Published online Jan 21, 2009. doi: 10.3748/wjg.15.339
Revised: December 11, 2008
Accepted: December 18, 2008
Published online: January 21, 2009
AIM: To test whether clamping during liver surgery predisposes to hepatic vein thrombosis.
METHODS: We performed a retrospective analysis of 210 patients who underwent liver resection with simultaneous inflow and outflow occlusion. Intraoperatively, flow in the hepatic veins was assessed by Doppler ultrasonography during the reperfusion phase. Postoperatively, patency of the hepatic veins was assessed by contrast-enhanced CT angiography, when necessary after 3-6 mo follow up.
RESULTS: Twelve patients (5.7%) developed intraoperative liver remnant swelling. However, intraoperative ultrasonography did not reveal evidence of hepatic vein thrombosis. In three of these patients a kinking of the common trunk of the middle and left hepatic veins hindering outflow was recognized and was managed successfully by suturing the liver remnant to the diaphragm. Twenty three patients (10.9%) who developed signs of mild outflow obstruction postoperatively, had no evidence of thrombi in the hepatic veins or flow disturbances on ultrasonography and contrast-enhanced CT angiography, while hospitalized. Long term assessment of the patency of the hepatic veins over a 3-6 mo follow-up period did not reveal thrombi formation or clinical manifestations of outflow obstruction.
CONCLUSION: Extrahepatic dissection and clamping of the hepatic veins does not predispose to clinically important thrombosis.
- Citation: Arkadopoulos N, Stafyla V, Marinis A, Koutoulidis V, Theodoraki K, Theodosopoulos T, Vassiliou I, Dafnios N, Fragulidis G, Smyrniotis V. Does clamping during liver surgery predispose to thrombosis of the hepatic veins? Analysis of 210 cases. World J Gastroenterol 2009; 15(3): 339-343
- URL: https://www.wjgnet.com/1007-9327/full/v15/i3/339.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.339
Vascular control during liver resection typically involves hepatic inflow occlusion, either continuous or intermittent. However, more complex resections may require both inflow and outflow occlusion, the latter usually being achieved with extraparenchymal control of the major hepatic veins at the hepatocaval junction. This maneuver can significantly reduce backflow bleeding during parenchymal transection and facilitate resection of tumors close to the roots of the major hepatic veins and even reconstruction of a hepatic vein in the liver remnant[1].
Three techniques for vascular control have been widely used: the Pringle maneuver (PM), the selective hepatic vascular exclusion (SHVE) and the total hepatic vascular exclusion (THVE). The PM[2] is performed by encircling and clamping the hepatoduodenal ligament[1]. Although this is well tolerated, it should always be kept in mind that backflow bleeding and air embolization might occur during parenchymal transection[3]. THVE includes clamping of the hepatoduodenal ligament and occlusion of the suprahepatic and infrahepatic inferior vena cava (IVC)[4]. This technique has the advantage of a bloodless surgical field, but the serious hemodynamic instability it may cause, makes it inappropriate for 20%-30% of patients. SHVE entails disconnection of the liver from the retrohepatic IVC and inflow occlusion combined with extrahepatic control of hepatic veins. This technique offers bloodless liver transection without the above-mentioned disadvantages of PM and THVE[56].
Dissection and clamping of the hepatic veins during the application of SHVE may predispose the major hepatic veins to thrombi formation through the induction of venous stasis and endothelial injury coupled with coagulation disturbances. The scarcity of studies addressing this issue prompted us to test our hypothesis that dissection and clamping of the hepatic veins is associated with an increased risk of thrombosis and liver outflow obstruction.
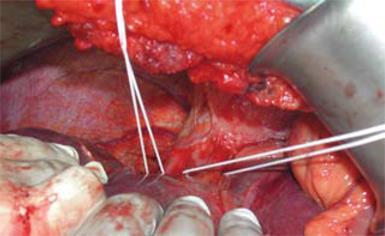
Between 1997 and 2007, 210 consecutive patients underwent hepatectomy with SHVE[1]. Briefly, in all cases and irrespective of the type of planned hepatectomy, the abdomen was accessed via a bilateral subcostal incision and the liver was fully mobilized after transection of its ligaments. The liver was then disconnected from the retrohepatic IVC by dividing the short perforator hepatic veins. On the right side, dissection along the anterior surface of the IVC continued until the right hepatic vein was isolated, while on the left side, the venous trunks of the left and middle hepatic veins were also dissected free from the surrounding tissues. Control of liver inflow was attained by clamping the porta hepatis with a Satinsky clamp and by occluding any accessory hepatic artery with bulldog clamps. Liver outflow control was achieved by clamping the trunks of the right, middle and left hepatic veins separately (Figure 1). The transection plane was defined with an intraoperative ultrasonographer (Aloka SSD-1400, model IP-1235V, ALOKA CO., LTD., Japan), in order to secure tumor-free margins > 1 cm. Liver splitting was performed using either the clamp crushing technique or by sharp transection with a knife. Hemostasis was achieved by suturing all vascular orifices on the cut surface with 3-0 and 4-0 prolene. After completion of the liver resection, outflow was released first, followed by liver inflow[56]. Following reperfusion, hemostasis was completed using additional stitches. Flow in the hepatic artery, the portal vein, the hepatic veins and the IVC was assessed by intraoperative Doppler ultrasonography. The negative findings in the first 120 consecutive patients prompted us to restrict our Doppler study to the hepatic veins. Thorough imaging of all liver vasculature was then reserved for cases with liver remnant swelling or signs of hyporeperfusion. All operations were performed by the same surgical team, directed by the senior author V.S. All patients had normal imaging of the hepatic veins before liver resection, either on contrast-enhanced CT or MRI.
Postoperative Doppler ultrasonography of the portal vein, hepatic artery and the hepatic veins was performed at the bedside, if the patient exhibited at least one of the following findings: (a) clinically worsening ascites on any postoperative day; (b) persistent or worsening cholestasis (conjugated bilirubin > 3 mg/dL) after the 3rd postoperative day; (c) worsening elevation of transaminases after the 2nd postoperative day (AST and/or ALT > 1st postoperative day levels) or (d) persistent or worsening prolongation of INR > 2 after the 3rd postoperative day.
All patients admitted after 2002 (117 patients-56%) received perioperative thromboprophylaxis with low molecular weight heparin for a median duration of 10 d (range: 4-14 d), according to our new institutional protocol which was initiated at that time. Post-discharge, all patients were invited for a follow-up abdominal CT scan, 3-6 mo after surgery. Helical CT (Philips, The Netherlands) of the abdomen was performed before and after intravenous administration of iodinated contrast material. Contrast enhanced images were obtained in the arterial and portal venous phase and images were evaluated for the presence of hepatic venous thrombosis by two independent observers. Data collection was performed in a prospective manner.
Clinical, intraoperative and postoperative parameters of the patients are summarized in Table 1. Intraoperatively, 12 (5.7%) patients developed unexpected swelling of the liver remnant. Intraoperative Doppler ultrasonography did not reveal evidence of hepatic vein thrombosis in any of these cases. In three of these patients kinking of the common trunk of the middle and left hepatic veins was recognized and was managed by suturing the liver remnant to the diaphragm. In the remaining nine patients, extensive work-up did not show thrombi formation and the liver remnant swelling was attributed to the fact that the portal flow was disproportional to the small liver remnant.
| Clinical, intraoperative and postoperative parameters | |
| Age (yr) | 63 (1-86) |
| Indication for hepatectomy | |
| Malignancy | 173 |
| Benign (hemangioma, hydatid cyst, etc) | 37 |
| Type of hepatectomy | |
| Right hepatectomy | 92 |
| Left hepatectomy | 40 |
| Minor hepatectomy (1-2 segments) | 78 |
| Intraoperative data | |
| Warm ischemic time (min) | 39 (25-61) |
| Blood loss (mL) | 420 (140-3100) |
| Transfusions of pRBCs (U) | 0 (0-14) |
| Outcome | |
| Morbidity (pleural effusion, bile leak, chest infection, post-operative bleeding, wound infection, postoperative liver failure) | 34% |
| Mortality | 3 (1.5%)1 |
| Hospital stay (d) | 12 (4-37) |
Twenty three (10.9%) patients fulfilled the previously mentioned criteria for postoperative Doppler ultrasonography at the bedside. All these patients were also examined with contrast enhanced CT-angiography. The hepatic veins were visualized in all cases and no evidence of thrombosis was found.
Forty two (20%) patients underwent contrast-enhanced CT scans during their postoperative hospitalization for reasons unrelated to suspected hepatic vein thrombosis (diagnostic work-up for fever, bile collection and chest infection). In all cases, hepatic vein imaging did not reveal thrombi formation or recanalization processes.
Finally, 200 patients (95%) underwent a follow up contrast-enhanced CT scan of the liver at a median time of 150 d (range: 92-205 d) after surgery, without evidence of thrombotic processes or stricture of the hepatic veins.
Our study showed that dissection and clamping of the hepatic veins in hepatectomies under inflow and outflow vascular occlusion of the liver was not complicated with hepatic vein thrombosis either intraoperatively or postoperatively during a 6-mo follow-up period.
Postoperative hepatic venous outflow obstruction is extremely rare in large series of hepatectomies[78]. On the contrary, in liver transplantation the incidence is high, especially with the piggyback technique (0.5%-2.5%) which has a mortality rate of 24%[9–14]. The main causative factors are either technique-related or are associated with coagulation disturbances generated by the underlying disease and graft function.
The only study addressing the risk of hepatic vein thrombosis in liver resection is by Arita et al[15], who showed that 10 out of 821 liver resections performed using the intermittent Pringle maneuver developed hepatic vein thrombosis. It is worth noting that the authors had to resort to thrombectomy in the most severe cases. The exposure of major hepatic veins to a length of 3 cm or more and the use of an ultrasonic dissector were postulated to be predisposing factors for vein thrombosis.
Although our technique could be considered more thrombogenic, since it includes dissection and clamping of hepatic veins, the lack of thrombosis in our series is in surprising contrast to the findings of Arita et al, who performed only intermittent inflow occlusion. It is possible that the use of the ultrasonic dissection technique used by Arita et al could, as the authors themselves admitted, have contributed to the thrombogenic effect, which was further aggravated when the energy was delivered close to the major hepatic veins[15].
Our results are in agreement with the findings of most major clinical series of hepatectomies performed under vascular control, in which hepatic venous thrombosis is scarcely if ever mentioned[1617]. Although SHVE could be considered more thrombogenic, since it involves injurious manipulation of hepatic veins, the lack of confirmed cases of vein thrombosis in our study can be attributed to short warm ischemic time and sharp transection of the liver surface with the scalpel, a technique that is less traumatic to venous epithelium compared to other ablative techniques. Avoidance of radiofrequency ablation in our series may also have contributed to our favorable results, since this technique has been recently associated with damage to the liver remnant[18] and hepatic vein thrombosis[19]. Venous endothelial trauma has been known to cause platelet aggregation and degranulation, vasoconstriction, thrombin activation and diminished fibrinolysis[20]. Therefore, we can not exclude the possibility that some of our patients may have developed small, undetected thrombi postoperatively. However, such thrombi remain clinically silent and resolve spontaneously without increasing morbidity or mortality. It is also possible that ischemia reperfusion of the liver mobilizes mechanisms that attenuate thrombi formation locally. Studies addressing the coagulation-fibrinolysis system during liver resection indicate that the balance leans towards fibrinolysis[2122].
Regarding diagnosis of hepatic venous thrombosis, Doppler ultrasound is a readily available and inexpensive tool[2324]. It is, however, operator-dependent and its diagnostic accuracy may be compromised by the presence of bowel gas or ascites. On CT, vein thrombosis is seen as a lack of enhancement on post-contrast images, often associated with peripheral rim enhancement. In hepatic veno-occlusive disease, CT reveals patchy hepatic parenchymal enhancement with lack of normal visualization of the hepatic veins[25–27].
In conclusion, our analysis of a large cohort of patients confirms that extrahepatic dissection and clamping of the hepatic veins for up to one hour is a safe procedure that does not predispose to clinically important thrombosis. Although the technique of selective vascular exclusion used in our series is not advocated for routine use in liver surgery, we suggest that concerns about venous thrombosis are unjustified and should not be a limiting factor in the application of this useful technique, whenever necessary.
Dissection and clamping of the hepatic veins during liver resection may predispose the major hepatic veins to thrombi formation. In this study we test our hypothesis that dissection and clamping of the hepatic veins is not associated with an increased risk of thrombosis and liver outflow obstruction.
Postoperative hepatic venous outflow obstruction is extremely rare in large series of hepatectomies. On the contrary, in liver transplantation the incidence is high, especially with the piggyback technique (0.5%-2.5%) which has a mortality rate of 24%. The main causative factors are either technique-related or are associated with coagulation disturbances generated by the underlying disease and graft function.
The only study addressing the risk of hepatic vein thrombosis in liver resection is by Arita et al, who showed that 10 out of 821 liver resections performed with the intermittent Pringle maneuver developed hepatic vein thrombosis. Our study showed that dissection and clamping of the hepatic veins in hepatectomies under inflow and outflow vascular occlusion of the liver was not complicated with hepatic vein thrombosis either intraoperatively or postoperatively during a six-month follow-up period.
Although the technique of selective vascular exclusion used in our series is not advocated for routine use in liver surgery, the authors suggest that concerns about venous thrombosis are unjustified and should not be a limiting factor in the application of this useful technique, whenever necessary.
Pringle maneuver: performed by encircling and clamping the hepatoduodenal ligament; Selective hepatic vascular exclusion: inflow occlusion combined with extrahepatic control of hepatic veins; Total hepatic vascular exclusion: clamping of the hepatoduodenal ligament and occlusion of the suprahepatic and infrahepatic vena cava (IVC).
This is an interesting paper about hepatic vein thrombosis.
| 1. | Smyrniotis V, Farantos C, Kostopanagiotou G, Arkadopoulos N. Vascular control during hepatectomy: review of methods and results. World J Surg. 2005;29:1384-1396. |
| 3. | Hatano Y, Murakawa M, Segawa H, Nishida Y, Mori K. Venous air embolism during hepatic resection. Anesthesiology. 1990;73:1282-1285. |
| 4. | Huguet C, Addario-Chieco P, Gavelli A, Arrigo E, Harb J, Clement RR. Technique of hepatic vascular exclusion for extensive liver resection. Am J Surg. 1992;163:602-605. |
| 5. | Smyrniotis VE, Kostopanagiotou GG, Contis JC, Farantos CI, Voros DC, Kannas DC, Koskinas JS. Selective hepatic vascular exclusion versus Pringle maneuver in major liver resections: prospective study. World J Surg. 2003;27:765-769. |
| 6. | Smyrniotis VE, Kostopanagiotou GG, Gamaletsos EL, Vassiliou JG, Voros DC, Fotopoulos AC, Contis JC. Total versus selective hepatic vascular exclusion in major liver resections. Am J Surg. 2002;183:173-178. |
| 7. | Pan ZY, Yang Y, Zhou WP, Li AJ, Fu SY, Wu MC. Clinical application of hepatic venous occlusion for hepatectomy. Chin Med J (Engl). 2008;121:806-810. |
| 8. | Gurusamy KS, Kumar Y, Sharma D, Davidson BR. Methods of vascular occlusion for elective liver resections. Cochrane Database Syst Rev. 2007;121:CD006409. |
| 9. | Wang SL, Sze DY, Busque S, Razavi MK, Kee ST, Frisoli JK, Dake MD. Treatment of hepatic venous outflow obstruction after piggyback liver transplantation. Radiology. 2005;236:352-359. |
| 10. | Ng SS, Yu SC, Lee JF, Lai PB, Lau WY. Hepatic venous outflow obstruction after piggyback liver transplantation by an unusual mechanism: report of a case. World J Gastroenterol. 2006;12:5416-5418. |
| 11. | Perkins J. Hepatic venous outflow obstruction after piggyback orthotopic liver transplantation. Liver Transpl. 2006;12:159-160. |
| 12. | Nishida S, Nakamura N, Vaidya A, Levi DM, Kato T, Nery JR, Madariaga JR, Molina E, Ruiz P, Gyamfi A. Piggyback technique in adult orthotopic liver transplantation: an analysis of 1067 liver transplants at a single center. HPB (Oxford). 2006;8:182-188. |
| 13. | Sze DY, Semba CP, Razavi MK, Kee ST, Dake MD. Endovascular treatment of hepatic venous outflow obstruction after piggyback technique liver transplantation. Transplantation. 1999;68:446-449. |
| 14. | Pitre J, Panis Y, Belghiti J. Left hepatic vein kinking after right hepatectomy: a rare cause of acute Budd-Chiari syndrome. Br J Surg. 1992;79:798-799. |
| 15. | Arita J, Kokudo N, Hasegawa K, Sano K, Imamura H, Sugawara Y, Makuuchi M. Hepatic venous thrombus formation during liver transection exposing major hepatic vein. Surgery. 2007;141:283-284. |
| 16. | Kimura F, Miyazaki M, Suwa T, Sugiura T, Shinoda T, Itoh H, Nakagawa K, Ambiru S, Shimizu H, Yoshitome H. Evaluation of total hepatic vascular exclusion and pringle maneuver in liver resection. Hepatogastroenterology. 2002;49:225-230. |
| 17. | Zhou W, Li A, Pan Z, Fu S, Yang Y, Tang L, Hou Z, Wu M. Selective hepatic vascular exclusion and Pringle maneuver: a comparative study in liver resection. Eur J Surg Oncol. 2008;34:49-54. |
| 18. | Mitsuo M, Takahiro T, Yasuko T, Masayasu A, Katsuya O, Nozomi S, Yoshihide O, Isamu K. Radiofrequency (RF)-assisted hepatectomy may induce severe postoperative liver damage. World J Surg. 2007;31:2208-2212; discussion 2213-2214. |
| 19. | Akahane M, Koga H, Kato N, Yamada H, Uozumi K, Tateishi R, Teratani T, Shiina S, Ohtomo K. Complications of percutaneous radiofrequency ablation for hepato-cellular carcinoma: imaging spectrum and management. Radiographics. 2005;25 Suppl 1:S57-S68. |
| 20. | Chung I, Lip GY. Virchow's triad revisited: blood constituents. Pathophysiol Haemost Thromb. 2003;33:449-454. |
| 21. | Meijer C, Wiezer MJ, Hack CE, Boelens PG, Wedel NI, Meijer S, Nijveldt RJ, Statius Muller MG, Wiggers T, Zoetmulder FA. Coagulopathy following major liver resection: the effect of rBPI21 and the role of decreased synthesis of regulating proteins by the liver. Shock. 2001;15:261-271. |
| 22. | Tsuji K, Eguchi Y, Kodama M. Postoperative hypercoagulable state followed by hyperfibrinolysis related to wound healing after hepatic resection. J Am Coll Surg. 1996;183:230-238. |
| 23. | Boozari B, Bahr MJ, Kubicka S, Klempnauer J, Manns MP, Gebel M. Ultrasonography in patients with Budd-Chiari syndrome: diagnostic signs and prognostic implications. J Hepatol. 2008;49:572-580. |
| 24. | Chong WK, Beland JC, Weeks SM. Sonographic evaluation of venous obstruction in liver transplants. AJR Am J Roentgenol. 2007;188:W515-W521. |
| 25. | Karaosmanoglu D, Karcaaltincaba M, Akata D, Ozmen M, Akhan O. CT, MRI, and US findings of incidental segmental distal hepatic vein occlusion: a new form of Budd-Chiari syndrome? J Comput Assist Tomogr. 2008;32:518-522. |
| 26. | Lupescu IG, Dobromir C, Popa GA, Gheorghe L, Georgescu SA. Spiral computed tomography and magnetic resonance angiography evaluation in Budd-Chiari syndrome. J Gastrointestin Liver Dis. 2008;17:223-226. |
| 27. | Meng XC, Zhu KS, Qin J, Zhang JS, Wang XH, Zou Y, Zhang YQ, Shan H. Clinical significance of multislice spiral CT scans in hepatic veins occlusion in Budd-Chiari syndrome. Chin Med J (Engl). 2007;120:100-105. |