Published online Aug 7, 2009. doi: 10.3748/wjg.15.3621
Revised: May 25, 2009
Accepted: June 1, 2009
Published online: August 7, 2009
AIM: To investigate the correlation between the expression levels of interleukin (IL)-6 and proteins in tight junctions (TJs) in the esophageal mucosa of rats modeling different types of reflux esophagitis (RE), and the ability of aluminum phosphate to protect against RE-induced mucosal damage via these proteins.
METHODS: Male SPF Wistar rats aged 56 d were divided randomly into acid RE, alkaline RE, mixed RE, and control groups. Various surgical procedures were performed to establish rat models of acid RE. At 14 d after the procedure, some of the rats started aluminum phosphate treatment. Transmission electron microscopy (TEM) was used to observe the morphological features of TJs and desmosomes in the esophageal epithelium. Immunohistochemical methods and Western blotting were used to measure expression of claudin 1, occludin, ZO-1, JAM-1, DSG-1 and IL-6; reverse transcription polymerase chain reaction (RT-PCR) was used to measure expression of mRNA of claudin 1, occludin, ZO-1, JAM-1, DSG-1 and IL-6.
RESULTS: At day 14 after the procedures, an RE model was established in all subsequently sacrificed rats of groups A, B and C. By both gross and microscopic observation, the mucosa was damaged and thickened as the disease progressed. With TEM observation, a widened intercellular space was noticed, with significantly fewer desmosomes. Immunohistochemistry showed significantly higher levels of all proteins in all RE models compared to control rats at 3 d after operation (65.5% ± 25.6% vs 20.5% ± 2.1%, P < 0.05, respectively). At 14 d after operation, along with continuing hyperplasia in the basal layer, the expression of TJ proteins in individual cells gradually decreased (12.4% ± 2.1% vs 20.5% ± 2.1%, P < 0.05, respectively). Western blottings and RT-PCR showed a directly proportional increase in IL-6 levels in relation to TJ proteins, as compared to controls (0.878 ± 0.024 vs 0.205 ± 0.021 and 0.898 ± 0.022 vs 0.205 ± 0.021, P < 0.05, respectively). Upon treatment with aluminum phosphate, however, these protein levels were restored to normal gradually over 30-60 d in rats with acid RE (30.4% ± 2.1% vs 20.5% ± 2.1%, P > 0.05, treated vs untreated, respectively). These levels increased in the rat with alkaline RE, and this increase was accompanied by continued hyperplasia in comparison with controls (85.5% ± 25.6% vs 20.5% ± 2.1%, P < 0.05, respectively). Furthermore, the expression of TJ proteins was not correlated significantly with that of IL-6 in this group.
CONCLUSION: These findings indicate that TJ proteins are highly expressed as an early molecular event involved in RE development, and that IL-6 is an inflammatory factor in this process.
- Citation: Li FY, Li Y. Interleukin-6, desmosome and tight junction protein expression levels in reflux esophagitis-affected mucosa. World J Gastroenterol 2009; 15(29): 3621-3630
- URL: https://www.wjgnet.com/1007-9327/full/v15/i29/3621.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3621
Gastro-esophageal reflux disease (GERD) is a common condition that has an impact on many millions of people worldwide, and is gaining more clinical recognition as a significant gastrointestinal disease. Lifestyle factors, such as obesity, high-fat diet, and physical inactivity, boost the incidence of GERD. GERD occurs when stomach and duodenal contents reflux into the esophagus. Reflux-induced damage and inflammation in the esophageal mucosa are referred to as reflux esophagitis (RE).
The pathogenic process of RE is highly complex. However, the current consensus among scientists is that RE mainly results from damage induced by reflux of the defending esophageal mucosa system. An imbalance between offensive and defensive factors in the esophagus leads to the development of RE. The epithelial defense system consists of three parts: pre-epithelial defense, epithelial defense and post-epithelial defense. In 2002, the German cellular biologist Langbein et al[1] reported that, in addition to desmosomes, tight junctions (TJs) could be found in the esophageal epithelium; and both were involved in maintaining the cellular barrier. Since then, TJs and desmosomes are considered to be important contributors to the epithelial defense of the esophageal mucosa. Other contributors include intercellular lipids, mucin and the epithelial transportation system (such as Na+-H+ exchanger and Na+-dependent Cl--HCO3- exchanger). Recent studies showed that TJs created a circular substance-blocking barrier between adjacent cells. TJs also function in cell adhesion, cell polarity and permeability, and in signal transduction resulting in cell proliferation and differentiation. Intercellular TJs consist mainly of claudins, occludins, JAM and ZOs. In 2003, Calabrese et al[2] discovered in their studies that the intercellular space of the lower esophageal epithelium in patients with GERD was three times as wide as that in normal controls, and suggested 0.74 &mgr;m as the threshold between normal and abnormal intercellular space. In 2004-2005, Asaoka et al[3] reported for the first time that expression of TJ proteins was altered during esophagitis; the expression level of claudin-3 decreased while that of claudin-1 increased[4]. Such changes have a determinant effect on the permeability of the esophageal epithelium. Therefore, further investigation is warranted using experiments testing the hypothesis that altered expression of TJ proteins and desmosome proteins results in failure of TJs and desmosomes, and thus leads to widened intercellular space and contributes to RE. In the investigation into RE, the use of human samples has methodological limitations. Hence, we created animal models of RE in rats, and sought to analyze the changes in intercellular space, changes in the distribution and expression of TJ proteins and desmosome proteins, and their correlation with the expression level of interleukin (IL)-6 in the pathogenic process of RE. Moreover, some rats were treated with aluminum phosphate to determine its efficacy in treating RE.
To explore the role of TJ and desmosome proteins in the pathogenesis of RE, we established three animal models of RE on the Wistar rat background. We performed three types of surgical procedures on these rats, and measured the distribution and expression profiles of claudin 1, occludin, ZO-1, JAM-1, DSG-1 and IL-6 in the esophageal mucosa of the various RE rats, untreated and treated with aluminum phosphate.
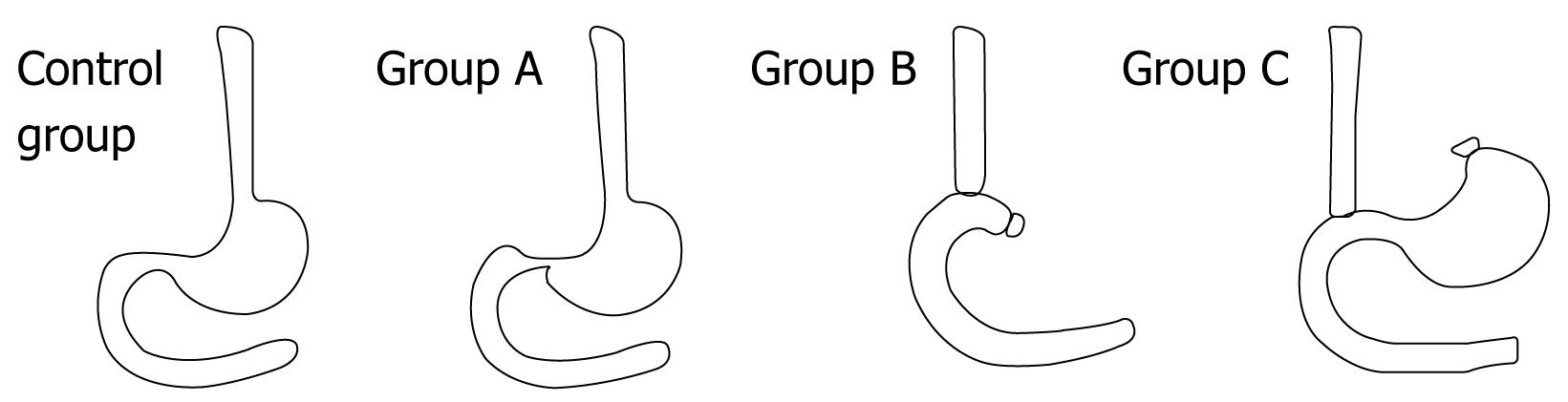
Two-hundred and twenty male SPF Wistar rats aged 56 d were purchased as laboratory animals at Shengjing Hospital of China Medical University, acclimatized and fed with standard chow and water ad libitum for 14 d in an animal room with controlled room temperature (24 ± 2°C). Following the surgical procedures, the rats were fasted for 24 h and were given water ad libitum. Before surgery, the rats were divided into four experimental groups. The control group was comprised of 10 rats, while the experimental groups A, B and C, respectively modeling acid RE, alkaline RE and mixed RE, were comprised of 70 rats each. Prior to surgery, the rats were anesthetized with chloral hydrate (Qingdao Yulong Algae Co., Ltd, Shangdong, China) 0.3% mL/100 mg via abdominal injection. The control rats underwent laparotomy, modified from the methods described by Yu et al[5], without any further surgical procedures[3]. After laparotomy, a pyloric stenosis was created by wrapping and ligating the pylorus with 1-0 silk thread around a metal tube (diameter: 2 mm) which was retrieved slowly after ligation. To enhance gastric reflux, a 0.5 cm longitudinal incision was made by cardiomyotomy in the esophageal-gastric junction. The incision was deep enough to expose the mucosa.
Group A rats underwent partial pyloric ligation (which is also known as incomplete pyloric ligation) and cardiomyotomy, while those in group B were treated with total gastrostomy plus end-to-side esophago-duodenal anastomosis. In the group B rats, the gastric artery and vein were separated and ligated, and then the cardia and pylorus were ligated and incised. The stomach was removed, and the cardia was then connected to the pylorus by end-to-side anastomosis[6]. The group C rats underwent cardiomyotomy and cardia ligation plus end-to-side esophago-duodenal anastomosis. To initiate surgery, the group C rats were placed in a supine position and an incision was made in the medium of upper abdomen. The lower esophagus was separated and incised and the vagus nerve was preserved. The cardia end of the stomach was disclosed with purse-string sutures, and the stomach was excluded. A 0.4-0.5 cm longitudinal incision was made on the duodenal wall at 1.0 cm from the pyloric end. The lower esophagus was connected to the duodenum with end-to-side anastomosis[7].
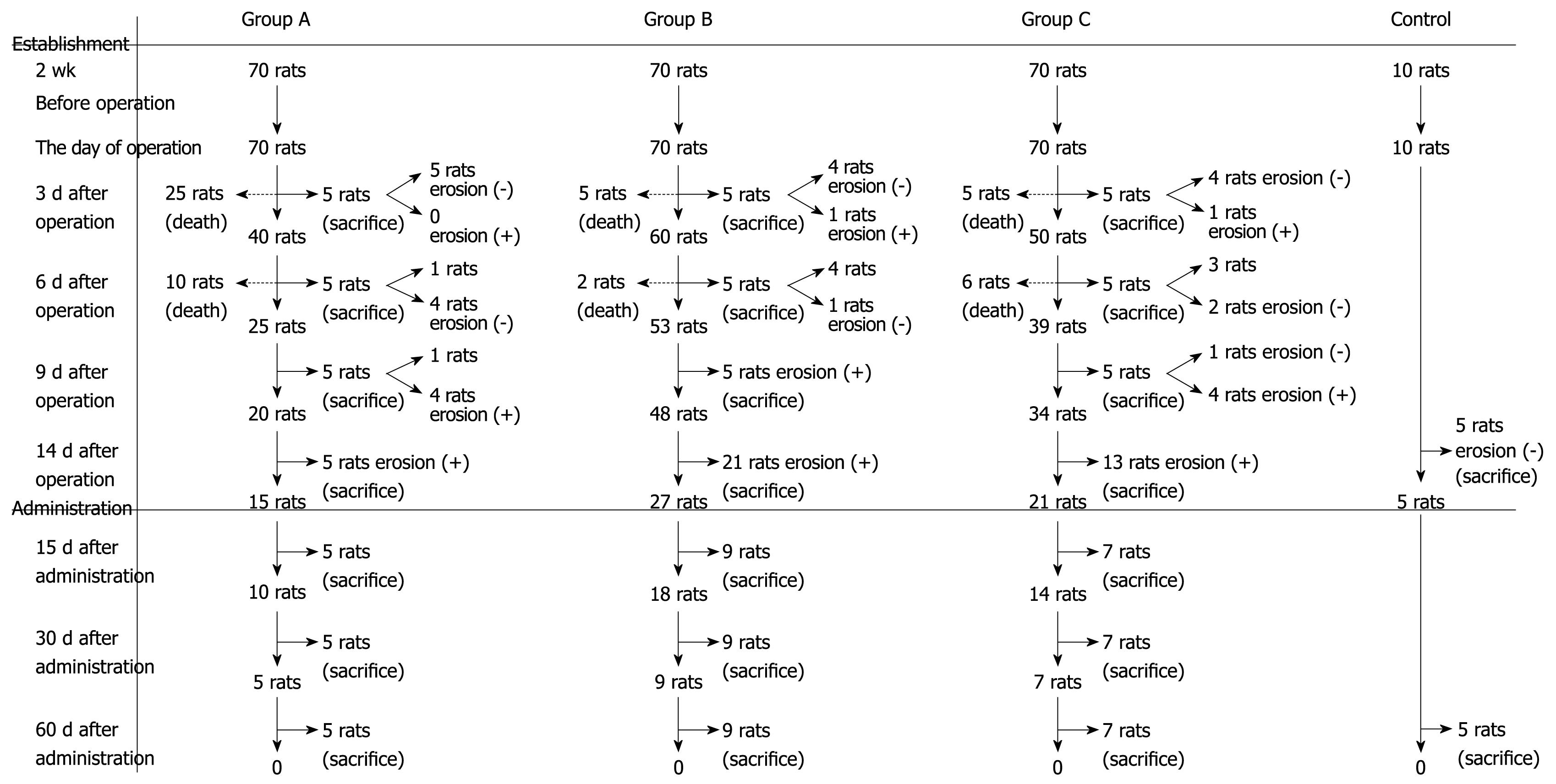
On post-procedure days 3, 6, 9 and 14, subgroups of the rats were sacrificed and the middle-low esophagi were dissected for evaluating the experimental success rates. The severity of the damage in esophagi was examined according to the diagnostic criteria of RE as determined by the Chinese Medical Association Digestive Endoscopy Society (1999).
One week following the procedure, 35 rats in group A, seven rats in group B and 21 rats in group C died. Five rats from groups A, B and C were sacrificed at post-procedure days 3, 6 and 9, respectively. At day 14, five rats in group A, five rats in the control group, 21 rats in group B and 13 rats in group C were sacrificed, and the middle-lower esophagus was obtained for calculating the success rate of the model.
Aluminum phosphate treatment: The remaining rats were fed 0.5 g aluminum phosphate (Boryung Pharmaceutical Co., Ltd. Fusan, Korea) in water, twice daily for 15 successive days. Five rats in group A, five rats in control group, nine rats in group B and seven rats in group C were sacrificed at post-treatment days 30 or 60. The survival, incidence of esophagitis, and average change in body mass in these model groups and in the control group were calculated.
At post-procedure days 3, 6, 9 and 14, the lower esophagi were dissected for mucosal damage evaluation based on the Chinese Medical Association Digestive of Erosive Esophagitis (1999), and epithelial thickness was measured and evaluated as an indication of damage. Esophageal tissues were then fixed in 10% buffered-formalin, shaped into 4 mm tissue masses, dehydrated with gradient ethanol, clarified with xylene, embedded in paraffin and sectioned into 4 &mgr;m slices, which were then stained with HE solution. The stained slices were then observed under the optical microscope, and a diagnosis was made based on the pathological features according to diagnostic criteria of RE by the Chinese Medical Association Digestive Endoscopic Society (1999), revealing the incidence of RE in these animal models[8].
At days 30 or 60 following aluminum phosphate treatment initiation, the rats were sacrificed and a TEM (TEM; 2600 J EME2000X, Hitachi) was used to observe the morphological features of TJs and desmosomes in the esophageal epithelium. Samples were obtained from the lower esophagus at 5 cm from the lower end, fixed in glutaraldehyde solution at 4°C, and then clarified, dehydrated, embedded and sectioned into ultra-thin slices. Ten slices, prepared with samples obtained from different sites of the same esophagus, were selected for each esophagus and then observed under TEM. The LEICA image analyzing system was used to measure the intercellular space and calculate the number of desmosomes. Ten pictures were taken for each sample. In each picture, an intact cell was selected and an intercellular space was calculated by detecting the vertical distance between the selected cell and its adjacent cells in 10 randomly selected directions, with each direction at least 1.0 &mgr;m apart. 100 intercellular spaces were measured in 10 pictures and average width of intercellular space was calculated. We counted the number of desmosomes in the intercellular spaces of the 10 selected cells, and totaled and averaged these values[9].
Immunohistochemical methods were used to measure the expression levels of claudin 1, occludin, ZO-1, JAM-1, DSG-1 and IL-6. For these assays, the middle-lower esophagus was obtained and fixed in 10% buffered-formalin, shaped into 4.0 mm tissue masses, dehydrated with gradient ethanol, clarified with xylene, embedded in paraffin and sectioned into 4.0 &mgr;m slices, which were then dewaxed with xylene, debenzolized with anhydrous ethanol and dehydrated with gradient ethanol, and then treated with 3.0% hydrogen peroxide and rinsed with water. After being restored at high pressure and high temperature, blocking agent (10% normal goat serum) was added to dissolve the slices at room temperature. Thirty minutes later, rabbit anti-IL-6, claudin 1, ZO-1, JAM-1 and DSG-1 antibodies were added at a dilution of 1:80, and goat anti-occludin antibody added at a 1:100 dilution at 4°C. The slices were then incubated overnight and washed with phosphate-buffered saline (PBS) 3 ×, 10 min each, the next day. At room temperature, general secondary antibody was added, and the slices were incubated at 37°C for 30 min. The slices were washed with PBS 3 ×, for 10 min each time. Streptavidin-peroxidase was added at 37°C. Thirty minutes later, the slices were washed again with 3 × PBS for 10 min each time. The slices were then stained with 3,3-Diaminobenzidine for 5 min, rinsed with water, and then stained with hematoxylin for another 5 min and rinsed with water again. The slices were then treated with acidic ethanol, and washed with running water for 30 min, dehydrated in gradient ethanol, and clarified with xylene, and sealed with gum. These slices of mucosa of RE were observed under the microscope for the measurement of expression of IL-6, claudin 1, occludin, ZO-1, JAM-1 and DSG-1.
Western blotting was used to measure the expression levels of claudin 1, occludin, ZO-1, JAM-1, DSG-1 and IL-6 (all from Santa Cruz Biotechnology, Santa Cruz, CA). For these assays, the esophageal tissue (100 mg) was obtained and added to 1.0 ml of cell lysis buffer [Tris/HCL (100 mmol/L, pH 7.5), NaCl (100 mmol/L), 0.5% sodium deoxycholate, ethylene diamine tetra-acetate (EDTA, 1 mmol/L), 1% NP40, 0.1% sodium dodecyl sulfate (SDS) and protease inhibitor]. Total protein was extracted, and the concentration was measured by the Lowry method. The extracted protein (50 &mgr;g) was added to 15% SDS-polyacrylamide gel for electrophoresis (100 V for 1.5 h). When the bromophenol blue reached the bottom of the gel, the protein was blotted onto a nitrocellulose membrane. The blots were immersed in 5.0% milk (prepared with milk powder) for 2 h. Then at 4°C, rabbit anti-IL-6, claudin 1, ZO-1, JAM-1 and DSG-1 antibodies at dilutions of 1:80, and goat anti-occludin antibody at dilutions of 1:100 were added to the blots, which were then incubated overnight. The next day, the membrane was washed, and the corresponding alkaline phosphate-labeled secondary antibodies were added at room temperature. The membrane was then stained with O-Dianisidine Tetrazotized and β-Naphthyl acid phosphate, and analyzed with an electrophoresis gel imaging system.
Using the grey scale measurement of the electrophoresis gel imaging system, the expression levels of IL-6, claudin 1, occludin, ZO-1, JAM-1 and DSG-1 were calculated and expressed as percentages relative to the β-actin control (absorbance of these proteins/absorbance of β-actin).
RT-PCR was employed for cognate mRNA detection and quantitation. Total RNA was extracted with a Trizol Kit, (Invitrogen, Carlsbad, CA), measured, and verified with an ultra-violet spectrophotometer. Primers were designed by Primer Premier 5.0 (Premier Biosoft International, Palo Alto, CA) according to mRNA sequences (by GenBank) of IL-6, claudin 1, occludin, ZO-1, JAM-1, DSG-1 and β-actin (as control). The primer sequences are shown in Table 1. RT-PCR was performed using the Takara RNA LA PCR kit (Takara, Kyoto, Japan) according to the manufacturers’ instructions. RT-PCR was catalyzed under the following temperatures: 5 min at 94°C; 35 cycles of 30 s at 94°C, 30 s at 59°C and 1 min at 72°C; finally 5 min at 72°C. 5 &mgr;g of the PCR product was added to 2 &mgr;L loading buffer, and electrophoresed in a 1.5% agarose gel at 100 V for 1.5 h. Analysis was carried out using a gel imaging system (Glyko, Novato, CA, USA).
| Primers | Upstream primers | Downstream primers | Amplification |
| JAM-1 | 5'-ACTGCCGTCCAGGTTC-3' | 5'-TTCGCCACTATCAAAGG-3' | 524 bp |
| Claudin-1 | 5'-GGATGTCCTGCGTTTC-3' | 5'-CACAGCCAAGACCCTC-3' | 674 bp |
| Occludin | 5'-CCACTATGAAACCGACTA-3' | 5'-CCAGCAACCAGCATC-3’ | 399 bp |
| Zo1 | 5'-CTCGGGCATTATTCG-3' | 5'-GCTTCCTGGCACTTTT-3' | 205bp |
| Dsg1 | 5'-CCTGCTGCTTGCTTT-3' | 5'-GGTTATTGGGCTCGTC-3' | 538bp |
| Il-6 | 5'-CTCCATCTGCCCTTCA-3' | 5'-CCAGGATAGAGCCACCAAT-3' | 586bp |
| β-actin | 5'-GGTCCTTAGCCACTCC-3' | 5'-CCAGGATAGAGCCACCAAT-3' | 701bp |
Data were processed with a statistical package SPSS version 10.0 (SPSS Inc, USA).
The means between two groups were compared for differences using the student’s t-test; comparison of the ratio between two samples was tested with the chi-square test. The data were reported as mean ± SD. A 95% confidence interval for the average weight of rats was calculated. In the analysis of correlation between expression of proteins, differences were considered significant at the P < 0.05 level.
At post-procedure day 14, the RE model was established in all group A-, B- and C-sacrificed rats. By gross and microscopic observation, we ascertained that the mucosa was damaged and thickened as the disease progressed. Figure 1 helps illustrate the establishment of the animal models with the three modified procedures. In the modified RE models, the survival of group A was 50% at day 14 after procedures (n = 35/70); 90% (n = 63/70) for group B, and 70% (n = 49/70) for group C. At 14 d after the procedures, the RE models were established in all the rats (n = 39/39). Survival and incidence of RE are shown in Figure 2. At days 3, 6 and 9 after the procedures, the average weight of rats in the model groups continuously decreased as compared to the control group. In aluminum phosphate-treated rats, the average weight improved as treatment progressed and was comparable to the control group at 1 mo afterward.
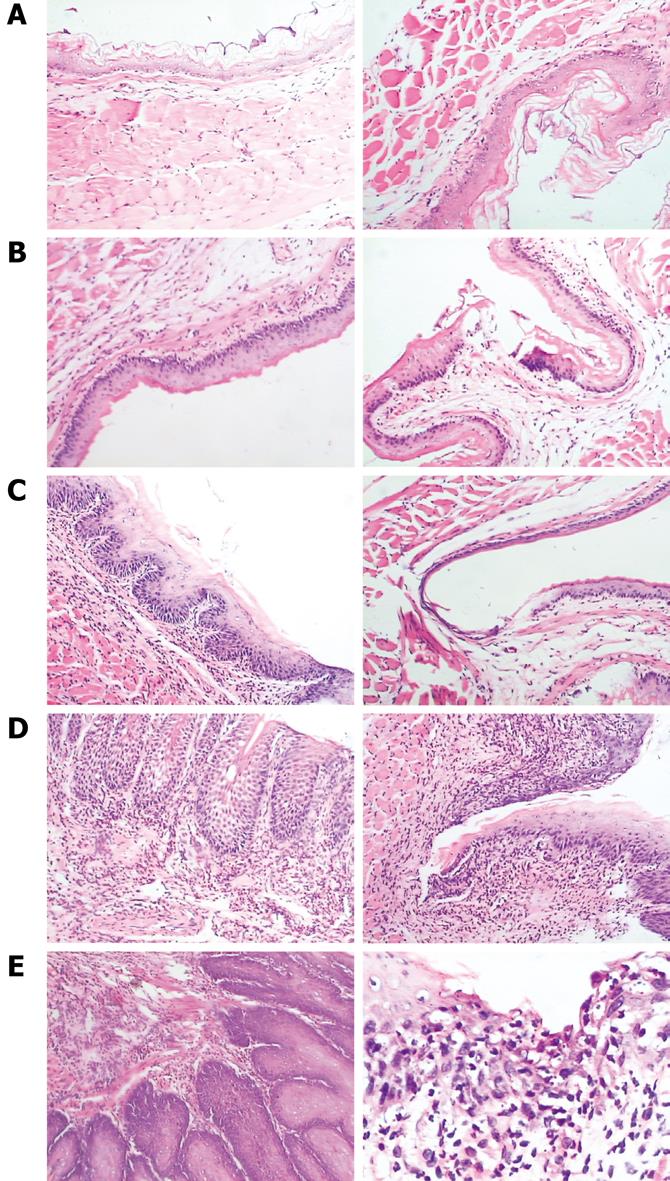
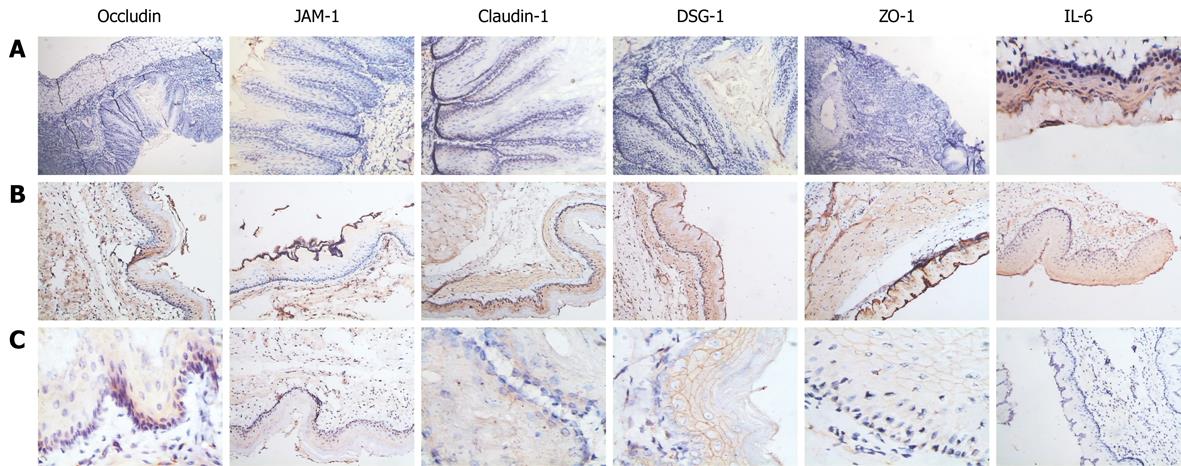
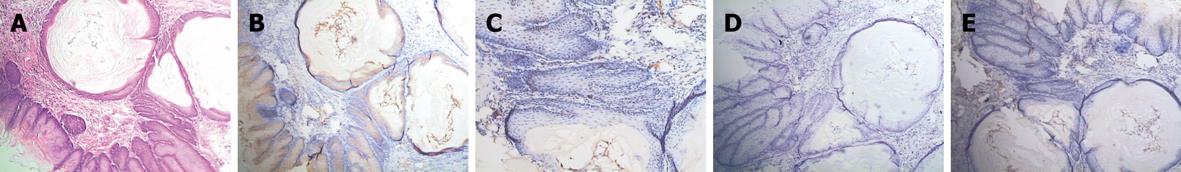
In RE models, erosions of various sizes could be seen in the mucosa, while no erosion was detected in the control group. In most cases, esophagitis occurred in the middle-low part of esophagus. Histologically, the normal esophagus revealed a thin epithelial layer with squamous cells (Figure 3A). In experimental RE models, the epithelium was markedly thickened and the lamina propria papillae were elongated into the epithelium. Basal cell hyperplasia and inflammatory cell infiltration were prominent in the lamina propria and the thickness of the esophageal epithelium increased with time in RE models (Figure 3B-E). In the control group, no expression of IL-6 was detected; claudin-1, occludin, ZO-1 and JAM-1 could be seen on the cellular membrane of the esophageal epithelial cells, mostly in the spinous and granular layers, while DSA-1 and JAM-1 could be seen on the cellular membrane of all mucosal layers. Amongst these proteins, DSG-1 had the highest expression level. In RE model rats, expression of IL-6 was only seen in inflammatory cells; the expression levels of claudin-1, occludin, ZO-1, JAM-1 and DSG-1 increased in both the cellular membrane and cytoplasm of spinous and granular layers in the mucosa around erosions, and these proteins were seen in cytoplasm as well as in membrane. However, as hyperplasia continued in the basal cells, expression of claudin-1, occludin, ZO-1, JAM-1 and DSG-1 decreased in individual cells of the spinous and granular layers (Figure 4A-C). One month after treatment started, the histological changes of RE disappeared in acid RE rats, with comparable expression levels of the proteins to the control group. Treatment was not effective for alkaline RE rats, in which hyperplasia and elevated expression of IL-6 continued and vesicles could be seen. But expression of these proteins was significantly lower in individual cells as compared to control group (Figure 5).
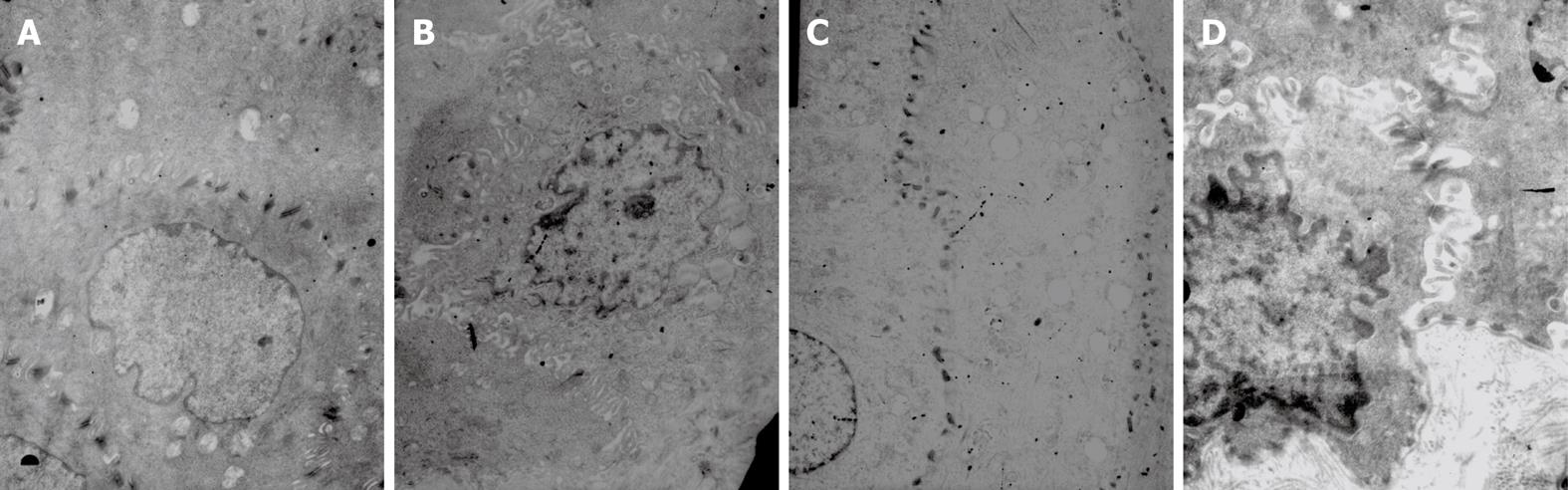
As shown with TEM, the nucleus and cellular membrane were generally intact. However, a swollen cellular membrane within the intercellular space was noted; intercellular space was significantly wider in some cells, with fewer or even no desmosomes in these widened spaces. The number of desmosomes per unit area decreased as well. In RE rats, the width of the intercellular space was 2.39 ± 0.42 &mgr;m. As for the control rats, the width was 0.63 ± 0.21 &mgr;m, which was significantly smaller as compared to that in the RE rats (P < 0.05). The average number of desmosomes was 0.124 ± 0.044/&mgr;m2, which was also significantly smaller than that observed in the control group (0.221 ± 0.031/&mgr;m2, P < 0.05) (Figure 6).
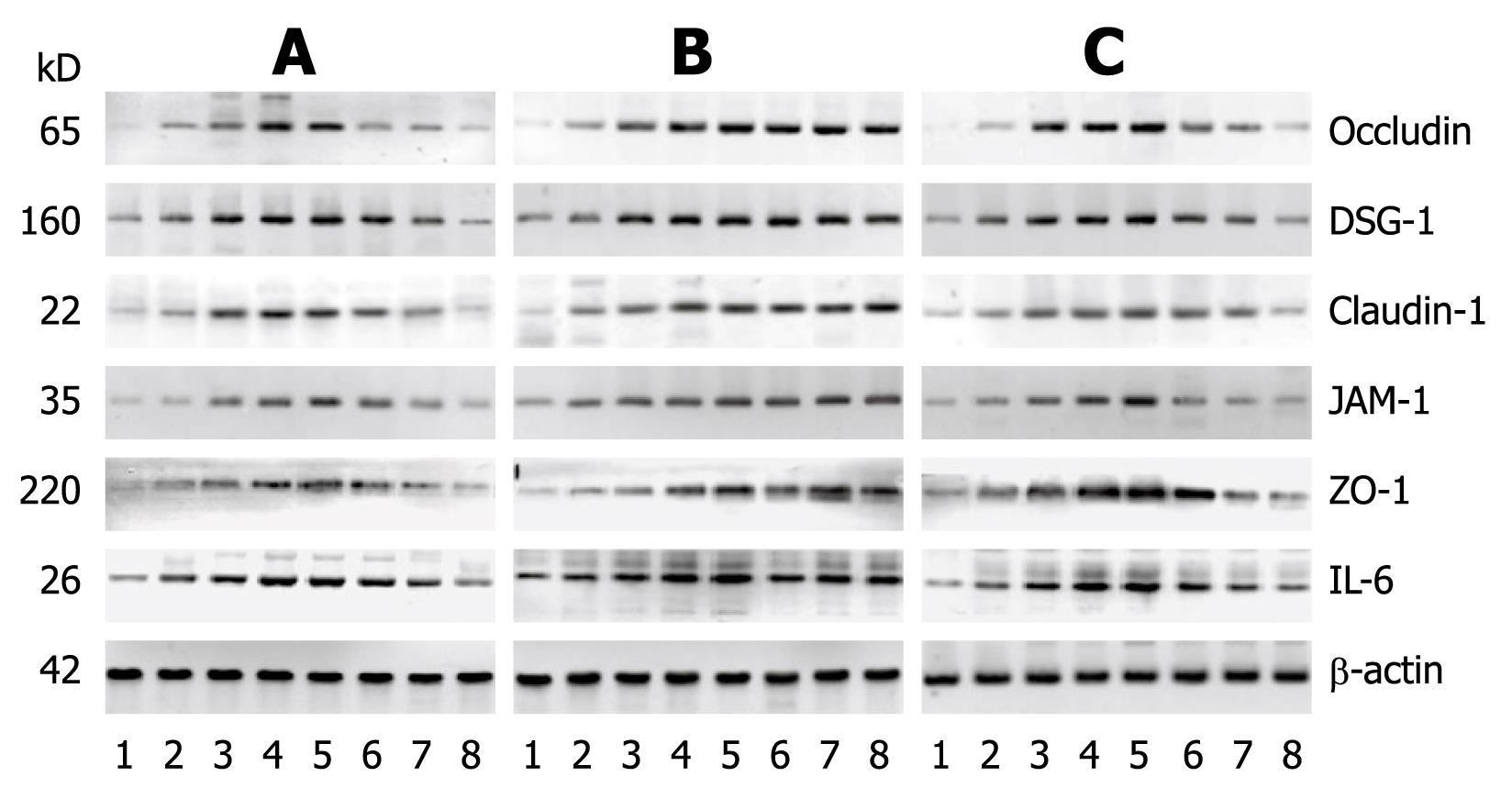
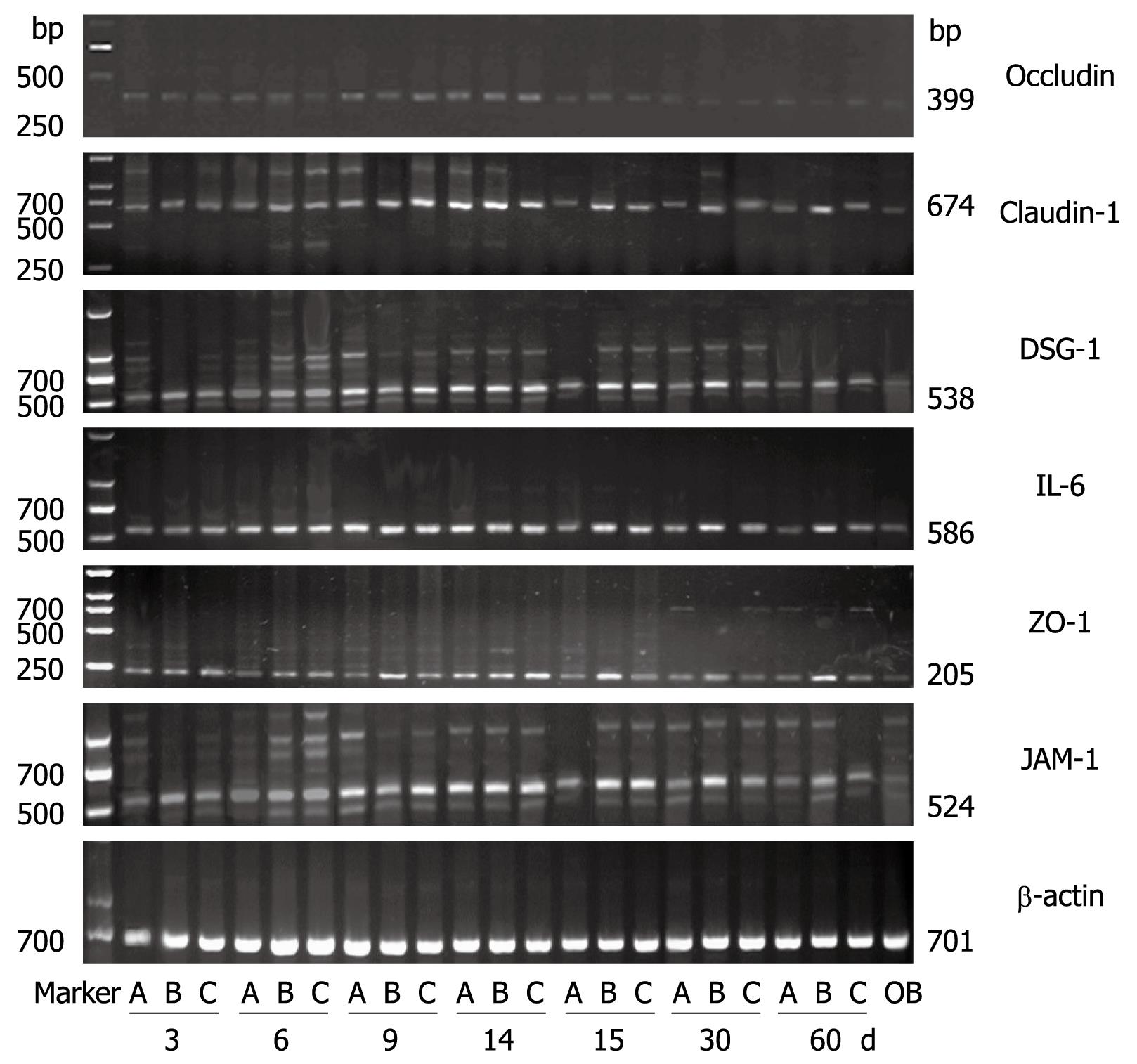
Compared to the control group, the Western blottings revealed that the expression levels of IL-6, claudin-1, occludin, ZO-1, JAM-1 and DSG-1 increased with time in the RE groups. In the aluminum phosphate-treated acid RE rats, the expression levels of claudin-1, occludin, ZO-1, JAM-1 and DSG-1 gradually decreased as treatment progressed and reverted to normal levels 30 d after treatment initiation. As for the mixed RE rats, the expression levels of IL-6, claudin-1, occludin, ZO-1, JAM-1 and DSG-1 returned to normal within 60 d after treatment initiation, while the expression levels of these proteins in the alkaline RE rats were markedly high at all time points during the study (Figures 7 and 8). The results of the Western blotting and RT-PCRs suggested that as the expression level of IL-6 increased, those of claudin-1, occludin, ZO-1, JAM-1 and DSG-1 increased as well and became more prominent as the disease progressed. Within 30 d after initiation of the aluminum phosphate treatment, the Western blotting and RT-PCR results indicated that the expression levels of IL-6, claudin-1, occludin, ZO-1, JAM-1 and DSG-1 in the acid RE rats (group A) decreased gradually and became comparable to that of the normal control group. Hyperplasia and widening of the intercellular spaces were restored as observed by microscopy. At day 60, the Western blotting and RT-PCR results suggested that the expression levels of these proteins decreased gradually in the mixed RE rats (group C), but increased dramatically in the alkaline reflux group (group B).
Numerous studies have described the establishment of animal models of RE. The success rate was about 10% when the rat model was created by using the method described by Yu et al[5]. Most rats died of reflux-related complications, such as asphyxia, pulmonary edema and pulmonary hemorrhage. Therefore, we modified their method by using a 2.0 mm metal tube in the partial pyloric ligation instead of a 1.5 mm tube. Furthermore, in our study, the tube was not inserted into the stomach as it was in Qiang Yu’s method, which helped avoid artificial gastric perforation. These modifications increased the success rate to 50% (n = 35/70). Two weeks after the procedures, the success rate was 100% (n = 5/5) as confirmed by histological examination. RE models created with the other procedures were even more stable, with higher survival levels of 70% and 90%. Prominent hyperplasia and erosion appeared at post-procedure day 6. At post-procedure day 14, the RE model was established in all examined rats[8].
Histological assessment of the esophagitis models showed that the epithelium was significantly thickened; the lamina propria mucosa was markedly elongated into the epithelium. Inflammatory cell infiltration was noted in lamina propria. Such histological findings were extremely similar to those seen in examples of human RE. Thus, these models can help us deduce the development of the process of erosion. In our study, no prominent erosions were noticed within 6 d after the procedure. However, microscopic observation revealed that the thickness of the esophageal mucosa had already increased at this time point, and continued to increase with time. This finding suggested that irritants such as inflammation had promoted cellular proliferation and epithelial hyperplasia before the erosions occurred[1011]. Moreover, we found that expression levels of claudin-1, occludin, ZO-1, JAM-1 and DSG-1 in the model rats were significantly higher than that of the control group; however, as hyperplasia of basal cells occurred, expression of these proteins in the individual cells decreased. These findings suggested elevated expression of these proteins was an early molecular event in the pathogenesis of RE, which occurred significantly earlier than hyperplasia. In the immunohistochemical staining, we noted that DSG-1 could be found on the membrane in all layers of the mucosa and had the highest expression level among these proteins, which suggests that the desmosome is the main cell junction in the esophageal epithelium. With TEM, we found that as RE progressed, the swollen cellular membrane within the intercellular space was noted in model rats in comparison with control rats; the intercellular space was significantly widened in some cells, with fewer or even no desmosomes in these spaces, which was consistent with the findings of Calabrese et al[2] and Tobey et al[12] in 2003. Western blottings and RT-PCR revealed that, as the expression of the inflammatory factor IL-6 increased in the model rats, the expression levels of claudin-1, occludin, ZO-1, JAM-1 and DSG-1 in esophageal mucosa increased as well. With the information from immunohistochemical assays, we found that the expression level of these TJ proteins in the model rats was significantly higher than that in control rats as early as 3 d after the procedures, but the Western blotting and RT-PRC suggested that the total amount of these proteins remained the same at this time point. When elevated expression levels of these proteins were detected by these assays, we could see hyperplasia in the basal cells under the microscope, while expression of these proteins decreased in the individual cells. These findings suggest that hyperplastic cell populations require more cell junctions and more cell junction proteins, but production of these proteins has started to decrease in individual cells, which leads to failure of cell junctions in these cells. A possible explanations is: when reflux occurs and TJs and desmosomes in the intercellular space are damaged, the cells begin to express more TJ and desmosome proteins in response in an effort to repair the damaged TJs and desmosomes; if damage persists and reflux starts to provoke hyperplasia, even more proteins are needed in the formation of cell junctions in these new cells. At this point, the production of TJ proteins and desmosome proteins falls short of the need, which leads to the failure of this protein compensating mechanism. Subsequent decreased expression of these proteins in individual cells leads to failure of cell junctions and further widening of the intercellular space and elevated permeability in the esophageal epithelium, resulting in damaged epithelial defense and the pathological changes of RE, such as swollen mucosa, erosion and ulcer. These findings also suggest that elevated expression of claudin-1, occludin, ZO-1, JAM-1 and DSG-1 is a part of the pathogenesis of RE and an early molecular event in the development and progression of RE, and also one protective mechanism against the damage in RE.
In 2005, Calabrese et al[2] found that widening of the intercellular spaces in squamous cells of the lower esophagus in patients with GERD could be restored after being treated with omeprazole for 3-6 mo. In our study, we treated RE rats with aluminum phosphate and it emerged that the three groups of rats responded differently to the treatment, with the most significant efficacy noticed in acid RE rats. Western blotting and RT-PCR assays suggested the expression of claudin-1, occludin, ZO-1, JAM-1 and DSG-1 decreased in these rats accordingly and gradually. At 1 mo after treatment started, expression of these proteins reverted to normal levels. Microscopic observation revealed that swelling and hyperplasia of the basal cells were restored, and the intercellular space was comparable to the normal controls. Within 60 d after treatment started, expression of these proteins gradually decreased in mixed RE rats, with milder hyperplasia under the microscope as compared to alkaline RE rats; swollen mucosa, erosion and ulcer disappeared as well. However, no significant treatment benefit was seen in alkaline RE rats. Western blotting and RT-PCR assays revealed that with persistent elevated expression of IL-6, claudin-1, occludin, ZO-1, JAM-1 and DSG-1, hyperplasia continued and vesicles developed. Microscopically, we found that expression of cell junction proteins was significantly lower in individual cells compared to those of control group. Aluminum phosphate is a weak acidic insoluble gel with a high level of absorbance. It creates a colloid membrane that lasts for 1.5 h on the surface of the esophageal and gastric mucosa. It can also repair damaged digestive mucosa by preventing erosions caused by esophageal reflux and gastric acid, and by preventing self-digestion induced by digestive juice. Therefore, it is more effective in treating acid RE.
Result the four TJ proteins and one desmosome protein in our study revealed positive correlation between two of these proteins, which suggests that positive regulation and synergism might occur in the pathogenesis of esophagitis and that these proteins function as a group. Furuse et al[13] found TJs in occludin gene-deleted rats, indicating that these four TJ proteins work in synergism in maintaining cell junctions. Claudins, the main components of TJs, are potent adhesive proteins that create a cell junction and seal the intercellular space[14]. They also play an important role in specific functions of TJs, such as fence and barrier functions. Altered expression of claudins might lead to malformation of TJs. Occludins are mainly seen in TJ fibrils and are involved in signal transduction in the formation of TJs[15–17]. ZO-1 is a bridge that connects the TJs to intracellular structures[18–20], while JAM has a role in regulating permeability of TJs[2122]. Desmosomes, a kind of cell junction that specifically localizes in epithelium and mainly consists of DSG, is a structure that facilitates connection and signal transduction between cells. Inspired by the pathogenesis of inflammatory bowel disease[23–25] as described by Gassler et al[26], we deduce that from the outset, esophageal reflux might destroy the structure of claudin-1 and occludin, and then ZO-1 fails to connect claudin-1 to the cellular membrane resulting in the re-distribution of ZO-1 and damage to the TJs and a subsequent increase in the permeability of esophageal epithelium; in response, expression of JAM and DSG increases, leading to more damage to the desmosomes, and eventually RE. These findings have certain implications in explaining the molecular mechanism of RE.
Statistical analysis was done to investigate the correlation between IL-6 and TJ proteins, and desmosome proteins. The results revealed no correlation. IL-6, an inflammatory factor in the development and progression of RE, is a pleiotropic cytokine produced by monocytes/macrophages. Jia et al[27] indicated that the IL-6 level in patients with active ulcerative colitis (UC) was markedly higher than that in patients with inactive disease and normal controls, but was not related to site and scale of the lesion. Martinez de Haro et al [11] found that the IL-6 level in normal mucosa of patients was higher than in normal controls but levels were not significantly different between normal mucosa and affected mucosa obtained from the same patient. These data suggest that the IL-6 level might reflect the activity of UC and RE to a certain extent.
Hamaguchi et al[28] found that in chronic esophagitis rats, expression of cytokines and adhesive molecules increased. Although currently, we don’t see any connection between cytokines and TJ proteins, we might assume that inflammatory cytokines and subsequent damage to the tissue might have regulated the permeability of esophageal epithelium in esophagitis. Cytokines might damage the barrier function and increase the permeability of the epithelium.
In summary, in molecular cell biology and microscopic technology which accelerate research in this field[2930], we now have an increasingly profound understanding of the function and structure of TJ, TJ proteins’ regulation of the TJ formation and structure, as well as the roles of epithelial permeability and TJs in epithelial defense. Simonovic et al[31] and Jin et al[32] proposed that phosphorylation of occludins was the determinant factor in the distribution of these proteins, but its regulating mechanism, as well as the biological mechanism underlying the feedback regulation of these proteins, are still unclear and need to be further explored in more studies.
Tight junction (TJ) and desmosome proteins in esophageal epithelium create a circular substance-blocking barrier between adjacent cells. These proteins function in cell adhesion, contribute to cell polarity and permeability and facilitate signal transduction in cell proliferation and differentiation. We investigated the correlation between the expression levels of IL-6 and the proteins in TJs, including claudin 1, occludin, ZO-1, JAM-1 and DSG-1, in the esophageal mucosa of rats modeling different types of reflux esophagitis (RE), and the ability of aluminum phosphate to protect against RE-induced mucosal damage via these proteins.
Further investigations are warranted using experiments testing the hypothesis that altered expression of TJ proteins (claudin 1, occludin, ZO-1, JAM-1) and desmosome proteins (DSG-1) results in failure of TJs and desmosomes, which thus leads to widened intercellular space and contributes to RE. Moreover, some rats were treated with aluminum phosphate to determine its efficacy in treating RE.
These findings indicate that increased expression of TJ proteins, as an early molecular event, is involved in RE development and that IL-6 is an inflammatory factor in this process. Aluminum phosphate significantly protects esophageal mucosa by enhancing its defense against damage. However, efficacy of the drug is related to pH value of the reflux: it is effective in treating acid RE rather than alkaline RE.
GERD occurs when stomach and duodenal contents reflux into the esophagus. Reflux-induced damage to, and inflammation in, the esophageal mucosa are referred to as RE. TJs create a circular substance-blocking barrier between adjacent cells. TJs also function in cell adhesion, cell polarity and permeability, and in signal transduction resulting in cell proliferation and differentiation. Intercellular TJs consist mainly of claudins, occludins, JAM and ZOs.
This interesting study was to determine the changes of IL-6 and various TJ proteins in the esophageal mucosa induced by RE. Authors conducted these studies in rats and nicely correlated between the expression pattern of claudin, occludin, ZO-1 and JAM-1 proteins in different types of RE and the ability of aluminum phosphate to protect against RE. Results presented in this manuscript showed that there is a significant difference between RE induced and sham-control groups.
| 1. | Langbein L, Grund C, Kuhn C, Praetzel S, Kartenbeck J, Brandner JM, Moll I, Franke WW. Tight junctions and compositionally related junctional structures in mammalian stratified epithelia and cell cultures derived therefrom. Eur J Cell Biol. 2002;81:419-435. |
| 2. | Calabrese C, Fabbri A, Bortolotti M, Cenacchi G, Areni A, Scialpi C, Miglioli M, Di Febo G. Dilated intercellular spaces as a marker of oesophageal damage: comparative results in gastro-oesophageal reflux disease with or without bile reflux. Aliment Pharmacol Ther. 2003;18:525-532. |
| 3. | Asaoka D, Miwa H, Hirai S, Ohkawa A, Kurosawa A, Kawabe M, Hojo M, Nagahara A, Minoo T, Ohkura R. Altered localization and expression of tight-junction proteins in a rat model with chronic acid reflux esophagitis. J Gastroenterol. 2005;40:781-790. |
| 4. | Miwa H, Asaoka D, Hojo M, Iijima K, Sato N. [GERD and tight junction proteins of the esophageal mucosa]. Nippon Rinsho. 2004;62:1441-1446. |
| 5. | Yu Q, Yuan HX, Cui NQ. Improved technique for the rat model of acid reflux esophagitis. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2002;10:74-75, 78. |
| 6. | Chen X, Yang G, Ding WY, Bondoc F, Curtis SK, Yang CS. An esophagogastroduodenal anastomosis model for esophageal adenocarcinogenesis in rats and enhancement by iron overload. Carcinogenesis. 1999;20:1801-1808. |
| 7. | Ireland AP, Peters JH, Smyrk TC, DeMeester TR, Clark GW, Mirvish SS, Adrian TE. Gastric juice protects against the development of esophageal adenocarcinoma in the rat. Ann Surg. 1996;224:358-370; discussion 370-371. |
| 8. | Wang W, Li Z, Xu G, Wan X, Duan Y, Zou D. [Carcinogenesis effects of gastric and duodenal refluxate on esophageal mucosa]. Zhonghua Neike Zazhi. 2000;39:821-824. |
| 9. | Liu SH, Xiong LS, Lin JK, Wu JL, Lu DY, Hu PJ, Chen MH. Ultrastructural investigation in esophageal mucosa of non-erosive gastroesophageal reflux disease. Zhonghua Xiaohua Zazhi. 2006;26:18-21. |
| 10. | Oberg S, Peters JH, DeMeester TR, Lord RV, Johansson J, DeMeester SR, Hagen JA. Determinants of intestinal metaplasia within the columnar-lined esophagus. Arch Surg. 2000;135:651-655; discussion 655-656. |
| 11. | Martinez de Haro L, Ortiz A, Parrilla P, Munitiz V, Molina J, Bermejo J, Rios A. Intestinal metaplasia in patients with columnar lined esophagus is associated with high levels of duodenogastroesophageal reflux. Ann Surg. 2001;233:34-38. |
| 12. | Tobey NA, Hosseini SS, Argote CM, Dobrucali AM, Awayda MS, Orlando RC. Dilated intercellular spaces and shunt permeability in nonerosive acid-damaged esophageal epithelium. Am J Gastroenterol. 2004;99:13-22. |
| 13. | Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539-1550. |
| 14. | Colegio OR, Van Itallie C, Rahner C, Anderson JM. Claudin extracellular domains determine paracellular charge selectivity and resistance but not tight junction fibril architecture. Am J Physiol Cell Physiol. 2003;284:C1346-C1354. |
| 15. | Nusrat A, Chen JA, Foley CS, Liang TW, Tom J, Cromwell M, Quan C, Mrsny RJ. The coiled-coil domain of occludin can act to organize structural and functional elements of the epithelial tight junction. J Biol Chem. 2000;275:29816-29822. |
| 16. | Chen YH, Lu Q, Goodenough DA, Jeansonne B. Nonreceptor tyrosine kinase c-Yes interacts with occludin during tight junction formation in canine kidney epithelial cells. Mol Biol Cell. 2002;13:1227-1237. |
| 17. | Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36:1206-1237. |
| 18. | Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994;127:1617-1626. |
| 19. | Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199-208. |
| 20. | Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745-29753. |
| 21. | Ostermann G, Weber KS, Zernecke A, Schröder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151-158. |
| 22. | Barton ES, Forrest JC, Connolly JL, Chappell JD, Liu Y, Schnell FJ, Nusrat A, Parkos CA, Dermody TS. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104:441-451. |
| 23. | Ranaldi G, Marigliano I, Vespignani I, Perozzi G, Sambuy Y. The effect of chitosan and other polycations on tight junction permeability in the human intestinal Caco-2 cell line(1). J Nutr Biochem. 2002;13:157-167. |
| 24. | Mine Y, Zhang JW. Surfactants enhance the tight-junction permeability of food allergens in human intestinal epithelial Caco-2 cells. Int Arch Allergy Immunol. 2003;130:135-142. |
| 25. | Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Mechanisms of diarrhea in collagenous colitis. Gastroenterology. 2002;123:433-443. |
| 26. | Gassler N, Rohr C, Schneider A, Kartenbeck J, Bach A, Obermüller N, Otto HF, Autschbach F. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216-G228. |
| 27. | Jia BL, Hou XH. Relationship between IL-6 and ulcerative colitis. Weichangbingxue He Ganbingxue Zazhi. 2004;13:217-221. |
| 28. | Hamaguchi M, Fujiwara Y, Takashima T, Hayakawa T, Sasaki E, Shiba M, Watanabe T, Tominaga K, Oshitani N, Matsumoto T. Increased expression of cytokines and adhesion molecules in rat chronic esophagitis. Digestion. 2003;68:189-197. |
| 29. | Zeissig S, Bürgel N, Günzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56:61-72. |
| 30. | Ara N, Iijima K, Asanuma K, Yoshitake J, Ohara S, Shimosegawa T, Yoshimura T. Disruption of gastric barrier function by luminal nitrosative stress: a potential chemical insult to the human gastro-oesophageal junction. Gut. 2008;57:306-313. |
| 31. | Simonovic I, Rosenberg J, Koutsouris A, Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2000;2:305-315. |
| 32. | Jin M, Barron E, He S, Ryan SJ, Hinton DR. Regulation of RPE intercellular junction integrity and function by hepatocyte growth factor. Invest Ophthalmol Vis Sci. 2002;43:2782-2790. |