Published online Aug 7, 2009. doi: 10.3748/wjg.15.3636
Revised: June 18, 2009
Accepted: June 25, 2009
Published online: August 7, 2009
AIM: To study the efficacy of low-dose imipramine in relieving symptoms associated with the irritable bowel syndrome (IBS).
METHODS: A randomized, double-blind trial of 25 mg imipramine vs matched placebo for 12 wk was performed. Doubling the dose was allowed once at week 2 in case of an unsatisfactory early response. Primary efficacy variables were subjective global symptom relief and quality of life (QoL) using SF-36 at week 12.
RESULTS: One hundred and seven patients were enrolled by advertisement or referral by general practitioners and 56 (31 imipramine: 25 placebo) completed the 16-wk study. Baseline characteristics were comparable. A high overall dropout rate was noted in the imipramine and placebo arms (47.5% vs 47.9%, P > 0.05), a mean of 25.0 and 37.4 d from enrollment, respectively (P < 0.05). At the end of 12 wk, there was a significant difference in global symptom relief with imipramine over placebo (per-protocol: 80.6% vs 48.0%, P = 0.01) and a trend on intent-to-treat (ITT) analysis (42.4% vs 25.0%, P = 0.06). This improvement was evident early and persisted to week 16 (P = 0.024 and 0.053 by per-protocol and ITT analyses, respectively). Mean cumulative and component-specific SF-36 scores improved in the imipramine group only (per-protocol, P < 0.01). Drug-related adverse events leading to patient dropout were more common in the imipramine group (25.4% vs 12.5%, P > 0.05).
CONCLUSION: Imipramine may be effective in the treatment of IBS patients and is associated with improved QoL. Careful patient selection, initiation of a low dose with gradual escalation and monitoring for side effects may result in an improved therapeutic response.
- Citation: Abdul-Baki H, El Hajj II, ElZahabi L, Azar C, Aoun E, Skoury A, Chaar H, Sharara AI. A randomized controlled trial of imipramine in patients with irritable bowel syndrome. World J Gastroenterol 2009; 15(29): 3636-3642
- URL: https://www.wjgnet.com/1007-9327/full/v15/i29/3636.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3636
Irritable bowel syndrome (IBS) is a common disorder of the gastrointestinal (GI) tract characterized by abdominal pain or discomfort and altered bowel habits. The exact pathophysiology of IBS remains unclear but is thought to involve altered intestinal motility and increased visceral sensitivity as a result of a dysregulated bidirectional communication between the enteric nervous system and the brain, the so-called brain-gut axis. The role of tricyclic antidepressants (TCAs) in the treatment of IBS has been systematically reviewed by the American Gastroenterology Association and the American College of Gastroenterology[12]. TCAs have been found to improve abdominal pain in IBS patients; however there was inadequate evidence to support an effect on improvement of global IBS symptoms. Notably, most randomized, controlled trials of TCAs in IBS were completed before the publication of the ROME II committee recommendations for the study design of treatment trials for IBS and suffered from suboptimal study design, small sample size, and short treatment duration[34].
The exact mechanism of action of TCAs is not known, but may be mediated via potentiation of adrenergic synapses by blocking uptake of the neurotransmitters, norepinephrine and serotonin, at nerve endings[56]. Their reported benefit in IBS appears, however, to be unrelated to an antidepressant effect given that a response is commonly noted early and with doses generally well below the effective antidepressant doses. Potential beneficial mechanisms of TCAs in IBS include a reduction in visceral pain sensitivity and, to a lesser extent, their anticholinergic effects.
The aim of this study was to evaluate the efficacy and safety of imipramine hydrochloride, a tertiary amine TCA, in patients with IBS who have failed to respond satisfactorily to antispasmodics. The primary study endpoint was global symptom relief as assessed by the patient at the end of the 12-wk treatment. Secondary endpoints were symptom relief at week 16 and changes in quality of life (QoL) as measured using the short form SF-36 questionnaire.
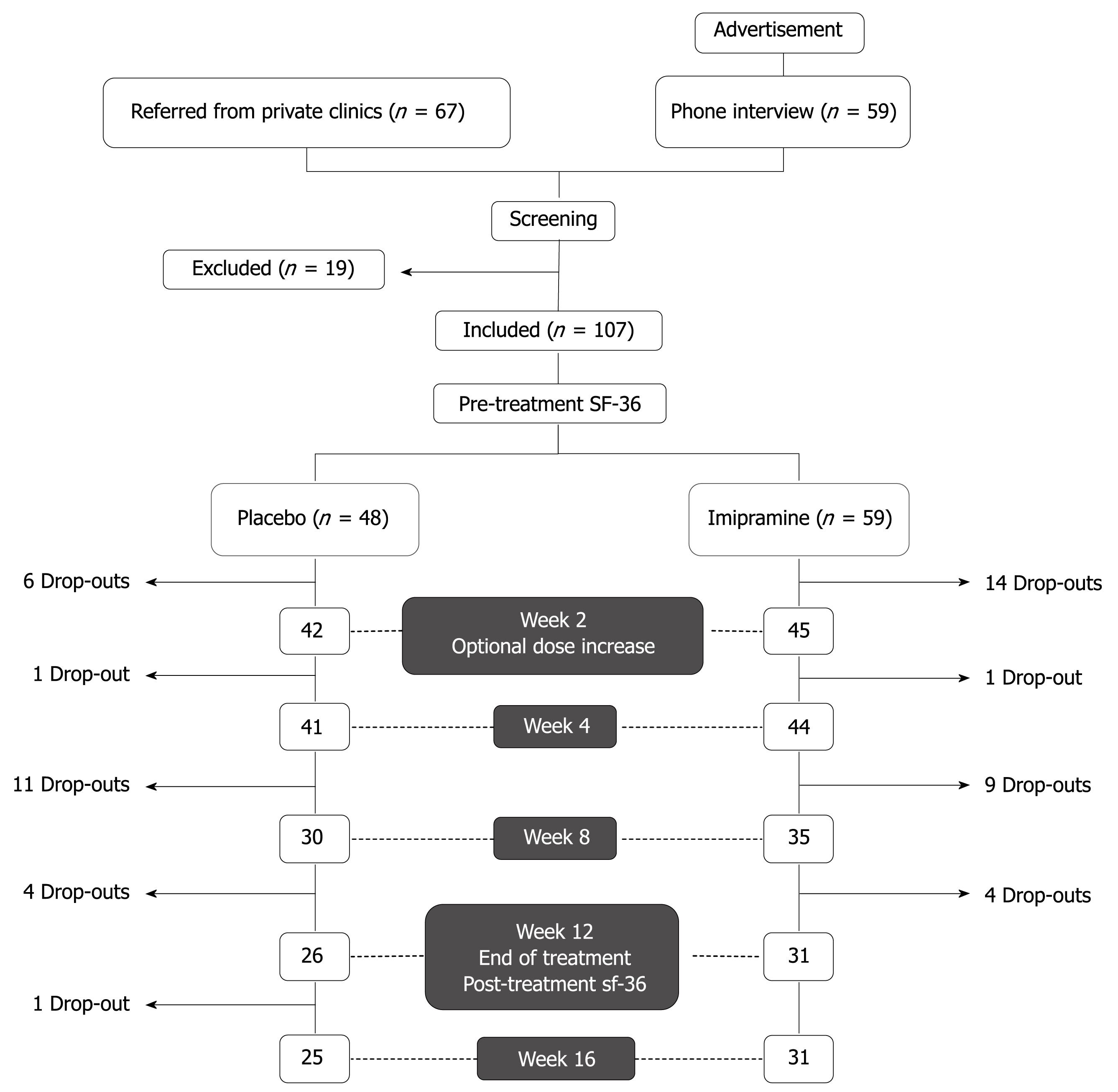
Figure 1 shows the general design of the study. Study subjects were recruited by either advertisements posted in clinics and pharmacies or by referral from primary care or specialty clinics at the American University of Beirut Medical Center (AUBMC). A preliminary telephone interview was conducted for patients who answered to posted advertisements. Those who satisfied inclusion criteria were asked to present for interview and examination by a general practitioner to confirm adherence to study inclusion criteria: (1) fulfillment of the Rome II criteria for the diagnosis of IBS and (2) history of an unsatisfactory response to one or more prescription antispasmodics available on the Lebanese market (trimebutine, mebeverine hydrochloride, otilonium bromide, or alverine citrate). Exclusion criteria were age below 18 years, allergy to imipramine, history of hematochezia or melena, constitutive symptoms (such as fever and weight loss), severe constipation (less than one bowel movement per 3 d), pregnancy, history of cardiac arrhythmias, use of any drug that could influence bowel function within 1 mo of entering the study (such as tegaserod, laxatives, antibiotics, or probiotics), known lactose intolerance, use of antidepressants or presence of signs and symptoms suggestive of clinical depression, or any evidence of advanced organic or psychiatric disease that may impact compliance or adherence to the study protocol. Similarly, patients who were referred from primary care or specialty clinics were screened for inclusion and exclusion criteria and records of previous medical investigations relating to the patient’s complaint were carefully reviewed to exclude organic disease.
After initial evaluation and assessment, written informed consent was obtained, and patients were asked to complete a pre-treatment QoL questionnaire, the SF-36, a reliable and valid measure of QoL[7–9]. A word-for-word Arabic language translation of the SF-36 was available for non-English speaking patients; however the translated version was not subjected to validation studies.
All subjects were randomized by an independent investigator using a computer-generated random numbers table with a 1.2 to 1 stratification in favor of imipramine. The randomization key was kept under lock until the completion of the study.
Study drugs were provided in opaque envelopes as imipramine (Tofranil, Novartis Pharma AG, Basle, Switzerland) 25 mg tablets, one tablet daily before bedtime for 84 d (12 wk) or matching placebo tablets.
Patients were contacted by phone at day 7 and day 14 of treatment to report any side effects. At day 14, patients who reported unsatisfactory global improvement of symptoms were given the choice of either continuing the treatment as before or doubling the daily dose (one tablet twice daily). The decision was left to the patients based on their level of tolerance to side effects, if any (the change, however, had to be effected once and on, or starting at, day 14).
The study’s main variable was the subjective feeling of global symptom relief as reported by the subjects in response to the following question: “Have your symptoms improved satisfactorily since starting the study drug?” Patients were contacted on weeks 4, 8 and 12 of treatment to answer this question. At week 12, patients were requested to complete a post-treatment SF-36 questionnaire. An off-treatment follow-up was done at week 16 to answer the same global relief question mentioned above. Compliance was checked by pill count. The trial was approved by the Institutional Research Board of the AUBMC.
Sample size calculation was estimated based on the assumption of a 60% response to imipramine vs a 30% response to placebo. The estimated sample size was 56 patients per arm. Projecting a 20%-30% dropout rate, the sample size was calculated at 70 patients per arm. The data were entered and analyzed using SPSS version 11.5. Frequency tables and cross-tabulations were derived in order to depict any associations between the different variables. Analysis of the primary end-point (global symptom relief) was done according to an intent-to-treat (ITT) basis. The paired samples t-test and the independent-samples t-test were used to compare the QoL scores before and after treatment. The change in SF-36 scores between week 12 (end of treatment) and baseline was calculated only in patients who completed the study and had paired SF-36 scores (PP analysis). A P-value at or below 0.05 was considered as the cut-off point for statistical significance.
From December 2004 to May 2006, 67 patients were referred from private clinics and 59 patients answered to posted advertisements. Because of significant thinning of patient recruitment late in the study period as well as extenuating political circumstances in Lebanon, the study was closed in June 2006 before the preset sample size could be reached. Of the 136 screened subjects, 107 met the criteria for enrollment; 59 were randomized to imipramine and 48 to placebo. Since enrollment was stopped prematurely, the power calculation was performed ad hoc. Assuming a 60% response rate to imipramine vs a 30% response rate to placebo, with α = 0.05 (two-tailed), the above sample size allowed us to detect a significant difference between the two groups with a calculated power of 88.4%.
Both groups were comparable with regard to age, sex, and symptoms (Table 1). The mean age in the imipramine arm was 42.6 ± 12.4 years vs 45.3 ± 13.8 years for the placebo arm, with a slight male predominance. Fifty-seven individuals of the total patient sample (52.5% vs 54.2%) had undergone endoscopic procedures with no abnormal findings. The remaining patients had undergone other diagnostic testing based on their primary physician’s recommendations (blood count, inflammatory markers, stool studies, and imaging) which were non-revealing.
| Imipramine (n = 59) | Placebo (n = 48) | |
| Mean age (yr) | 42.6 ± 12.4 | 45.3 ± 13.8 |
| Male sex | 33 (55.9) | 29 (60.4) |
| Type of recruitment | 38 (64.4) referrals | 29 (60.4) referrals |
| Bloating/distention | 57 (96.6) | 46 (95.8) |
| Abdominal pain | 58 (98.3) | 47 (97.9) |
| Flatulence | 45 (76.3) | 40 (83.3) |
| Constipation | 17 (28.8) | 15 (31.3) |
| Diarrhea | 11 (18.6) | 7 (14.6) |
| Mixed pattern | 14 (23.7) | 15 (31.3) |
| Mean baseline SF-36 score | 98.6 ± 21.3 | 102.8 ± 16.6 |
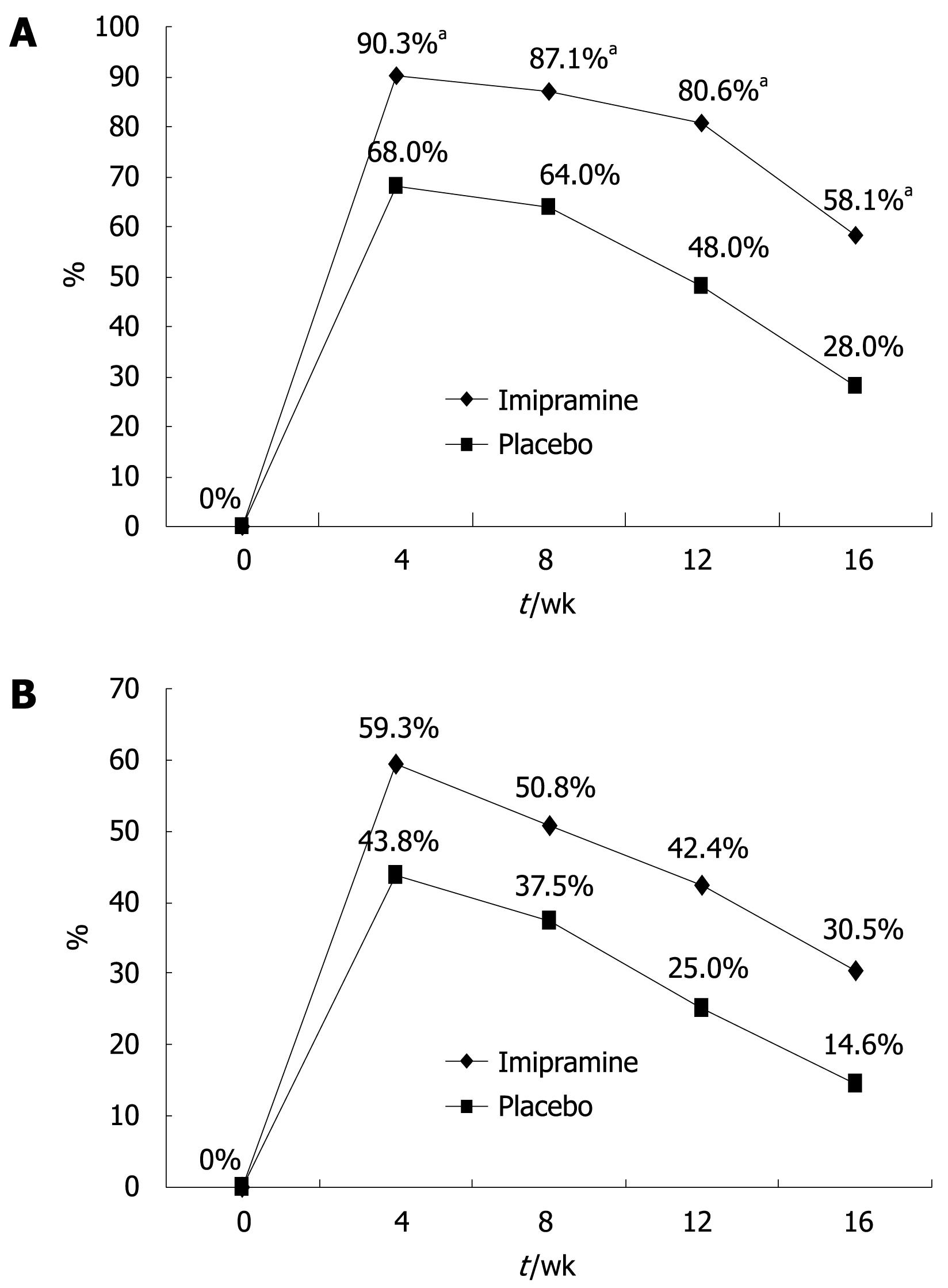
All patients fulfilled the Rome II criteria for IBS and had a history of at least one previous treatment using off-the-counter or prescribed antispasmodic medications with unsatisfactory results. The patients’ global relief of symptoms during the different intervals of the trial is shown in Figure 2. The ITT population included all 107 patients while the per-protocol analysis was based on results from the 56 patients (31 on imipramine and 25 on placebo) who completed the 12-wk active treatment period and the 4-wk off-treatment follow-up). The imipramine group reported relief of baseline symptoms in 80.6% of patients at the end of the 12-wk treatment period as compared to 48.0% in the placebo arm (P = 0.01). The highest rate of relief was achieved at week 4 in both groups: 90.3% for imipramine and 68.0% for placebo (P = 0.037). Per-protocol analysis revealed a significantly greater improvement of baseline symptoms in the imipramine arm vs placebo group at all checkpoints of the 12-wk trial including 4 wk after stopping treatment (P = 0.024). ITT analysis of all 107 patients who received at least one tablet of the assigned treatment showed higher rates of global symptom relief in favor of imipramine at all study points; however, the difference was not statistically significant (42.4% vs 25% at week 12, P = 0.06, Figure 2).
The dropout rate was similar in both study groups: 28/59 (47.5%) for imipramine and 23/48 (47.9%) for placebo. Subjects receiving imipramine dropped out of the trial earlier than in the placebo arm with a mean dropout time of 25.0 ± 17.9 d vs 37.4 ± 20.8 d, respectively (P = 0.026). The reasons for dropout were loss to follow-up, premature withdrawal of treatment without side effects (at or before 2 wk of treatment), protocol violation, and side effects (Table 2). Premature withdrawal accounted for 60.9% of dropouts in the placebo group. Fourteen of the 28 dropouts (50%) on imipramine reported that side effects were the main reason for withdrawal with a predominance of anticholinergic side effects (Table 3). Of those 14 patients, only two had doubled the dose of medication (to 50 mg imipramine) after a sub-optimal response at 2 wk. In general, the rate of side effects was higher in the imipramine group (64.4% vs 39.6%, respectively, P = 0.01). However, when comparing the rates of side effects which led to subject dropout, the difference was not statistically significant (25.4% vs 12.5%, P = 0.094).
| Imipramine (n = 59) | Placebo (n = 48) | P-value | |
| Total dropouts | 28 (47.5) | 23 (47.9) | NS |
| Premature withdrawal | 8 (13.6) | 14 (29.2) | < 0.05 |
| Lost to follow-up | 3 (5.1) | 3 (6.3) | NS |
| Protocol violation | 3 | 0 | NS |
| Side effects | 14 (23.7) | 6 (12.5) | 0.094 |
| Side effect | Number of patients (n = 14) |
| Sleep disturbance | 3 |
| Urologic symptoms | 2 |
| Palpitations | 2 |
| Anxiety | 1 |
| Dry mouth | 1 |
| Dizziness | 3 |
| Flushing & sweating | 1 |
| Constipation | 1 |
Sixteen patients (27.1%) from the imipramine group compared to 19 (39.6%) patients from the placebo arm opted for doubling of the dose at day 14 of treatment (P = 0.188). There was no association between increasing the dose in the imipramine group and global symptomatic improvement, development of side effects, or adverse events leading to dropout; however, it was observed that 8/16 (50%) of patients whose treatment dose was doubled dropped out of the trial as compared to 15/38 (39.5%) patients maintained on the original dosage (P = 0.038). Furthermore, 41.8% (28/67) of patients recruited from clinics dropped out compared to 57.5% (23/40) of self-referred patients (P = 0.12), independent of assigned treatment (P = 0.10 for imipramine; P = 0.60 for placebo).
The change in QoL was assessed by asking patients to complete the SF-36 questionnaire upon onset of treatment and at completion of therapy (week 12). Only patients with paired completed SF-36 questionnaires were included in this analysis (PP group, n = 56). Before beginning treatment, the mean SF-36 scores were similar for imipramine and placebo groups (96.1 ± 25.0 vs 102.2 ± 17.0, P = 0.307). After treatment, the mean SF-36 scores for the imipramine and placebo groups increased to 113.7 ± 19.4 and 108.6 ± 15.9, respectively (P = 0.3). The mean percent difference in SF-36 scores before and after treatment for imipramine was 11.8% ± 13.2% compared to 4.3% ± 9.0% for placebo (P = 0.02). Further analysis of SF-36 results showed a trend for greater improvement in all components of the questionnaire in the imipramine arm compared to placebo (Figure 3).
The current ROME II committee recommendations for conducting randomized, controlled trials in IBS have stressed the use of the Rome criteria to identify patients with IBS, and a randomized, parallel-trial, double-blinded design with no placebo run-in, with minimum treatment duration of 8-12 wk, follow-up of symptoms after treatment is stopped, and assessment of compliance with therapy. Moreover, trials should include baseline assessment of symptoms, account for patient disposition (discontinuations, withdrawals, etc), provide sample size calculation and enroll an adequate number of patients, with the primary outcome being improvement of global IBS symptoms based on patient assessment and/or use of a validated scale to assess IBS symptoms[3]. To date, 10 randomized, controlled trials and two crossover studies have evaluated the effectiveness of TCAs in the treatment of IBS[10–18]. The largest and best study to date on TCAs was done by Drossman et al[19], investigating the role of desipramine in patients with functional disorders. However, the study involved patients with functional abdominal disorders (IBS, functional abdominal pain, painful constipation, and unspecified functional bowel disorders) and was therefore not restricted to IBS. Although the findings of that study supported a role of TCAs in functional bowel disorders, it is difficult to draw a firm conclusion regarding IBS given the inhomogeneous study population. On the other hand, few of the other trials used the Rome criteria to identify patients with IBS, measured compliance, or presented sample size calculations. Most had small sample sizes, and only one trial was more than 8 wk in duration. A most recent study by Rajagopalan et al[11] met most recommendations for optimal study design but again suffered from a small sample size (20 patients per arm of whom nine dropped out from each group), and the lack of patient follow-up after the treatment was stopped. Our study meets almost all of the above requirements and is the largest randomized trial to date investigating the role of TCAs in patients with IBS. The study findings provide support for the efficacy of TCAs in the treatment of IBS, particularly after failure of antispasmodics, commonly used as first-line agents in IBS.
The fact that antidepressants are more consistent in improving global measures than specific GI symptoms has raised concerns about their true effect in functional GI disorders including IBS[20] where changes in symptoms are weak predictors of changes in well-being following treatment with TCAs[21]. Although controversial[22], the use of global symptom improvement as the primary end point may arguably be more important because of the wide and varied symptomatology of IBS and functional abdominal symptoms, and the varying importance that patients place on particular symptoms. This helps overcome inherent disadvantages of symptom score systems, which measure physiologic epiphenomena such as stool characteristics and subtypes of IBS[23], but do not address the impact of this on global well-being.
The mechanism of action of TCAs in IBS remains unclear but appears to primarily involve a modulation of the brain-gut neurologic axis. Using functional magnetic resonance imaging, Morgan et al[24] have shown that rectal pain following balloon distention induced significant activation of the perigenual anterior cingulated cortex, right insula and right prefrontal cortex, and that amitriptyline use was associated with reduced pain-related cerebral activation in some centers, but only during stress. This effect was thought to occur in the central nervous system, rather than peripherally, to blunt pain and other symptoms exacerbated by stress in IBS. Another putative mechanism of action of TCAs is by modulation of gut motility via action on peripheral muscarinic receptors and/or on ATP-sensitive K+ channels in interstitial cells of Cajal[2526]. Identifying an anxiety or affective disorder is not necessary for initiating TCAs since they appear to have an analgesic advantage on somatic pain independent of psychiatric effects. Response to TCAs may in fact be attenuated in the presence of active psychiatric illness[2728]. Because of their side effects, particular effort must be taken to initiate treatment with TCAs. In our study, major side effects leading to premature dropout were more common in the imipramine group and were primarily anticholinergic side effects. The incidence of adverse events was similar to previously published trials of TCAs in patients with functional GI disorders or neuropathic pain[29]. This emphasizes the need to warn patients about potential side effects of TCAs and weigh these against any expected or perceived benefit.
Health-related QoL is affected significantly in patients with IBS compared to the general population. The health-related QoL is associated with perceived IBS severity defined by the overall disease limitations rather than symptoms and appears to improve in treatment responders and correlates with symptom improvement[3031]. For that reason, the SF-36 model, a general health status instrument, was used in our study to assess QoL. We found that the post-treatment SF-36 mean total score was significantly higher than the pre-treatment score in the imipramine group only with a notable improvement in all components of the health-related QoL instrument used.
The limitations of this study include the single center nature of the study, the lack of formal assessment of baseline co-morbid psychiatric distress and the possibility of unblinding given the untoward, primarily anticholinergic adverse events of TCA in some patients. This potential unblinding is unlikely, however, to have biased the measured patient-specific endpoints of global symptom relief or the SF-36 components. Other limitations include the fact that dose escalation was allowed at week 2 and the high dropout rate noted in both the imipramine and placebo arms (47.5% vs 47.9%, respectively) as well as the inability to meet the preset sample size for the study. The restriction of dose escalation to a single early time point (at 2 wk) was felt to best reflect the real-life clinical situation where dose adjustments are often made in the dose of TCAs according to patient response and possible drug-related adverse events. The high dropout rate in our trial can be explained by several factors. First, the IBS patient population is hard to study because of an existing high degree of psychosomatization. Second, and perhaps more importantly, these patients have an often unrealistic expectation of rapid symptomatic relief or even cure, and a 12-wk period may therefore constitute an unduly long period of time to “experiment” with a placebo or a drug that they may perceive as investigational and possibly ineffective. It is for this reason that most large studies on functional GI disorders have included a 2-4 wk run-in phase wherein patients who are more likely to continue the trial period are consequently selected. However, and despite these run-in or screening periods, dropout rates in these large trials remain in the order of 20%-25%[32–34]. Lastly, and perhaps most importantly, adherence may have been further reduced in our patient population for cultural reasons such as the unfamiliar concept of a “placebo” control arm and the fact that the use of the active drug in this particular study (a medication approved for the use of depression) may suggest or connote a “psychiatric” label to the patients’ condition. These factors, as well as the single center nature of the study, may have contributed to our inability to reach the preset sample size and to the premature closure of the study. The above notwithstanding, this study remains the largest placebo-controlled trial of TCAs in patients with IBS as defined by strict clinical criteria.
In conclusion, this randomized, double-blind, placebo-controlled trial provides evidence in support of the efficacy of imipramine in reducing symptoms of IBS and providing global relief. Symptom improvement is associated with improved QoL. Careful patient selection and education about the role of TCAs, the rationale for their use, and the recognition of potential side effects are important considerations. Gradual dose escalation, perhaps in small increments of 10 mg, and close monitoring are likely to result in a better therapeutic response.
Tricyclic antidepressants (TCAs) were found to improve abdominal pain in irritable bowel syndrome (IBS) patients. This trial aimed to study the effect of imipramine, a TCA, on global symptom relief in patients with IBS.
TCAs have been shown in a few trials to improve abdominal pain in patients with IBS; however there was inadequate evidence to support an effect on improvement of global IBS symptoms. Notably, most randomized, controlled trials of TCAs in IBS were completed before the publication of the ROME II committee recommendations for the study design of treatment trials for IBS and suffered from suboptimal study design, small sample size, and short treatment duration.
This trial did not include a run-in-phase in order to simulate the real-life scenario in the clinic, and to obtain a valid assessment of the utility and compliance with TCAs. It was rigorously designed and included a formal assessment of quality of life (QoL) indicators and followed all the recommendations of the Rome committee on the optimal design of IBS trials. Only a few published trials on TCAs have done so and all included less than 20 patients per arm, making this trial the largest ever conducted on the use of TCAs in patients with IBS.
IBS remains a common intestinal disorder causing significant discomfort and poor QoL to patients who have the diagnosis. TCAs have been shown to improve abdominal pain in patients with IBS; however, there is insufficient evidence of global symptom relief. The search for an optimal treatment to improve symptoms and QoL in IBS remains ongoing.
IBS is a common disorder of the gastrointestinal tract characterized by abdominal pain or discomfort and altered bowel habits. The exact pathophysiology of IBS remains unclear; however, it is thought to involve altered intestinal motility as a result of dysregulated communication between the brain and enteric nervous system. The mechanism by which TCAs affect this neural communication is yet unknown.
Few trials have adequately studied the effect of TCAs on global relief of symptoms in patients with IBS. The aim of this study was to evaluate the efficacy and safety of imipramine in patients with IBS who have failed to respond satisfactorily to antispasmodics.
| 1. | Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology. 2002;123:2108-2131. |
| 2. | Brandt LJ, Bjorkman D, Fennerty MB, Locke GR, Olden K, Peterson W, Quigley E, Schoenfeld P, Schuster M, Talley N. Systematic review on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S7-S26. |
| 3. | Veldhuyzen van Zanten SJ, Talley NJ, Bytzer P, Klein KB, Whorwell PJ, Zinsmeister AR. Design of treatment trials for functional gastrointestinal disorders. Gut. 1999;45 Suppl 2:II69-II77. |
| 4. | American College of Gastroenterology Functional Gastrointestinal Disorders Task Froce. Evidence-based position statement on the management of irritable bowel syndrome in North America. Am J Gastroenterol. 2002;97:S1-S5. |
| 5. | Linner L, Arborelius L, Nomikos GG, Bertilsson L, Svensson TH. Locus coeruleus neuronal activity and noradrenaline availability in the frontal cortex of rats chronically treated with imipramine: effect of alpha 2-adrenoceptor blockade. Biol Psychiatry. 1999;46:766-774. |
| 6. | Svensson TH, Usdin T. Feedback inhibition of brain noradrenaline neurons by tricyclic antidepressants: alpha-receptor mediation. Science. 1978;202:1089-1091. |
| 7. | Garratt AM, Ruta DA, Abdalla MI, Buckingham JK, Russell IT. The SF36 health survey questionnaire: an outcome measure suitable for routine use within the NHS? BMJ. 1993;306:1440-1444. |
| 8. | Stewart AL, Hays RD, Ware JE Jr. The MOS short-form general health survey. Reliability and validity in a patient population. Med Care. 1988;26:724-735. |
| 9. | Stewart AL, Greenfield S, Hays RD, Wells K, Rogers WH, Berry SD, McGlynn EA, Ware JE Jr. Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA. 1989;262:907-913. |
| 10. | Vahedi H, Merat S, Momtahen S, Kazzazi AS, Ghaffari N, Olfati G, Malekzadeh R. Clinical trial: the effect of amitriptyline in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27:678-684. |
| 11. | Rajagopalan M, Kurian G, John J. Symptom relief with amitriptyline in the irritable bowel syndrome. J Gastroenterol Hepatol. 1998;13:738-741. |
| 12. | Greenbaum DS, Mayle JE, Vanegeren LE, Jerome JA, Mayor JW, Greenbaum RB, Matson RW, Stein GE, Dean HA, Halvorsen NA. Effects of desipramine on irritable bowel syndrome compared with atropine and placebo. Dig Dis Sci. 1987;32:257-266. |
| 13. | Heefner JD, Wilder RM, Wilson ID. Irritable colon and depression. Psychosomatics. 1978;19:540-547. |
| 14. | Myren J, Groth H, Larssen SE, Larsen S. The effect of trimipramine in patients with the irritable bowel syndrome. A double-blind study. Scand J Gastroenterol. 1982;17:871-875. |
| 15. | Steinhart MJ, Wong PY, Zarr ML. Therapeutic usefulness of amitriptyline in spastic colon syndrome. Int J Psychiatry Med. 1981;11:45-57. |
| 16. | Tanum L, Malt UF. A new pharmacologic treatment of functional gastrointestinal disorder. A double-blind placebo-controlled study with mianserin. Scand J Gastroenterol. 1996;31:318-325. |
| 17. | Tripathi BM, Misra NP, Gupta AK. Evaluation of tricyclic compound (trimipramine) vis-a-vis placebo in irritable bowel syndrome. (Double blind randomised study). J Assoc Physicians India. 1983;31:201-203. |
| 18. | Talley NJ, Kellow JE, Boyce P, Tennant C, Huskic S, Jones M. Antidepressant therapy (imipramine and citalopram) for irritable bowel syndrome: a double-blind, randomized, placebo-controlled trial. Dig Dis Sci. 2008;53:108-115. |
| 19. | Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, Emmott S, Proffitt V, Akman D, Frusciante K. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19-31. |
| 20. | Talley NJ. Antidepressants in IBS: are we deluding ourselves? Am J Gastroenterol. 2004;99:921-923. |
| 21. | Clouse RE, Lustman PJ. Use of psychopharmacological agents for functional gastrointestinal disorders. Gut. 2005;54:1332-1341. |
| 22. | Whitehead WE, Palsson OS, Levy RL, Feld AD, VonKorff M, Turner M. Reports of “satisfactory relief” by IBS patients receiving usual medical care are confounded by baseline symptom severity and do not accurately reflect symptom improvement. Am J Gastroenterol. 2006;101:1057-1065. |
| 23. | Spiegel BM, Gralnek IM, Bolus R, Chang L, Dulai GS, Mayer EA, Naliboff B. Clinical determinants of health-related quality of life in patients with irritable bowel syndrome. Arch Intern Med. 2004;164:1773-1780. |
| 24. | Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601-607. |
| 25. | Gorard DA, Libby GW, Farthing MJ. Effect of a tricyclic antidepressant on small intestinal motility in health and diarrhea-predominant irritable bowel syndrome. Dig Dis Sci. 1995;40:86-95. |
| 26. | Choi S, Park CG, Kim MY, Lim GH, Kim JH, Yeum CH, Yoon PJ, So I, Kim KW, Jun JY. Action of imipramine on activated ATP-sensitive K(+) channels in interstitial cells of Cajal from murine small intestine. Life Sci. 2006;78:2322-2328. |
| 27. | Lancaster-Smith MJ, Prout BJ, Pinto T, Anderson JA, Schiff AA. Influence of drug treatment on the irritable bowel syndrome and its interaction with psychoneurotic morbidity. Acta Psychiatr Scand. 1982;66:33-41. |
| 28. | Clouse RE, Lustman PJ, Geisman RA, Alpers DH. Antidepressant therapy in 138 patients with irritable bowel syndrome: a five-year clinical experience. Aliment Pharmacol Ther. 1994;8:409-416. |
| 30. | Chang L. Review article: epidemiology and quality of life in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20 Suppl 7:31-39. |
| 31. | Hahn BA, Kirchdoerfer LJ, Fullerton S, Mayer E. Patient-perceived severity of irritable bowel syndrome in relation to symptoms, health resource utilization and quality of life. Aliment Pharmacol Ther. 1997;11:553-559. |
| 32. | Camilleri M, Northcutt AR, Kong S, Dukes GE, McSorley D, Mangel AW. Efficacy and safety of alosetron in women with irritable bowel syndrome: a randomised, placebo-controlled trial. Lancet. 2000;355:1035-1040. |
| 33. | Camilleri M, Kim DY, McKinzie S, Kim HJ, Thomforde GM, Burton DD, Low PA, Zinsmeister AR. A randomized, controlled exploratory study of clonidine in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2003;1:111-121. |
| 34. | Novick J, Miner P, Krause R, Glebas K, Bliesath H, Ligozio G, Ruegg P, Lefkowitz M. A randomized, double-blind, placebo-controlled trial of tegaserod in female patients suffering from irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2002;16:1877-1888. |