:5-10. |
| 4. | Jain A, Venkataramanan R, Sharma R, Kwong T, Orloff M, Abt P, Kashyap R, Tsoulfas G, Batzold P, Williamson M. Pharmacokinetics of tacrolimus in living donor liver transplant and deceased donor liver transplant recipients. Transplantation. 2008;85:554-560. |
| 5. | Taber DJ, Dupuis RE, Fann AL, Andreoni KA, Gerber DA, Fair JH, Johnson MW, Shrestha R. Tacrolimus dosing requirements and concentrations in adult living donor liver transplant recipients. Liver Transpl. 2002;8:219-223. |
| 6. | Venkataramanan R, Swaminathan A, Prasad T, Jain A, Zuckerman S, Warty V, McMichael J, Lever J, Burckart G, Starzl T. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29:404-430. |
| 7. | Kishino S, Ogawa M, Takekuma Y, Sugawara M, Shimamura T, Furukawa H, Todo S, Miyazaki K. The variability of liver graft function and urinary 6beta-hydroxycortisol to cortisol ratio during liver regeneration in liver transplant recipients. Clin Transplant. 2004;18:124-129. |
| 8. | Fukatsu S, Yano I, Igarashi T, Hashida T, Takayanagi K, Saito H, Uemoto S, Kiuchi T, Tanaka K, Inui K. Population pharmacokinetics of tacrolimus in adult recipients receiving living-donor liver transplantation. Eur J Clin Pharmacol. 2001;57:479-484. |
| 9. | Kishino S, Takekuma Y, Sugawara M, Shimamura T, Furukawa H, Todo S, Miyazaki K. Influence of continuous venovenous haemodiafiltration on the pharmacokinetics of tacrolimus in liver transplant recipients with small-for-size grafts. Clin Transplant. 2003;17:412-416. |
| 10. | Todo S, Fung JJ, Tzakis A, Demetris AJ, Jain A, Alessiani M, Takaya S, Day R, Gordon R, Starzl TE. One hundred ten consecutive primary orthotopic liver transplants under FK 506 in adults. Transplant Proc. 1991;23:1397-1402. |
| 11. | Yasuhara M, Hashida T, Toraguchi M, Hashimoto Y, Kimura M, Inui K, Hori R, Inomata Y, Tanaka K, Yamaoka Y. Pharmacokinetics and pharmacodynamics of FK 506 in pediatric patients receiving living-related donor liver transplantations. Transplant Proc. 1995;27:1108-1110. |
| 12. | Whitington PF, Kehrer BH, Whitington SH, Shneider B, Black DD. The effect of biliary enteroenterostomy on the pharmacokinetics of enterally administered cyclosporine in rats. Hepatology. 1989;9:393-397. |
| 13. | Marcos A, Olzinski AT, Ham JM, Fisher RA, Posner MP. The interrelationship between portal and arterial blood flow after adult to adult living donor liver transplantation. Transplantation. 2000;70:1697-1703. |
| 14. | Smyrniotis V, Kostopanagiotou G, Kondi A, Gamaletsos E, Theodoraki K, Kehagias D, Mystakidou K, Contis J. Hemodynamic interaction between portal vein and hepatic artery flow in small-for-size split liver transplantation. Transpl Int. 2002;15:355-360. |
| 15. | Payen DM, Fratacci MD, Dupuy P, Gatecel C, Vigouroux C, Ozier Y, Houssin D, Chapuis Y. Portal and hepatic arterial blood flow measurements of human transplanted liver by implanted Doppler probes: interest for early complications and nutrition. Surgery. 1990;107:417-427. |
| 16. | Sugawara Y, Makuuchi M, Kaneko J, Ohkubo T, Imamura H, Kawarasaki H. Correlation between optimal tacrolimus doses and the graft weight in living donor liver transplantation. Clin Transplant. 2002;16:102-106. |
| 17. | Lee JY, Hahn HJ, Son IJ, Suh KS, Yi NJ, Oh JM, Shin WG. Factors affecting the apparent clearance of tacrolimus in Korean adult liver transplant recipients. Pharmacotherapy. 2006;26:1069-1077. |
| 18. | Kishino S, Ohno K, Shimamura T, Furukawa H, Todo S. A nomogram for predicting the optimal oral dosage of tacrolimus in liver transplant recipients with small-for-size grafts. Clin Transplant. 2006;20:443-449. |
| 19. | Hashimoto Y, Sasa H, Shimomura M, Inui K. Effects of intestinal and hepatic metabolism on the bioavailability of tacrolimus in rats. Pharm Res. 1998;15:1609-1613. |
| 20. | Powis G, Jardine I, Van Dyke R, Weinshilboum R, Moore D, Wilke T, Rhodes W, Nelson R, Benson L, Szumlanski C. Foreign compound metabolism studies with human liver obtained as surgical waste. Relation to donor characteristics and effects of tissue storage. Drug Metab Dispos. 1988;16:582-589. |
| 21. | Ninomiya M, Harada N, Shiotani S, Hiroshige S, Minagawa R, Soejima Y, Suehiro T, Nishizaki T, Shimada M, Sugimachi K. Hepatocyte growth factor and transforming growth factor beta1 contribute to regeneration of small-for-size liver graft immediately after transplantation. Transpl Int. 2003;16:814-819. |
| 22. | Oyama T, Sadamori H, Matsukawa H, Murata H, Umeda Y, Watanabe Y, Ozaki M, Iwagaki H, Tanaka N, Yagi T. Small liver graft regenerates through immediate increase of HGF and IL-6--possible involvement of sinusoidal tensile/shear stress in small liver graft. Hepatogastroenterology. 2007;54:2078-2083. |
| 23. | Siewert E, Bort R, Kluge R, Heinrich PC, Castell J, Jover R. Hepatic cytochrome P450 down-regulation during aseptic inflammation in the mouse is interleukin 6 dependent. Hepatology. 2000;32:49-55. |
| 24. | Donato MT, Gómez-Lechón MJ, Jover R, Nakamura T, Castell JV. Human hepatocyte growth factor down-regulates the expression of cytochrome P450 isozymes in human hepatocytes in primary culture. J Pharmacol Exp Ther. 1998;284:760-767. |
| 25. | Abdel-Razzak Z, Loyer P, Fautrel A, Gautier JC, Corcos L, Turlin B, Beaune P, Guillouzo A. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol Pharmacol. 1993;44:707-715. |
| 26. | Niwa T, Shiraga T, Takagi A. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol Pharm Bull. 2005;28:1805-1808. |
| 27. | Butani L, Berg G, Makker SP. Effect of felodipine on tacrolimus pharmacokinetics in a renal transplant recipient. Transplantation. 2002;73:159-160. |
| 28. | Hosohata K, Masuda S, Katsura T, Takada Y, Kaido T, Ogura Y, Oike F, Egawa H, Uemoto S, Inui K. Impact of intestinal CYP2C19 genotypes on the interaction between tacrolimus and omeprazole, but not lansoprazole, in adult living-donor liver transplant patients. Drug Metab Dispos. 2009;37:821-826. |