Published online Jul 28, 2009. doi: 10.3748/wjg.15.3573
Revised: June 16, 2008
Accepted: June 23, 2008
Published online: July 28, 2009
The diagnosis of cystadenoma is rare, even more so when located in the extrahepatic bile duct. Unspecific clinical signs may lead this pathology to be misdiagnosed. The need for pathological anatomy in order to distinguish cystadenomas from simple biliary cysts is crucial. The most usual treatment nowadays is resection of the bile duct, together with cholecystectomy and Roux-en-Y reconstruction.
- Citation: Bartolome MAH, Ruiz SF, Romero IM, Lojo BR, Prieto IR, Alvira LG, Carreño RG, Esteban ML. Biliary cystadenoma. World J Gastroenterol 2009; 15(28): 3573-3575
- URL: https://www.wjgnet.com/1007-9327/full/v15/i28/3573.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3573
Cystadenoma is a benign tumor, although prone to malignant degeneration[1], supposedly originating in intrahepatic (and more rarely extrahepatic) embryonic tissue precursors of biliary epithelium. It is a non recurrent lesion, with only 125 cases reported in literature[2]. It may appear either as a uninodular or as a multinodular cystic lesion, and may attain large proportions.
Cystadenomas account for 4.6% of intrahepatic biliary cysts. They are more recurrent in middle-aged females (40-50 years old) with an incidence rate of 4:1 with respect to males. Cystadenomas are rarely found in extrahepatic bile ducts.
The etiology of cystadenomas remains unclear, although several theories have been suggested. Some authors consider this disease a premalignant lesion. Due to the usual absence of clinical symptoms, the most frequent diagnosis is by chance, as in the excision of a cystic lesion.
In this paper, we report a case of cystadenoma at the excision of a suspected choledochal cyst in an adult female.
Our patient was a 60-year-old woman with a history of high blood pressure under treatment. While a laparoscopic cholecystectomy was performed for recurrent episodes of biliary colic, a dilated bile duct was evidenced intraoperatively.
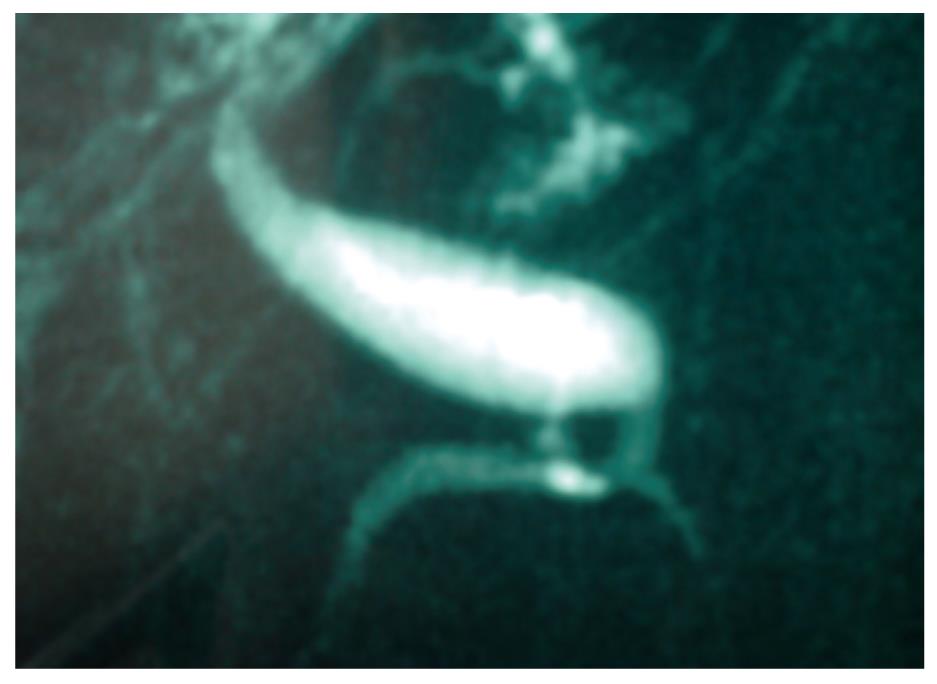
Upon this finding, an informed magnetic cholangioresonance was requested due to dilatation of the choledochal duct. However, it did not show any proximal or distal bile duct dilatation, which was most likely a normal variant (type-1 choledochal cyst according to Todany’s classification) (Figure 1). Blood tests with tumor markers were requested, and a CA19.9 of 51.8 was shown, whereas the rest of the tests were normal.
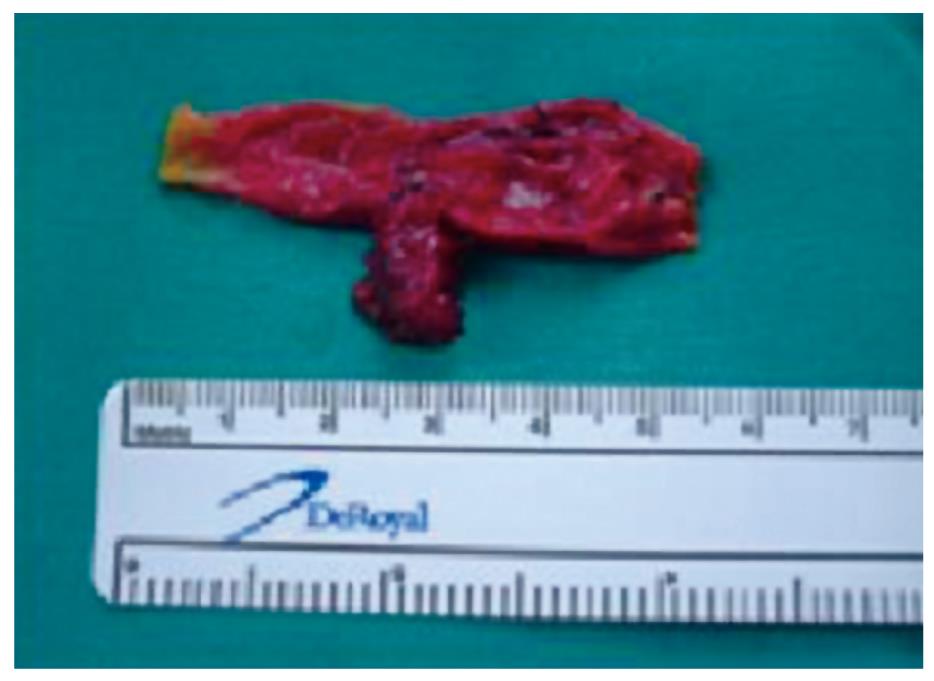
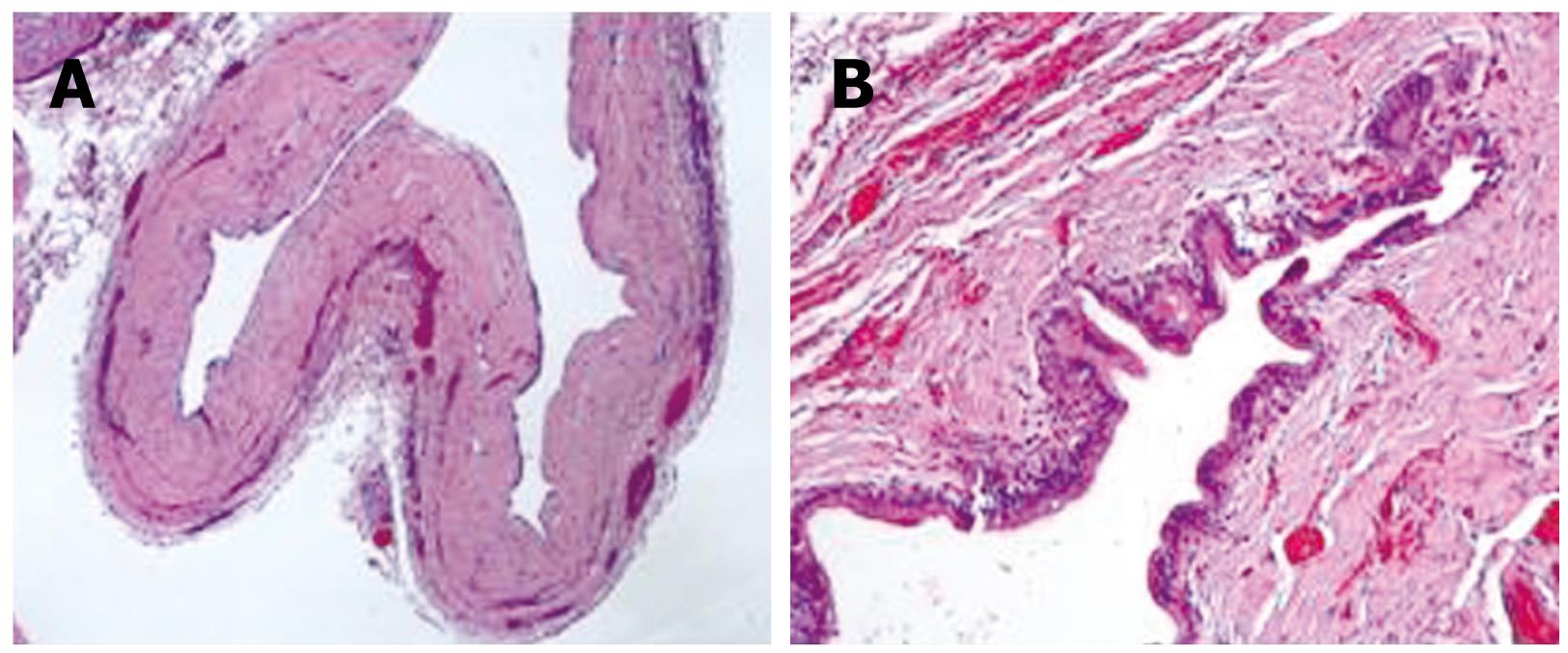
Surgery was performed as planned for the diagnosis of choledochal cyst. The patient underwent resection of the bile duct up to the pancreas joint and before the bifurcation of the hepatic duct, with terminolateral transmesocolic Roux-en-Y hepaticojejunal anastomosis. A type-1 choledochal cyst was discovered macroscopically (Figure 2). The sample was sent to the Pathological Anatomy Service, where it was reported as a 1.4 cm extrahepatic bile duct cystadenoma, with chronic inflammation and bile duct dilatation with regenerative epithelial atypia. No evidence of malignant degeneration was found (Figure 3).
The postoperative course was eventful, with anastomotic leak noted on hepatobiliary iminodiacetic acid (HIDA) scan, central venous catheter-related septicaemia and abscess formation shown by computed tomography (CT), which were solved with percutaneous drainage. Finally, the patient was discharged from the hospital asymptomatically.
Biliary cystadenomas arise in the liver in 90% of the cases, and they are much less frequent in the extrahepatic bile ducts, as in our patient. Intra and extrahepatic concomitant cases have also been found. Despite the report of some unilocular cystadenomas, multilocular ones are by far more common. Cystadenomas are usually located in the right lobe of liver, although they may also be present in both lobes or only in the left one of liver.
Macroscopically, their surface is usually flat, and may reach great proportions. The contents are mostly liquid, tending to become mucinous texture, including biliary pigment, hemosiderine and even purulent material if overinfected. Biliary communication has been rarely reported. In some patients, the tumor projects into the bile ducts. In our case, the projection was located in the choledochal wall.
On light microscopy, the inner surface was covered with a cuboidal-to-tall epithelium and some papillary and polypous excrescences, basally oriented nuclei with prominent nucleus and thick chromatin fibers, pale acidophil cytoplasm with mucine-filled vacuoles. According to some authors, this epithelium must be surrounded by a densely cellular mesenchymal stroma with plain muscular fibers and oval cells, which are typical of epithelia[34]. However, others claim that hepatic cystadenomas with such features are consistent only with females, males being different in stroma formation. That is why the latter scholars suggest the name of cystadenoma with mesenchymal stroma. Outside this cellular stroma, a dense layer of collagenous tissue separates it from the hepatic parenchyma[5].
The etiology of cystadenomas is unclear. Cystadenomas without mesenchymal stroma have been induced experimentally with aflatoxins in an animal model. This might lead to a possible malignant transformation of simple hepatic cystic lesions[67]. Coincidences between cystadenoma, gallbladder embryonic tissue, and main bile ducts tissue have also been found[3]. Stimuli such as ischemia or carcinogenic elements also produce this kind of lesions.
Cystadenoma may display a wide range of symptoms, although it is mainly asymptomatic. The most typical symptoms are a slowly growing abdominal mass, upper abdomen pain, dyspepsia, anorexia, nausea and fever. Jaundice by compression, protrusion, invasion of bile ducts or by secretion of dense mucinous material has been reported[8]. Invasion of the bile ducts may result in pancreatitis episodes. In our patient, we could only reach a diagnosis by the anatomopathological study of the sample, as the patient was asymptomatic except for recurrent biliary colics due to gallbladder lithiasis.
The most widely used diagnosis methods are ecography and tomography. They allow us to observe the cyst formation walls, intracystic projections and possible multilocular arrangement. Since magnetic cholangioresonance provides precise images of the lesion, it is thus the current reference test for tumor study[8–10].
For some scholars, ecologically-guided fine-needle aspiration puncture (FNAP) may be a good diagnostic method, but it may present drawbacks such as the danger of dissemination and its low diagnostic value[11]. CEA levels in cyst liquid help to differentiate cystadenomas from cystadenocarcinomas. Other tests, such as endoscopic retrograde cholangiopancreatography (ERCP), gammagraphy and angiography, may give indirect signs for diagnosis. In blood tests, high levels of CA 19.9 are inconsistent with relation to the lesion. In our case, the rise of this marker occurred inside a cystadenoma[12].
Treatment must be surgical whenever possible, due to a potential malignant degeneration of these lesions. The technique chosen for bile duct sites is complete resection of the bile duct, associating cholecystectomy and reconstruction with hepatic-jejunostomy in Roux-en-Y. When a partial resection has been done for other reasons and the sample shows evidence for a cystadenoma, complete resection of the bile duct and its reconstruction must be performed. However, this was not necessary for our case, as the bile duct was properly fully removed, and the gallbladder was previously removed.
In the hepatic lobes, enucleation must be the objective. The technique used should be personalised taking into account the placement and the patient in context[1314].
The patient follow-up is justified in order to avoid possible surgical complications in the bile duct, such as cholangitis, gallstones, estenosis of the anastomosis, and malignant degeneration. In hepatic cystadenomas, the high level of recurrence should be monitored in the postoperative follow-up[131516].
| 1. | Davies W, Chow M, Nagorney D. Extrahepatic biliary cystadenomas and cystadenocarcinoma. Report of seven cases and review of the literature. Ann Surg. 1995;222:619-625. |
| 2. | Florman SS, Slakey DP. Giant biliary cystadenoma: case report and literature review. Am Surg. 2001;67:727-732. |
| 3. | Wheeler DA, Edmondson HA. Cystadenoma with mesenchymal stroma (CMS) in the liver and bile ducts. A clinicopathologic study of 17 cases, 4 with malignant change. Cancer. 1985;56:1434-1445. |
| 4. | Koroglu M, Akhan O, Akpinar E, Oto A, Gumus B. Biliary cystadenoma and cystadenocarcinoma: two rare cystic liver lesions. JBR-BTR. 2006;89:261-263. |
| 5. | Zen Y, Fujii T, Itatsu K, Nakamura K, Konishi F, Masuda S, Mitsui T, Asada Y, Miura S, Miyayama S. Biliary cystic tumors with bile duct communication: a cystic variant of intraductal papillary neoplasm of the bile duct. Mod Pathol. 2006;19:1243-1254. |
| 6. | Cruickshank AH, Sparshott SM. Malignancy in natural and experimental hepatic cysts: experiments with aflatoxin in rats and the malignant transformation of cysts in human livers. J Pathol. 1971;104:185-190. |
| 7. | Bloustein PA. Association of carcinoma with congenital cystic conditions of the liver and bile ducts. Am J Gastroenterol. 1977;67:40-46. |
| 8. | Teoh AY, Ng SS, Lee KF, Lai PB. Biliary cystadenoma and other complicated cystic lesions of the liver: diagnostic and therapeutic challenges. World J Surg. 2006;30:1560-1566. |
| 9. | Kim HG. [Biliary cystic neoplasm: biliary cystadenoma and biliary cystadenocarcinoma]. Korean J Gastroenterol. 2006;47:5-14. |
| 10. | Buetow PC, Buck JL, Pantongrag-Brown L, Ros PR, Devaney K, Goodman ZD, Cruess DF. Biliary cystadenoma and cystadenocarcinoma: clinical-imaging-pathologic correlations with emphasis on the importance of ovarian stroma. Radiology. 1995;196:805-810. |
| 11. | Debenes B, Pauwels A, Levy VG; Kyste solitaire et polikystose hepatique de l'adulte cystadenome hepatique. Ed Tecinniques Encyc1 Med-Chir Paris France Hepatologie. 1992;18. |
| 12. | Mantke R, Ridwelski K, Rocken C, Pross M, Schulz HU, Lippert H. [Hepatobiliary cystadenoma]. Chirurg. 2001;72:277-280. |
| 13. | Veroux M, Fiamingo P, Cillo U, Tedeschi U, Brolese A, Veroux P, Basso S, Buffone A, D’Amico DF. Cystadenoma and laparoscopic surgery for hepatic cystic disease: a need for laparotomy? Surg Endosc. 2005;19:1077-1081. |
| 14. | Fiamingo P, Veroux M, Cillo U, Basso S, Buffone A, D’Amico DF. Incidental cystadenoma after laparoscopic treatment of hepatic cysts: which strategy? Surg Laparosc Endosc Percutan Tech. 2004;14:282-284. |
| 15. | Delis SG, Touloumis Z, Bakoyiannis A, Tassopoulos N, Paraskeva K, Athanassiou K, Safioleas M, Dervenis C. Intrahepatic biliary cystadenoma: a need for radical resection. Eur J Gastroenterol Hepatol. 2008;20:10-14. |
| 16. | Gonzalez M, Majno P, Terraz S, Morel P, Rubbia-Brandt L, Mentha G. Biliary cystadenoma revealed by obstructive jaundice. Dig Liver Dis. 2009;41:e11-e13. |