INTRODUCTION
Plasma cell neoplasms account for approximately 1%-2% of human malignancies and they are classically categorized into four groups: multiple myeloma (MM), plasma cell leukemias, solitary plasmacytomas of the bone (SPB), and extramedullary plasmacytomas (EMP). EMP represents 3% of these neoplasms and it is defined as a soft-tissue plasma cell tumor occurring in the absence of systemic signs of multiple myeloma[1]. Most cases of EMP arise in the upper aerodigestive tract (UAD); other sites of involvement include the gastrointestinal tract, breast, thyroid, testis, bladder, retroperitoneum, and lymph nodes. In non-UAD regions, the gastrointestinal tract represents 40% of cases[2]. We reported an unusual case of localized plasmocytoma associated with massive local deposition of amyloid in the duodenum.
CASE REPORT
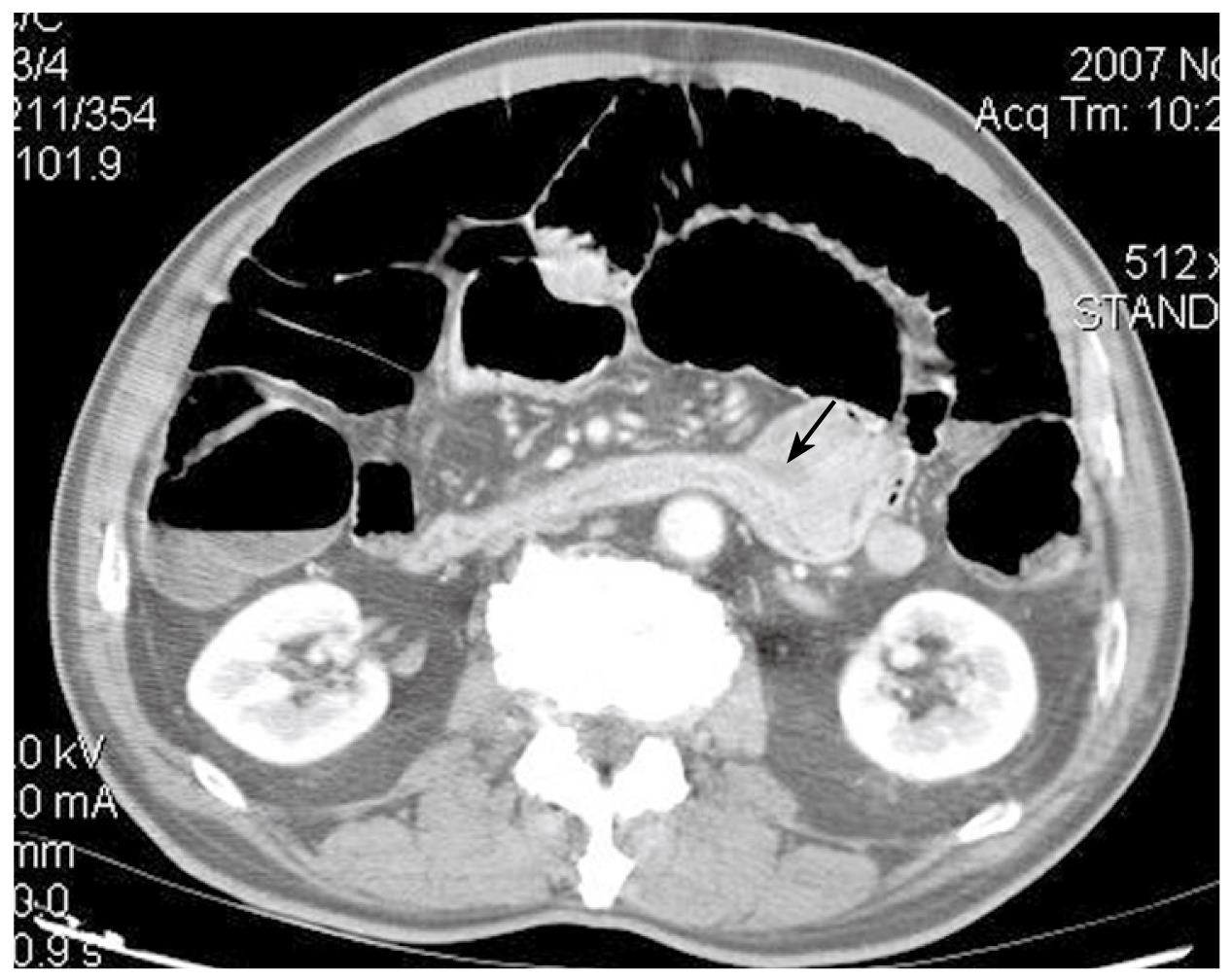
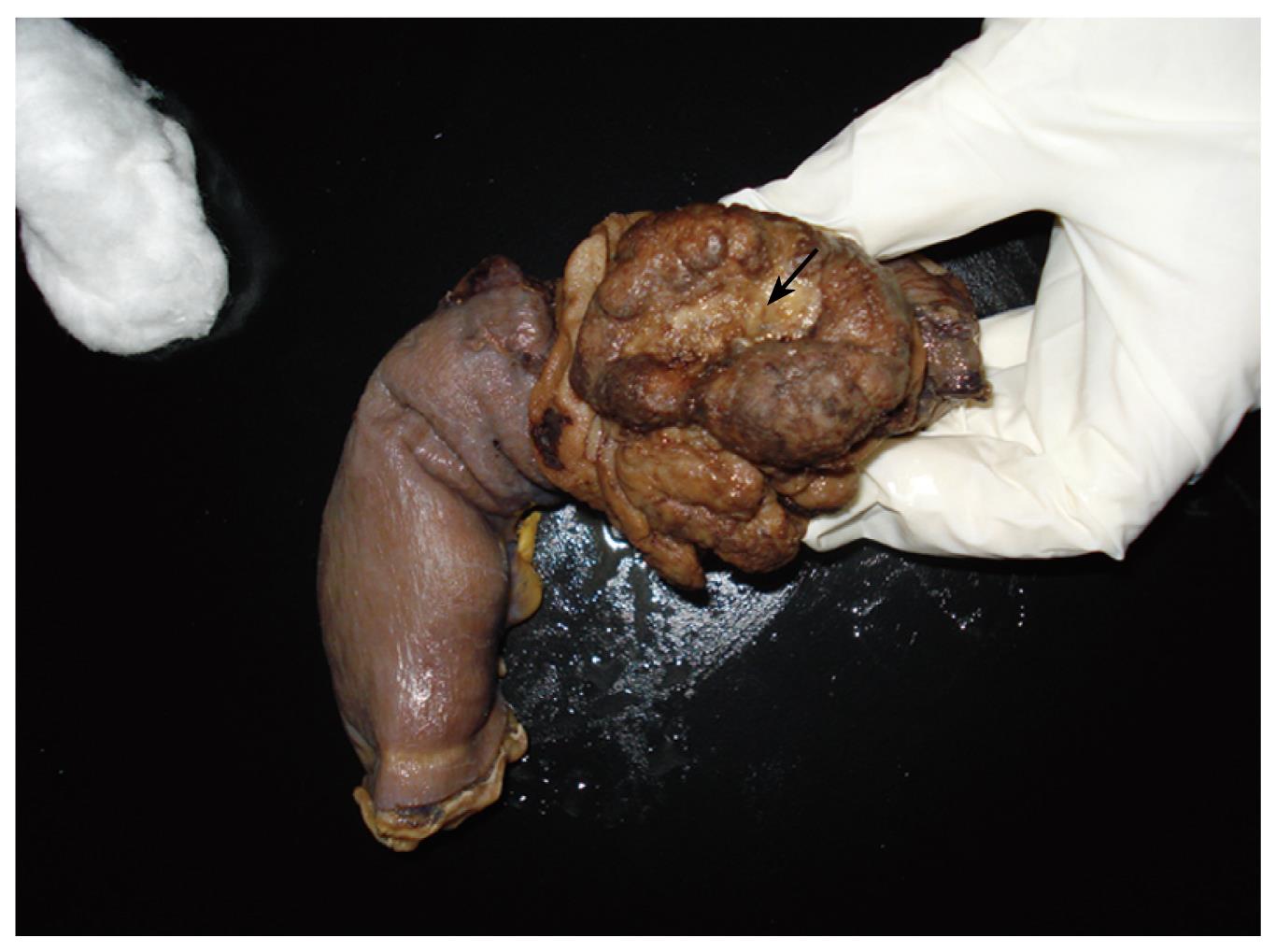
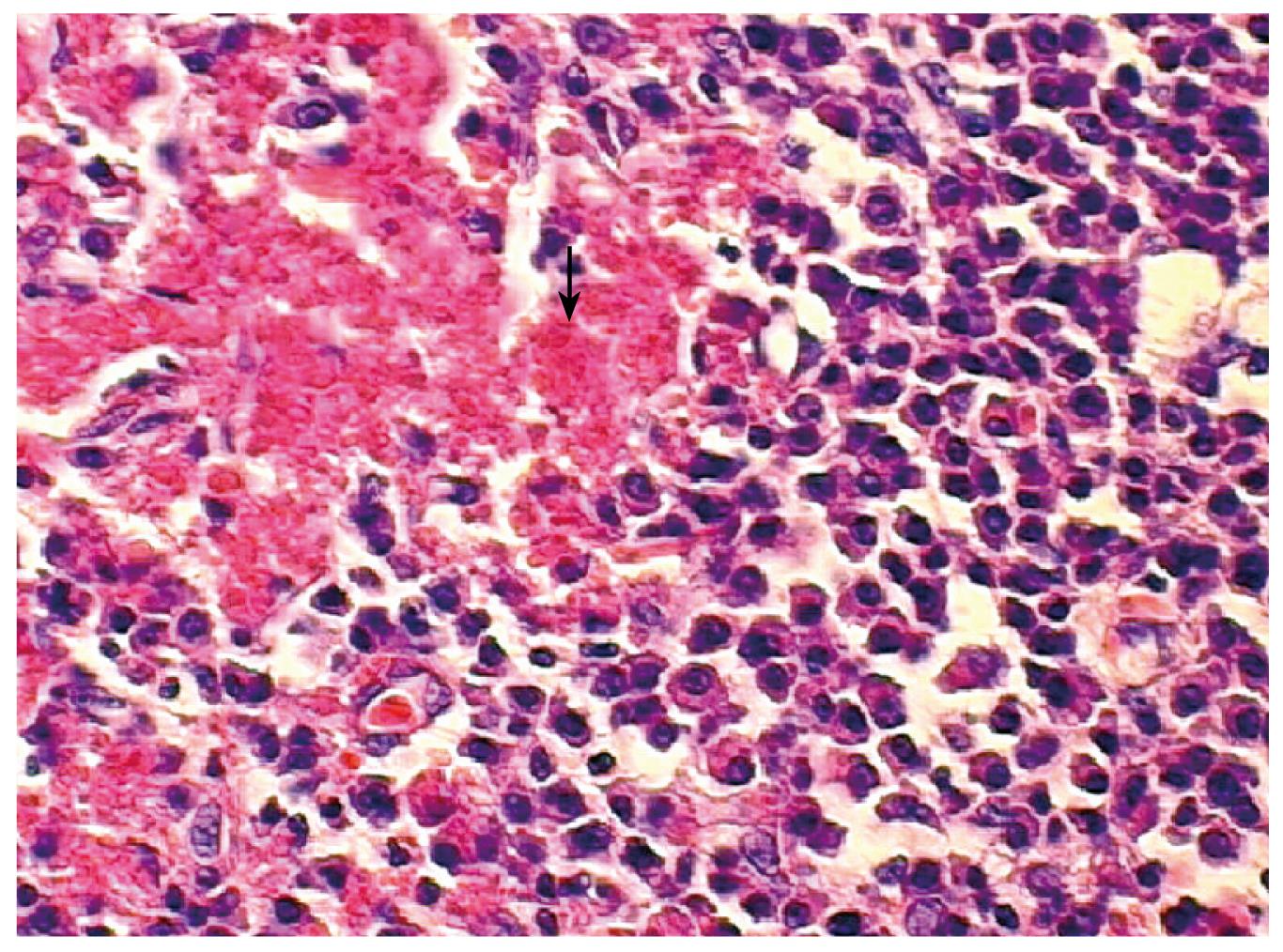
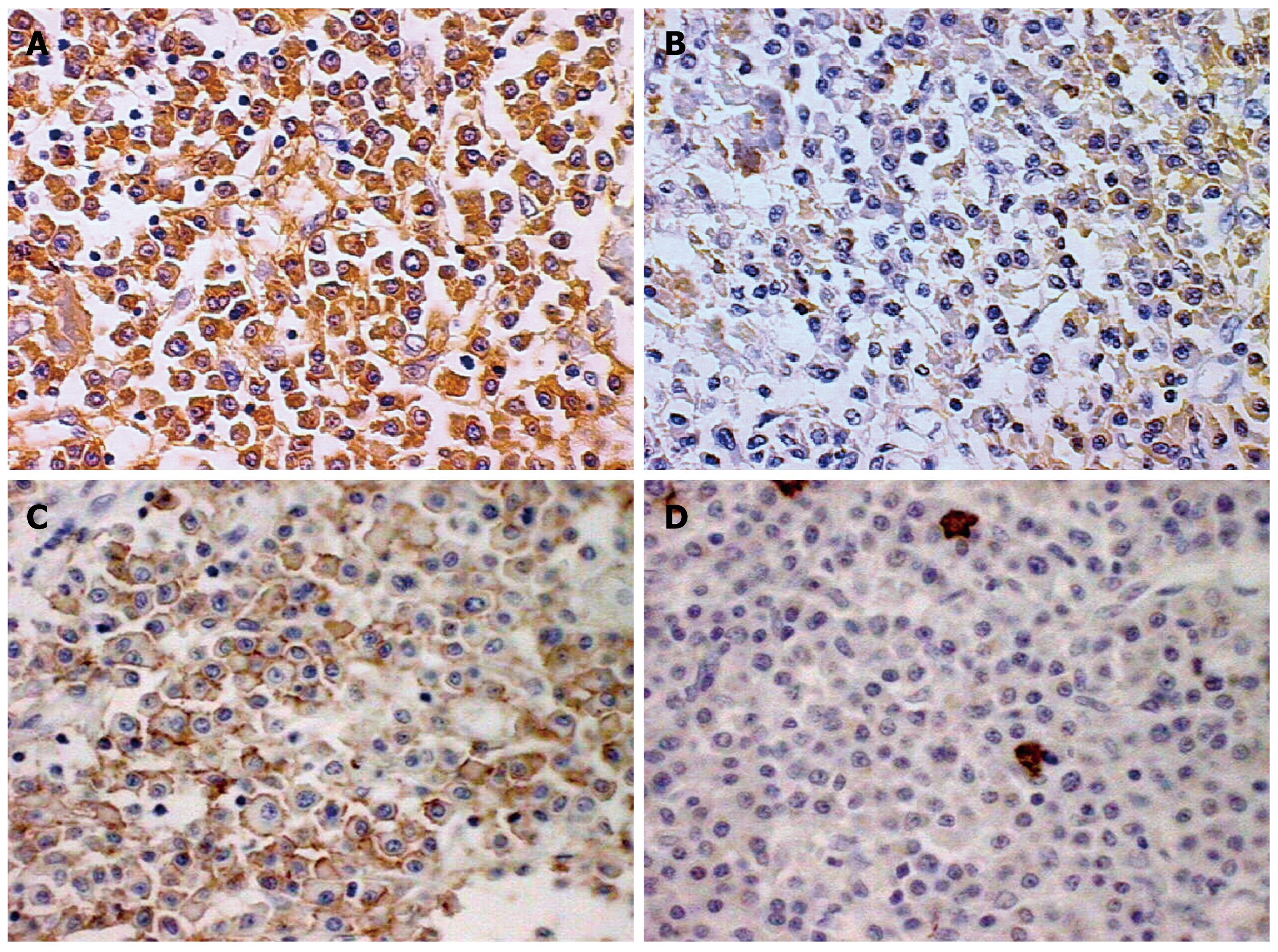
A 72-year-old Japanese man was admitted to our hospital presenting with a 3-mo history of epigastric pain, vomiting and weight loss. No abdominal mass was palpated on physical examination. An upper endoscopy revealed a mass in the duodenum. CT showed wall thickening of the fourth part of the duodenum (Figure 1). Lymphadenopathy was not seen. Multiple biopsies obtained from the lesion showed infiltration of plasma cells and lymphocytes, but they were not conclusive. The patient underwent resection of the fourth part of the duodenum and proximal segment of jejunum. On gross examination of the surgical specimen, the involved segment measured 5 cm in length (Figure 2). On histopathological examination, the lesion consisted of a dense and diffuse infiltrate of plasma cells and a few admixed lymphocytes with reactive follicles extending to the muscular propria. An extensive deposition of hyaline amorphous eosinophilic extracellular material was also observed (Figure 3). With Congo red stain, amyloid appeared red in normal light and apple-green in polarized light. Immunohistochemical stains revealed that a few plasmacytoid cells showed λ light chain staining, while most were κ light chain positive (Figure 4A and B). These cells also were positive for CD138 and CD56 but negative for CD20 and CD79 (Figure 4C and D). The scattered lymphocytes represented a mixture of CD3-positive T cells and CD20-positive B cells. The extracellular hyaline materials also showed reactivity with anti-kappa (but not lambda) immunoglobulin light chains. The findings were consistent with a plasma cell neoplasm. A subsequent workup for MM (serum and urinary protein electrophoresis, radiographic examination of the axial skeleton and a bone marrow aspirate and biopsy) was completely negative. Anemia and hypercalcemia were not seen. The patient showed no signs of local recurrence or dissemination of the disease after 12 mo follow-up.
Figure 1 CT showing a mass on the fourth part of the duodenum (arrow).
Figure 2 Gross appearance of the surgical specimen containing the tumor (arrow).
Figure 3 Histopathological examination displaying a dense and diffuse infiltrate of plasma cells and amyloid deposit (arrow).
(HE × 400).
Figure 4 Immunohistochemical findings (positive cells in brown, × 400).
A: Most plasmocytoid cells were positive for κ light chain; B: Few plasmacytoid cells showing λ light chain staining; Plasmocytoid cells were positive for CD56 (C) and negative for CD20 (D).
DISCUSSION
The involvement of gastrointestinal tract is common in MM but a localized plasmocytoma is rare and not suspected clinically. In previous reports[3–6] of duodenal EMP, males were more affected than females and the disease was most commonly seen in patients older than 50 years of age. These patients presented with nonspecific symptoms such as dyspepsia, vomiting, epigastric pain and weight loss. As in our case, in these previous reports radiological features were not suggestive and the diagnosis was revealed only by the findings of histopathological and immunohistochemical examination of the sample biopsy or surgical specimen, mainly the light chain restriction (demonstrating the monoclonality) and positivity for CD138 (indicating plasma cell lineage).
Treatment options for EMPs include surgical resection and radiation therapy, given the relative sensitivity of plasma cell neoplasms to radiation[27]. Alexiou et al[2] found no statistically significant difference in patient survival comparing surgery alone, surgery plus radiotherapy, or radiotherapy alone. Surgery alone, however, had the lowest recurrence rate. Our patient was not submitted to radiation after surgery. Of all of the plasma cell tumors, EMPs have the best prognosis. Progression to MM is more frequent in solitary osseous plasmacytoma than in EMP[89]. The prognosis of patients with EMP of the duodenum is uncertain as so few patients have been reported, but, of those cases reported in the literature, we found two that presented evolution to myeloma after surgery[1011].
Amyloidosis, a complication of plasmacytoma in the present case, is characterized histopathologically by the extracellular deposition of insoluble fibrillar proteins. In our case, amyloidosis was caused by the deposition of immunoglobulin light chains since both amyloid and plasma cells shared the same immunoglobulin light chain restriction (lambda-restriction). The absence of systemic amyloidosis in massive localized deposits, as in our case, may be explained by the secretion of abnormal, poorly soluble immunoglobulin molecules with a tendency toward local precipitation[12]. We did not find previous cases of amyloidosis associated with duodenal plasmocytoma, so the impact of associated amyloid accumulation on progression or response to treatment in our case cannot be determined. Amyloid deposition can be found in 15% to 40% of extramedullary plasmacytomas in the head and neck regions and, according to previous reports, without clinical significance[13].
In view of their indolent biological nature and their frequent involvement of anatomic sites containing mucosal lymphoid tissues, it has been proposed that extramedullary plasmacytomas represent a low-grade lymphoma of mucosal lymphoid tissues (MALT) with extensive plasmacytic differentiation. Results from a previous study support this hypothesis[14]; morphologic features of MALT lymphoma, including centrocytic-like cells, reactive follicles, and lymphoepithelial lesions, are often found in extramedullary plasmacytomas. Besides this, amyloid deposits have also been described in lymphomas[15]. However, according to others authors, some immunohistochemical findings on plasmocytoid cells are helpful in differentiating plasmacytoma from MALT lymphoma[1617]. These authors found that plasmocytoid cells were less likely to express CD56 in lymphoma than in myeloma. So, the positivity for CD56, in our case, suggests that it may indeed represent a true primary PC dyscrasia (plasmacytoma), with phenotypic feature similar to myeloma.
In conclusion, plasmocytoma localized in the duodenum is rare and may cause intestinal obstruction. The diagnosis is performed by histopathological examination and must be distinguished from lymphomas with extensive plasmacytic differentiation. In associations of EMP and amyloidosis, the patient must be followed up because of the possible systemic involvement of the neoplasm and amyloidosis in future.