Published online Jul 28, 2009. doi: 10.3748/wjg.15.3472
Revised: June 15, 2009
Accepted: June 22, 2009
Published online: July 28, 2009
Hepatitis C is recognized as a major threat to global public health. The current treatment of patients with chronic hepatitis C is the addition of ribavirin to interferon-based therapy which has limited efficacy, poor tolerability, and significant expense. New treatment options that are more potent and less toxic are much needed. Moreover, more effective treatment is an urgent priority for those who relapse or do not respond to current regimens. A major obstacle in combating hepatitis C virus (HCV) infection is that the fidelity of the viral replication machinery is notoriously low, thus enabling the virus to quickly develop mutations that resist compounds targeting viral enzymes. Therefore, an approach targeting the host cofactors, which are indispensable for the propagation of viruses, may be an ideal target for the development of antiviral agents because they have a lower rate of mutation than that of the viral genome, as long as they have no side effects to patients. Drugs targeting, for example, receptors of viral entry, host metabolism or nuclear receptors, which are factors required to complete the HCV life cycle, may be more effective in combating the viral infection. Targeting host cofactors of the HCV life cycle is an attractive concept because it imposes a higher genetic barrier for resistance than direct antiviral compounds. However the principle drawback of this strategy is the greater potential for cellular toxicity.
- Citation: Khattab MA. Targeting host factors: A novel rationale for the management of hepatitis C virus. World J Gastroenterol 2009; 15(28): 3472-3479
- URL: https://www.wjgnet.com/1007-9327/full/v15/i28/3472.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3472
Chronic hepatitis C virus (CHC) infects approximately 170 million individuals worldwide and is a major cause of mortality and morbidity[1].
Egypt has the highest hepatitis C virus (HCV) prevalence in the world (overall prevalence of HCV is 12% among the general population, reaches 40% in persons above 40 years of age and is more in rural areas)[23].
HCV is an RNA virus that belongs to the family Flaviviridae with six known genotypes (numbered 1-6) and more than 50 subtypes (e.g. 1a, 1b, 2, etc)[4]. In general, the genetic make-up of the HCV genotype varies by about 30%-35% between its different genotypes[56], and these differences in genotype are related to response to antiviral treatment.
Current treatment for patients with CHC is interferon-based therapies with ribavirin for 24-48 wk. Unfortunately, a sustained virological response (SVR) is achieved in only 42%-52% of treatment-naïve patients, and the rest of patients either show no response or experience a relapse when therapy is stopped[7], with a wide profile of side effects.
The mechanisms underlying the failure of interferon therapy are not well understood, but evidence indicates that in addition to viral factors, several host factors are also involved[8]. So, CHC patients still need a novel approach for treatment of HCV infection.
A major obstacle in combating HCV infection is that the fidelity of the viral replication machinery is notoriously low, thus enabling the virus to quickly develop mutations that resist compounds targeting viral enzymes[9]. Therefore, an approach targeting the host factors that are indispensable for the propagation of viruses might be an ideal target for the development of antiviral agents because of a lower rate of mutation compared to that of the viral genome, as long as they have no serious side effects to patients.
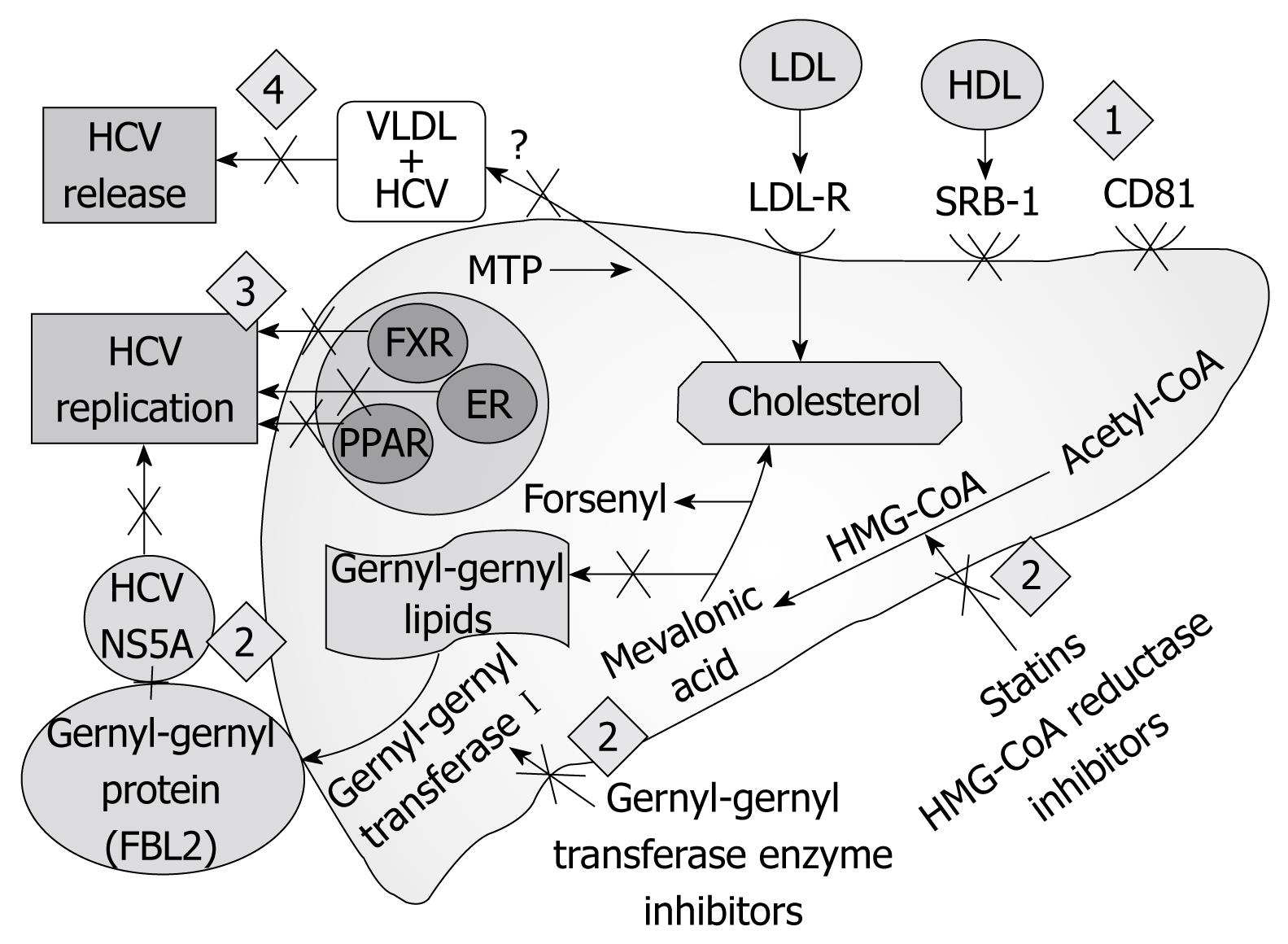
A unique aspect of HCV that has not been observed in other viruses is that the entire viral life cycle is associated with cholesterol metabolism in host cells. Thus, drugs that target cholesterol metabolism might be useful for treating HCV infection[10]. Also, drugs targeting the host proteins required for HCV infection, nuclear receptor or anti-receptor antibodies may be more helpful in combating the viral infection[11] (Table 1).
| Drug | Mechanism of action |
| Nitazoxanide | Induces PKR phosphorylation |
| HMG-CoA reductase inhibitors | Disruption of HCV replication; depletion of geranylgeranyl lipids |
| Antisense RNA drugs targeting apoB | Blocks the assembly and secretion of VLDL, inhibits release of HCV particles from infected cells |
| Microsomal triglyceride protein (MTP) inhibitors | Blocks the assembly and secretion of VLDL, inhibits release of HCV particles from infected cells |
| Insulin sensitizer | |
| Metformin | Insulin sensitivity by acting on hepatic AMP-activated protein kinase |
| Thiazolidindiones (pioglitazone) | Insulin sensitivity by activating peroxisome proliferator-activated receptors (PPARs) |
| Debio-025 | Inhibition of Cyclophilin B |
| NIM 811 | Inhibition of Cyclophilin B |
| Tamoxifen, other anti-estrogen drugs | Potentially suppresses genome replication |
| Small molecules (e.g. receptor mimics, soluble intracellular adhesion molecule-1) | Receptor and uptake inhibition |
| Receptor antibodies (e.g. Anti CD81) |
HCV circulates in the bloodstream in different forms; either free or in a complex with immunoglobulin or lipoprotein. Implicated lipoproteins are very-low-density lipoprotein (VLDL), intermediate-density lipoprotein, or low-density lipoprotein (LDL)[12]. HCV RNA is always found in at least one of these fractions and represents 8% to 95% of the total plasma HCV RNA[1314]. Entry into the host cell is the primary step in the HCV life cycle, which makes it an attractive target for antiviral therapies. Inhibition of viral entry can be accomplished at the level of cell receptor(s) or HCV pseudo-particles (HCVpp) but both approaches require an in-depth knowledge of interactions between host and virus[15].
Attachment and cell entry of HCV is pH dependent and is a clathrin-dependent endocytic pathway[1617]. Although the molecular details regarding how this virus enters a cell are unknown, CD81[18] and scavenger receptor class B type 1[19] seem to be the key receptor components that mediate viral entry. However, other potential receptors play a role in entry of HCV such as LDL receptor[20], negatively charged glycosaminoglycans, and recently, Evans et al[21] added another molecule to the list of HCV receptors, namely, the tight junction protein claudin-1 (CLDN1).
The rationale for anti-receptor antibodies as a drug target is based on them not being prone to the problems of viral variability and high density lipoprotein (HDL) attenuation of neutralizing activity[22].
Targeting viral receptors can be accomplished by various methods, including the design of small molecules that bind to proteins and prevent interaction(s) with HCV. The crystal structure of CD81 long extracellular loop enabled the design of small molecules that bind CD81 and prevent its association with HCV E2[23]. A recent presentation by Liu et al[24] identified compounds that displayed a dose-dependent inhibition of HCV infection.
Scavenger receptor BI (SR-BI): SR-BI is a lipoprotein receptor with the highest levels found in the liver and adrenal glands, responsible for the selective uptake of cholesteryl ester from HDL[25–27]. HCV particles have been reported to be complexed with lipoproteins; it is possible that HDL interacts with HCVpp, via protein/protein or lipid/protein interactions[28], suggesting an indirect interaction of virus with lipoprotein receptors[2930]. Recent studies have demonstrated the cell culture-derived HCV association with VLDL containing apolipoprotein B (ApoB) and apolipoprotein E, supporting earlier claims of lipo-viral particles in human plasma.
Previous observations implicated SR-B1 as important for infection by different HCV subtypes and support for this hypothesis is the fact that the same SR-B1 protein element is responsible for the recognition of different HCV E2 glycoproteins despite the high level of variability between their amino acid sequences, especially in the HVR1 region previously shown to be involved in interaction with SR-B1[1931].
HCV appears to use SR-BI during cell entry not merely as an additional site for the viral particle entry but also for exploiting its physiological activity, i.e. the capacity to mediate lipid transfer from HDL which is known to facilitate the entry of many different viruses, such as influenza virus, HIV, and HCV[3233]. However, HCVs are many times more sensitive to HDL-mediated infection enhancement than other cholesterol-sensitive viruses. Therefore, enhancement of viral infection might be dependent on the lipid exchange activity of SR-B1[28]. Recently, a novel function of SR-Bs for viral antigen uptake and recognition has been suggested; SR-BI may represent a cell-surface receptor for the recognition of viral antigens and be implicated in trafficking exogenous viral antigens toward the MHC class I presentation pathway. The SR-BI-viral antigen interaction may represent a novel target for therapeutic or preventive strategies aiming at the induction of efficient antiviral immune responses[34].
Moreover, HDL with SR-BI is the predominant enhancing factor in infectivity and the presence of HVR1 with HDL protects HCV from neutralizing antibodies as HDL can reduce the neutralizing effect of anti-HCV antibodies[3536]. This phenomenon might be responsible, at least in part, for the limited ability of the humoral immune response to control HCV infection in vivo, which raises concerns about the efficacy of anti-HCV antibodies for active or passive immunotherapy[37]. Thus, as an alternative to the development of anti-HCV antibodies, one could consider anti-SR-B1 human MAbs or anti-CD-81 capable of interfering with HCV infection as potential therapeutic leads. Agents involved in modulating the normal hepatocellular processes of lipid transport have been reported to have pleiotropic effects on HCV infectivity. Antibodies to ApoB have been shown to have antiviral activity[1329–3138–43]. Recent data show that BLT-4 and other inhibitors of SR-BI-mediated lipid transfer not only inhibit HCV entry but also fully restore the potency of neutralizing antibodies in infection assays conducted in the presence of HS/HDL, indicating an intriguing link between neutralization efficiency and stimulation of cell entry[2835]. However, it is too early to know whether the potential for vaccines and passive immunotherapy will be realized. Cholesterol-lowering drugs may be beneficial in patients with chronic hepatitis C by exerting effects on cholesterol metabolism and lipoprotein trafficking via SR-BI (See below).
CD81: Recently, Meuleman et al[11] showed that CD81 is a critical receptor for HCV infection in vivo. Prophylactic injection of monoclonal anti-CD81 antibodies prevented infection of human liver-uPA-SCID mice, however once an infection occurred, no significant difference in viremia was observed between anti-CD81-treated and control animals (irrelevant antibody). These results strongly support the use of CD81 as a clinical target for HCV prevention, especially in the context of orthotopic liver transplantation[11].
Targeting CD81, SR-BI or CLDN1 may be complicated by receptor-independent modes of virus transmission. In general, there are two primary routes of virus transmission: cell-free and cell-to-cell. Cell-free transmission begins when virus is released from an infected cell and enters the extracellular environment. The virion can bind to surface-expressed receptors on naïve or uninfected cells, become internalized, and initiate new rounds of infection. En route from one cell to the next, the virus may encounter neutralising antibodies or other components of the immune response that may limit infection. In the second route of transmission, the virus spreads directly from one cell to another and, in doing so, may bypass receptor-mediated attachment as well as the immune response.
Direct cell-to-cell transmission has been observed with several viruses, including HIV[44], human T-lymphotropic virus type 1[45] and vesicular stomatitis virus[46] and it was recently proposed that HCV may use this route in vitro[47]. Whether cell-to-cell HCV transmission occurs in vivo remains to be determined. If this mode of transmission exists in vivo, targeting cellular receptors alone may not be an effective antiviral therapy[48].
Targeting receptors as antiviral therapy may also be complicated by their ubiquitous expression in human tissue and their essential roles in cell biology.
HCV seems to be not only an infectious hepatotropic virus but also a metabolic disease[49] with a wide area of metabolic disarrangement, including lipid metabolism[50], glucose metabolism[51] and vitamin D metabolism[5253]. Metabolism refers to all the reactions by which living things break down nutrients to produce energy, along with those reactions by which they rebuild broken-down nutrients into complex molecules (e.g. DNA). Many viruses, including influenza, HIV and hepatitis, dramatically increase cellular metabolism. The fields of metabolomics and fluxomics have emerged to measure these patterns and to provide insight into diseases with a metabolic component, from diabetes to cancer to infectious diseases such as HCV. Many metabolic processes are essential to the survival of human cells, and so are not candidates for research efforts that would shut them down in an attempt to stop viral replication.
Recently, using the new fluxomic techniques, studies revealed that viral infection takes control of cellular metabolism and drives, among other things, marked increases in fatty acid synthesis. Interfering with glucose-to-fatty acid metabolism could stop viral replication, because fatty acid biosynthesis is not essential in adult humans. It does appear, however, to be essential to the ability of HCV to build their envelopes, reproduce and spread. So, targeting of host lipid metabolism by the existing anti-obesity drugs may represent a new way to block these metabolic changes and inhibit viral replication, and may therefore be a potential novel approach that could improve response rates to treatment[54]. There are at least two different molecular mechanisms representing a novel target for management of HCV through the modulation of cellular lipid and cholesterol metabolism. In vitro data suggest that statins, the widely used cholesterol-lowering drugs, may inhibit HCV RNA replication by depletion of geranylgeranyl lipids[5556]. It was recently demonstrated that dose-dependent strong antiviral effects exist for all the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, except for pravastatin, in vitro. Fluvastatin exhibited the strongest antiviral activity, followed by atorvastatin and simvastatin[57].
Recently, Bader et al[58] reported that fluvastatin inhibits HCV RNA replication in patients with CHC; the study provided evidence that fluvastatin is well tolerated in patients with CHC and at relatively low doses.
These findings, along with other data suggesting synergism with α-interferon, support ‘proof-of-concept’ for trials combining fluvastatin with standard pegylated interferon plus ribavirin. Cautious, prospective and randomized trials are needed before we can call statin therapy an adjuvant treatment panacea[54].
Another class of drugs designed for treating hypercholesterolemia blocks the assembly and secretion of VLDL. These drugs may also be effective in treating HCV infection because they inhibit release of HCV particles from infected cells[59]. In this regard, antisense RNA drugs targeting apoB[60] and several microsomal triglyceride protein (MTP) inhibitors[6162] have already been tested in clinical trials because of their ability to block VLDL secretion, thereby lowering the plasma levels of VLDL triglycerides and LDL cholesterol. Long-term treatment with MTP inhibitors led to the toxic accumulation of fat in livers[6162], thus hampering the approval of these drugs for the treatment of hypercholesterolemia on a long-term basis. However, short-term treatment (up to several weeks) reduced the plasma level of VLDL with only minor adverse effects, which disappeared after drug discontinuation[61]. It will be interesting to examine whether short-term treatment with MTP inhibitors is beneficial in treating HCV infection (Figure 1).
Another host cell factor involved in HCV RNA replication is the human protein cyclophilin B protein which interacts with the C-terminal region of NS5B and appears to stimulate its RNA binding activity[63]. The cyclophilin B inhibitor Debio-025 potently suppresses genotype 1 HCV replication in vivo[64].
Insulin resistance emerges as a very important host factor in patients with CHC, mainly because it has been related to steatosis development, fibrosis progression and non-response to peg-interferon plus ribavirin[65]. Insulin resistance is the main pathogenic factor in the development of steatosis in chronic hepatitis C; both viral-induced insulin resistance and metabolic insulin resistance could be implicated in the development of steatosis[66].
Insulin resistance, calculated by the homeostasis model assessment (HOMA), has been found to be one of the most important host factors related to the impermanence of virological response to combined therapy in chronic hepatitis C patients[67].
Recently, obesity has been identified as a modifiable host factor associated with a lower SVR. An elevated BMI is associated with reduced insulin sensitivity and HCV treatment outcome. This observation has led experts to suggest that managing insulin resistance might improve hepatitis treatment outcome and that insulin resistance seems to be a new target in the management of hepatitis C.
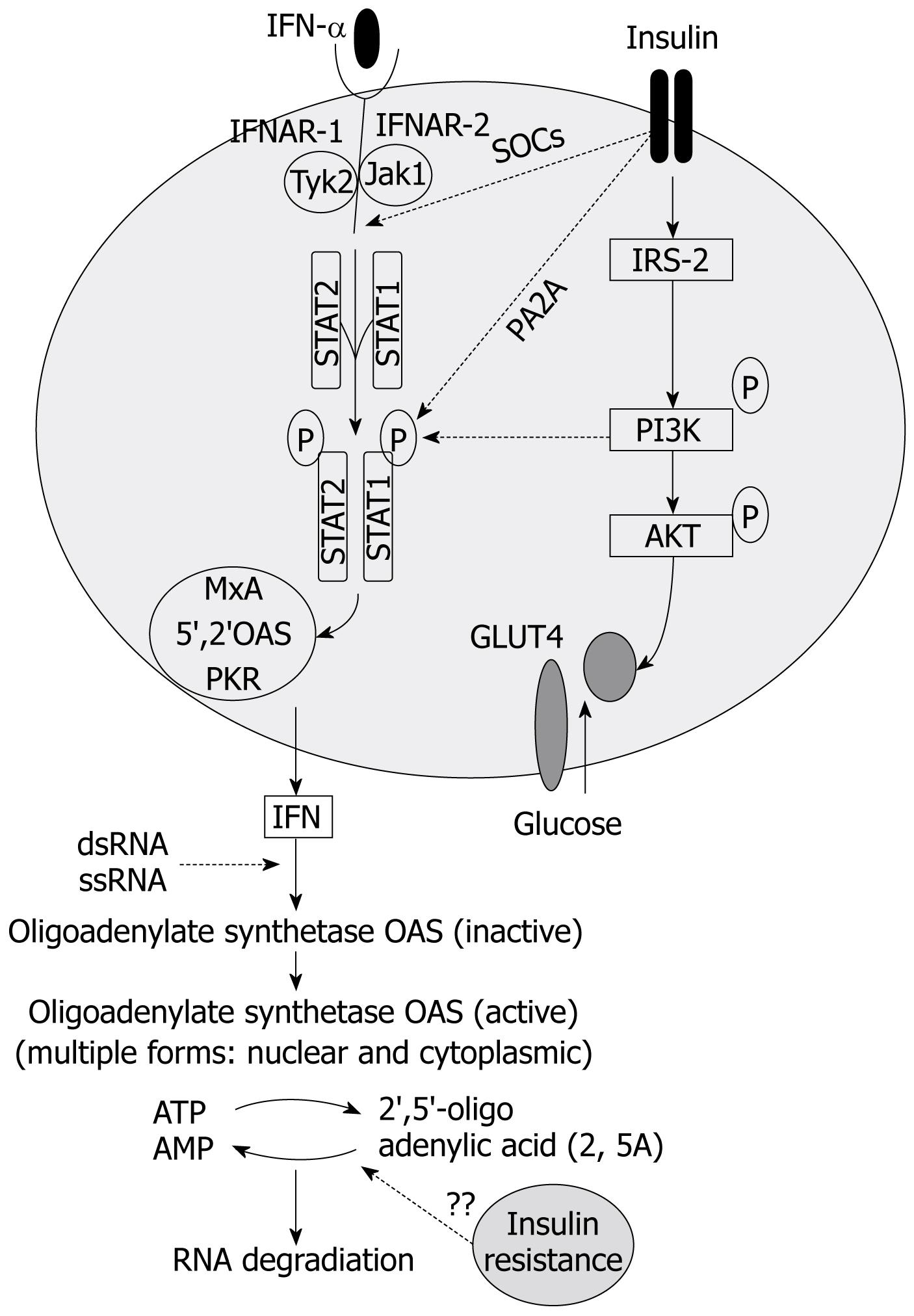
The rationale of increasing insulin sensitivity in patients with chronic hepatitis C is based on the premise that insulin resistant state directly or indirectly inhibits the antiviral action of interferon (IFN)-α-β, or increases the viral fitness making it more resistant to therapy, or both[868]. Since HCV appears to directly interfere with the glucose homeostasis, several studies have tried to analyze in detail the potential interactions between viral products and insulin signaling. Experimental data suggest a direct interference of HCV with the insulin signaling cascade via proteasome degradation of the insulin receptor substrates-1 and -2[6970]. HCV may also impair insulin signaling transduction indirectly, that is, through increased levels of proinflammatory cytokines such as tumor necrosis factor (TNF)-α[7172]. The interference with insulin signaling seems to proceed via HCV genotype-specific mechanisms and insulin resistance levels vary according to the infecting HCV genotype, although all genotypes induce insulin resistance. Interestingly, intracellular factors dysregulated by HCV and responsible for the insulin resistant phenotype may play promiscuous effects as they are also involved in regulating IFN-α signaling (Figure 2). These factors include some members of the suppressor of cytokine signalling (SOCS) family[697073] and the protein phosphatase 2A[74]. Thus, modulating the levels and/or the activity of these factors may not only reverse hepatic insulin resistance but also help in establishing the IFN-α-induced antiviral state at the site of HCV replication. This is one of the reasons for trying to restore insulin sensitivity in chronic hepatitis C patients, especially those who failed to respond to therapy. Although increasing insulin sensitivity may be a rational option in chronic hepatitis C patients, especially in those with metabolic syndrome, the modalities of this intervention, however, have not been established. In addition, it is unclear whether one should start the antiviral treatment together with the insulin sensitizer or only once the HOMA-IR score has been decreased to a level predicting sufficient SVR rate[67].
However, specific inhibitors of SOCS family members and of the protein phosphatase 2A are either not suitable for in vivo administration or are toxic. Alternatively, increasing insulin sensitivity may be achieved by modulating serum levels of specific cytokines, such as TNF-α, associated with insulin resistance[7172], but the administration of anti-TNF-α antibodies to chronic hepatitis C patients may be risky[75]. Insulin sensitizers not only increase insulin sensitivity but may also inhibit HCV replication by decreasing serum free fatty acid flow to hepatocytes; saturated and monounsaturated free fatty acids have indeed been shown to stimulate HCV replication in an in vitro model[57]. It is not clear whether the best approach would be using a thiazolidindione, activating peroxisome proliferator-activated receptors (PPARs) (see below), or a biguanide such as metformin, whose mechanism of action is specifically directed to the hepatic AMP-activated protein kinase.
Recently, metformin-based triple therapy has been shown to be safe, improving insulin sensitivity and increasing SVR rate by 10% in patients with hepatitis C genotype 1 and insulin resistance (HOMA > 2). This therapy was especially effective in females in whom metformin significantly raised the SVR rate[76].
The PPARs are nuclear factors (amongst others) involved in the regulation of glucose homeostasis. In addition to the direct effects on factors involved in lipid and glucose homeostasis[77–81], PPARs may have insulin sensitizing effects via their anti-inflammatory activity[8283]. Thus, treatment with PPAR agonists results in improved insulin sensitivity via diverse mechanisms, both direct and indirect, and both at the level of the liver and at the level of extrahepatic tissues[77]. The relationship between HCV replication, protein expression and PPARs has been the focus of some recent studies. However, the data available so far are quite scanty and concern only the HCV genotype 3a[77].
In a recent randomized, double-blind, placebo-controlled study, adding concurrent (PPAR-γ agonist) pioglitazone 30 mg QD to the standard of care (i.e. without a preceding administration as monotherapy) markedly increased the on-treatment virological response, but failed to increase the SVR after the end of treatment[84]. In a related but smaller and shorter study, another research team reported that pioglitazone given as an adjuvant to pegylated interferon/ribavirin in HCV genotype one patients improved viral kinetic response during the first 4 wk of therapy[85].
Also, in a recent study, the level of PPARα mRNA was found to be profoundly suppressed in the liver of chronic hepatitis C patients (about 85% compared to control livers)[86]. The suppression of PPAR-α leads to the upregulation of nuclear factor (NF)-κB. NF-κB has been shown to accelerate virus replication[87], and it has been speculated that activation of PPAR-α with subsequent NF-κB suppression leads to decreased HCV replication in hepatocytes[88]. Given the availability of potent agonists, PPARs may represent a novel pharmacological target in the treatment of liver lesions observed in chronic hepatitis C.
The bile acid receptors were found to play a role in bile acid-mediated promotion of HCV replication[89]. Furthermore, it was discovered that bile acids compromised the anti-HCV effect of IFN in the cells. These findings suggest a mechanism for persistent infections of HCV in hepatocytes and for the failure of IFN-based treatment for certain HCV patients[8990]. These data suggest a novel mechanism for bile acid-mediated gene regulation at virus and host levels. Importantly, these data may contribute to the finding of better regimens for the treatment of chronic HCV infections by including agents altering the bile acid-mediated FXR pathway[89].
ESR belongs to the steroid hormone receptor family of the nuclear receptor super family. There are two different forms of the estrogen receptor, usually referred to as α and β, each encoded by a separate gene[91]. The novel role of ESR α in regulation of HCV replication has been recently reported[92]. Tamoxifen and other anti-estrogens suppress genome replication, as part of ESR resides on the endoplasmic reticulum and interacts with HCV RNA polymerase NS5B, so ESR is suggested to serve as a potential novel target for anti-HCV therapies[92].
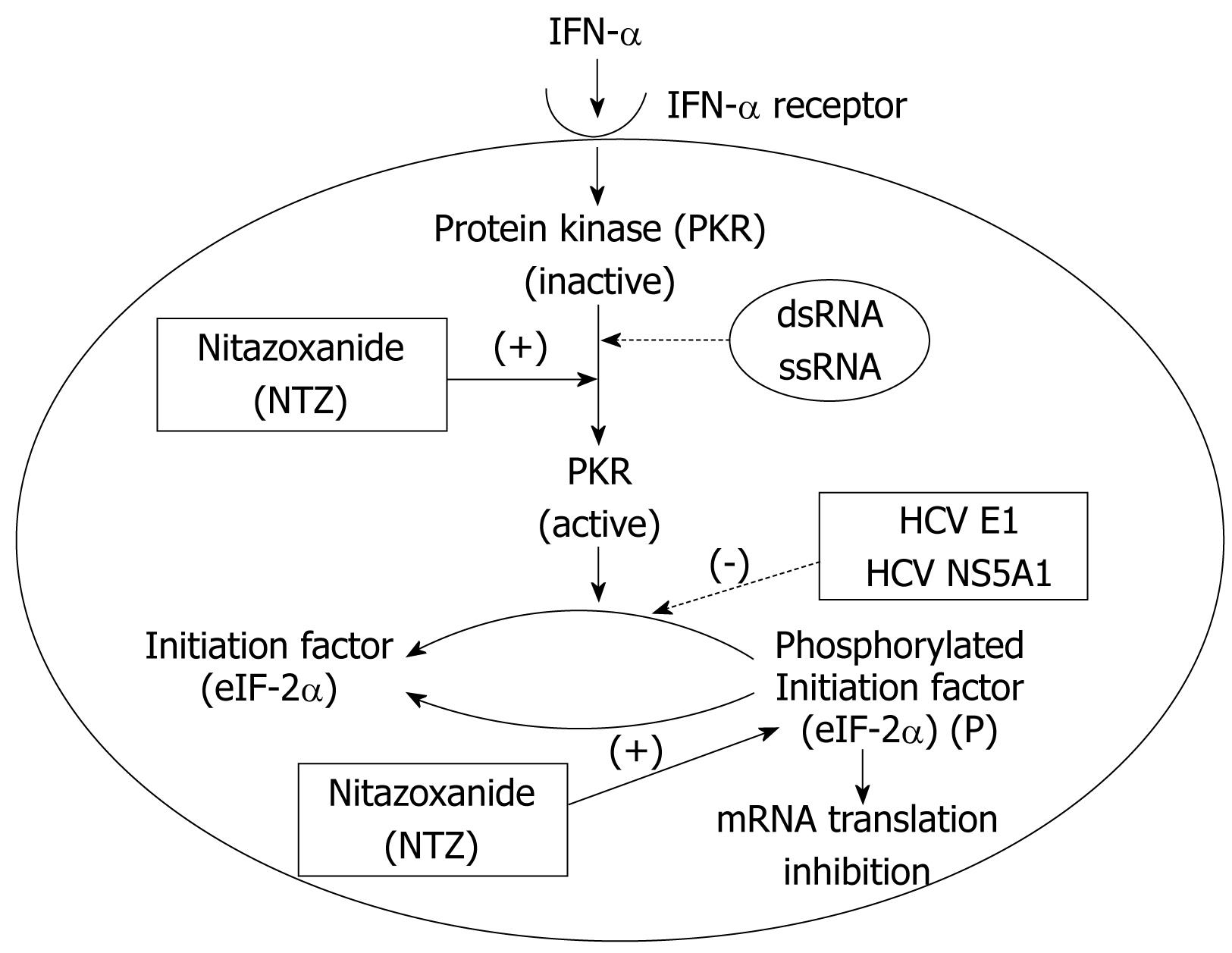
Nitazoxanide is an oral prodrug of a thiazolide (tizoxanide), and was approved for the treatment of protozoal infections[93]. In addition to having antiprotozoal and antibacterial activity, nitazoxanide coincidently was discovered to inhibit HCV replication[94] through a recently identified host-mediated mechanism of action. The antiviral mechanism of action of nitazoxanide appears to be different from the mechanism of action of nitazoxanide in protozoa and anaerobic bacteria. Recent studies suggest that nitazoxanide and other thiazolides selectively induce PKR phosphorylation, which leads to increased cell concentration of phosphorylated eIF2, a naturally occurring antiviral intracellular protein (Figure 3)[95]. This mechanism of action is only triggered when a cell is infected with HCV while nitazoxanide has no effect in uninfected cells, which provides a possible explanation for its very low rate of toxicity.
Furthermore, nitazoxanide does not appear to induce antiviral resistance, based on an attempt to produce a resistance to nitazoxanide and tizoxanide in HCV replicon-containing cell lines[96]. With serial exposure to nitazoxanide or tizoxanide, direct HCV viral resistance did not emerge, suggesting that the genetic barrier to the development of resistance to nitazoxanide is high. The drug has been recently studied in combination with the standard of care in 96 treatment-naive patients in Egypt infected with genotype 4 HCV infection. The combination of nitazoxanide, peginterferon α-2a, and ribavirin increased the percentage of patients with rapid and sustained virologic responses, compared with patients given peginterferon plus ribavirin, without an increase in adverse events[97].
Nitazoxanide, a novel protein kinase inducer, has the potential not only to increase the SVR rate but also potentially to shorten the duration of therapy.
In summary, the suboptimal response to the currently available standard therapy has led to an extensive search for novel therapies with new therapeutic approaches. Targeting host cofactors of the HCV life cycle by different strategies (inhibition of viral entry, targeting host metabolism, nuclear receptors and other principles) may be a novel rational option, especially because they impose higher genetic barriers for resistance than direct antiviral compounds. However, the principle drawback of these strategies is the greater potential for cellular toxicity.
| 1. | World Health Organization. Hepatitis C. Fact sheet number 164. Available from: URL: http://www.who.int/mediacentre/factsheets/fs164/en/. |
| 2. | Medhat A, Shehata M, Magder LS, Mikhail N, Abdel-Baki L, Nafeh M, Abdel-Hamid M, Strickland GT, Fix AD. Hepatitis c in a community in Upper Egypt: risk factors for infection. Am J Trop Med Hyg. 2002;66:633-638. |
| 3. | Habib M, Mohamed MK, Abdel-Aziz F, Magder LS, Abdel-Hamid M, Gamil F, Madkour S, Mikhail NN, Anwar W, Strickland GT. Hepatitis C virus infection in a community in the Nile Delta: risk factors for seropositivity. Hepatology. 2001;33:248-253. |
| 4. | Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41-52. |
| 5. | NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1-46. |
| 6. | Hnatyszyn HJ. Chronic hepatitis C and genotyping: the clinical significance of determining HCV genotypes. Antivir Ther. 2005;10:1-11. |
| 7. | Pawlotsky JM. Therapy of hepatitis C: from empiricism to eradication. Hepatology. 2006;43:S207-S220. |
| 8. | Gao B, Hong F, Radaeva S. Host factors and failure of interferon-alpha treatment in hepatitis C virus. Hepatology. 2004;39:880-890. |
| 9. | De Francesco R, Migliaccio G. Challenges and successes in developing new therapies for hepatitis C. Nature. 2005;436:953-960. |
| 10. | Aizaki H, Morikawa K, Fukasawa M, Hara H, Inoue Y, Tani H, Saito K, Nishijima M, Hanada K, Matsuura Y. Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection. J Virol. 2008;82:5715-5724. |
| 11. | Meuleman P, Hesselgesser J, Paulson M, Vanwolleghem T, Desombere I, Reiser H, Leroux-Roels G. Anti-CD81 antibodies can prevent a hepatitis C virus infection in vivo. Hepatology. 2008;48:1761-1768. |
| 12. | Monazahian M, Kippenberger S, Müller A, Seitz H, Böhme I, Grethe S, Thomssen R. Binding of human lipoproteins (low, very low, high density lipoproteins) to recombinant envelope proteins of hepatitis C virus. Med Microbiol Immunol. 2000;188:177-184. |
| 13. | Thomssen R, Bonk S, Propfe C, Heermann KH, Köchel HG, Uy A. Association of hepatitis C virus in human sera with beta-lipoprotein. Med Microbiol Immunol. 1992;181:293-300. |
| 14. | Thomssen R, Bonk S, Thiele A. Density heterogeneities of hepatitis C virus in human sera due to the binding of beta-lipoproteins and immunoglobulins. Med Microbiol Immunol. 1993;182:329-334. |
| 15. | Castet V, Moradpour D. A model for the study of hepatitis C virus entry. Hepatology. 2003;38:771-774. |
| 16. | Blanchard E, Belouzard S, Goueslain L, Wakita T, Dubuisson J, Wychowski C, Rouillé Y. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol. 2006;80:6964-6972. |
| 17. | Codran A, Royer C, Jaeck D, Bastien-Valle M, Baumert TF, Kieny MP, Pereira CA, Martin JP. Entry of hepatitis C virus pseudotypes into primary human hepatocytes by clathrin-dependent endocytosis. J Gen Virol. 2006;87:2583-2593. |
| 18. | Pileri P, Uematsu Y, Campagnoli S, Galli G, Falugi F, Petracca R, Weiner AJ, Houghton M, Rosa D, Grandi G. Binding of hepatitis C virus to CD81. Science. 1998;282:938-941. |
| 19. | Scarselli E, Ansuini H, Cerino R, Roccasecca RM, Acali S, Filocamo G, Traboni C, Nicosia A, Cortese R, Vitelli A. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 2002;21:5017-5025. |
| 20. | Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci USA. 1999;96:12766-12771. |
| 21. | Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wölk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801-805. |
| 22. | Catanese MT, Graziani R, von Hahn T, Moreau M, Huby T, Paonessa G, Santini C, Luzzago A, Rice CM, Cortese R. High-avidity monoclonal antibodies against the human scavenger class B type I receptor efficiently block hepatitis C virus infection in the presence of high-density lipoprotein. J Virol. 2007;81:8063-8071. |
| 23. | Kitadokoro K, Bordo D, Galli G, Petracca R, Falugi F, Abrignani S, Grandi G, Bolognesi M. CD81 extracellular domain 3D structure: insight into the tetraspanin superfamily structural motifs. EMBO J. 2001;20:12-18. |
| 24. | Liu C, Coco D, Dong HJ, Eksioglu E, Zhu H, Ostrov D; Novel antiviral small molecules that block hepatitis C virus cellular entry through the CD81 receptor. 14th International Symposium on Hepatitis C and Related Viruses; 2007 september 9-13; Glasgow, UK. . |
| 25. | Rhainds D, Brissette L. The role of scavenger receptor class B type I (SR-BI) in lipid trafficking. defining the rules for lipid traders. Int J Biochem Cell Biol. 2004;36:39-77. |
| 26. | Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518-520. |
| 27. | Nieland TJ, Ehrlich M, Krieger M, Kirchhausen T. Endocytosis is not required for the selective lipid uptake mediated by murine SR-BI. Biochim Biophys Acta. 2005;1734:44-51. |
| 28. | Bartosch B, Verney G, Dreux M, Donot P, Morice Y, Penin F, Pawlotsky JM, Lavillette D, Cosset FL. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J Virol. 2005;79:8217-8229. |
| 29. | Maillard P, Huby T, Andréo U, Moreau M, Chapman J, Budkowska A. The interaction of natural hepatitis C virus with human scavenger receptor SR-BI/Cla1 is mediated by ApoB-containing lipoproteins. FASEB J. 2006;20:735-737. |
| 30. | Nielsen SU, Bassendine MF, Burt AD, Martin C, Pumeechockchai W, Toms GL. Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J Virol. 2006;80:2418-2428. |
| 31. | Bartosch B, Vitelli A, Granier C, Goujon C, Dubuisson J, Pascale S, Scarselli E, Cortese R, Nicosia A, Cosset FL. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J Biol Chem. 2003;278:41624-41630. |
| 32. | Chazal N, Gerlier D. Virus entry, assembly, budding, and membrane rafts. Microbiol Mol Biol Rev. 2003;67:226-237, table of contents. |
| 33. | Rawat SS, Viard M, Gallo SA, Rein A, Blumenthal R, Puri A. Modulation of entry of enveloped viruses by cholesterol and sphingolipids (Review). Mol Membr Biol. 2003;20:243-254. |
| 34. | Barth H, Schnober EK, Neumann-Haefelin C, Thumann C, Zeisel MB, Diepolder HM, Hu Z, Liang TJ, Blum HE, Thimme R. Scavenger receptor class B is required for hepatitis C virus uptake and cross-presentation by human dendritic cells. J Virol. 2008;82:3466-3479. |
| 35. | Dreux M, Pietschmann T, Granier C, Voisset C, Ricard-Blum S, Mangeot PE, Keck Z, Foung S, Vu-Dac N, Dubuisson J. High density lipoprotein inhibits hepatitis C virus-neutralizing antibodies by stimulating cell entry via activation of the scavenger receptor BI. J Biol Chem. 2006;281:18285-18295. |
| 36. | Voisset C, Op de Beeck A, Horellou P, Dreux M, Gustot T, Duverlie G, Cosset FL, Vu-Dac N, Dubuisson J. High-density lipoproteins reduce the neutralizing effect of hepatitis C virus (HCV)-infected patient antibodies by promoting HCV entry. J Gen Virol. 2006;87:2577-2581. |
| 37. | Dustin LB, Rice CM. Flying under the radar: the immunobiology of hepatitis C. Annu Rev Immunol. 2007;25:71-99. |
| 38. | Dreux M, Boson B, Ricard-Blum S, Molle J, Lavillette D, Bartosch B, Pécheur EI, Cosset FL. The exchangeable apolipoprotein ApoC-I promotes membrane fusion of hepatitis C virus. J Biol Chem. 2007;282:32357-32369. |
| 39. | Grove J, Huby T, Stamataki Z, Vanwolleghem T, Meuleman P, Farquhar M, Schwarz A, Moreau M, Owen JS, Leroux-Roels G. Scavenger receptor BI and BII expression levels modulate hepatitis C virus infectivity. J Virol. 2007;81:3162-3169. |
| 40. | Kapadia SB, Barth H, Baumert T, McKeating JA, Chisari FV. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J Virol. 2007;81:374-383. |
| 41. | Zeisel MB, Fafi-Kremer S, Fofana I, Barth H, Stoll-Keller F, Doffoel M, Baumert TF. Neutralizing antibodies in hepatitis C virus infection. World J Gastroenterol. 2007;13:4824-4830. |
| 42. | André P, Komurian-Pradel F, Deforges S, Perret M, Berland JL, Sodoyer M, Pol S, Bréchot C, Paranhos-Baccalà G, Lotteau V. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76:6919-6928. |
| 43. | Andréo U, Maillard P, Kalinina O, Walic M, Meurs E, Martinot M, Marcellin P, Budkowska A. Lipoprotein lipase mediates hepatitis C virus (HCV) cell entry and inhibits HCV infection. Cell Microbiol. 2007;9:2445-2456. |
| 44. | Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283-293. |
| 45. | Bangham CR. The immune control and cell-to-cell spread of human T-lymphotropic virus type 1. J Gen Virol. 2003;84:3177-3189. |
| 46. | Vassalli JD, Lombardi T, Wohlwend A, Montesano R, Orci L. Direct cell-to-cell transmission of vesicular stomatitis virus. J Cell Sci. 1986;85:125-131. |
| 47. | Timpe JM, Stamataki Z, Jennings A, Hu K, Farquhar MJ, Harris HJ, Schwarz A, Desombere I, Roels GL, Balfe P. Hepatitis C virus cell-cell transmission in hepatoma cells in the presence of neutralizing antibodies. Hepatology. 2008;47:17-24. |
| 48. | Witteveldt J, Evans MJ, Bitzegeio J, Koutsoudakis G, Owsianka AM, Angus AG, Keck ZY, Foung SK, Pietschmann T, Rice CM. CD81 is dispensable for hepatitis C virus cell-to-cell transmission in hepatoma cells. J Gen Virol. 2009;90:48-58. |
| 49. | Koike K. Hepatitis C as a metabolic disease: Implication for the pathogenesis of NASH. Hepatol Res. 2005;33:145-150. |
| 50. | Jármay K, Karácsony G, Nagy A, Schaff Z. Changes in lipid metabolism in chronic hepatitis C. World J Gastroenterol. 2005;11:6422-6428. |
| 51. | Bahtiyar G, Shin JJ, Aytaman A, Sowers JR, McFarlane SI. Association of diabetes and hepatitis C infection: epidemiologic evidence and pathophysiologic insights. Curr Diab Rep. 2004;4:194-198. |
| 52. | Schiefke I, Fach A, Wiedmann M, Aretin AV, Schenker E, Borte G, Wiese M, Moessner J. Reduced bone mineral density and altered bone turnover markers in patients with non-cirrhotic chronic hepatitis B or C infection. World J Gastroenterol. 2005;11:1843-1847. |
| 53. | Nanda KS, Ryan EJ, Murray BF, Brady JJ, McKenna MJ, Nolan N, O'Farrelly C, Hegarty JE. The effect of chronic hepatitis C virus infection on bone disease in postmenopausal women. Clin Gastroenterol Hepatol. 2009;11:Jan 24. [Epub ahead of print]. |
| 54. | Torres DM, Harrison SA. HCV replication and statin pleotropism: an adjuvant treatment panacea? Am J Gastroenterol. 2008;103:1390-1392. |
| 55. | Ye J, Wang C, Sumpter R Jr, Brown MS, Goldstein JL, Gale M Jr. Disruption of hepatitis C virus RNA replication through inhibition of host protein geranylgeranylation. Proc Natl Acad Sci USA. 2003;100:15865-15870. |
| 56. | Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci USA. 2005;102:2561-2566. |
| 57. | Ikeda M, Abe K, Yamada M, Dansako H, Naka K, Kato N. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology. 2006;44:117-125. |
| 58. | Bader T, Fazili J, Madhoun M, Aston C, Hughes D, Rizvi S, Seres K, Hasan M. Fluvastatin inhibits hepatitis C replication in humans. Am J Gastroenterol. 2008;103:1383-1389. |
| 59. | Huang H, Sun F, Owen DM, Li W, Chen Y, Gale M Jr, Ye J. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci USA. 2007;104:5848-5853. |
| 60. | Burnett JR. Drug evaluation: ISIS-301012, an antisense oligonucleotide for the treatment of hypercholesterolemia. Curr Opin Mol Ther. 2006;8:461-467. |
| 61. | Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, Ikewaki K, Siegelman ES, Gregg RE. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356:148-156. |
| 62. | Chandler CE, Wilder DE, Pettini JL, Savoy YE, Petras SF, Chang G, Vincent J, Harwood HJ Jr. CP-346086: an MTP inhibitor that lowers plasma cholesterol and triglycerides in experimental animals and in humans. J Lipid Res. 2003;44:1887-1901. |
| 63. | Watashi K, Ishii N, Hijikata M, Inoue D, Murata T, Miyanari Y, Shimotohno K. Cyclophilin B is a functional regulator of hepatitis C virus RNA polymerase. Mol Cell. 2005;19:111-122. |
| 64. | Flisiak R, Horban A, Gallay P, Bobardt M, Selvarajah S, Wiercinska-Drapalo A, Siwak E, Cielniak I, Higersberger J, Kierkus J. The cyclophilin inhibitor Debio-025 shows potent anti-hepatitis C effect in patients coinfected with hepatitis C and human immunodeficiency virus. Hepatology. 2008;47:817-826. |
| 65. | Romero-Gómez M. Insulin resistance and hepatitis C. World J Gastroenterol. 2006;12:7075-7080. |
| 66. | Ratziu V, Munteanu M, Charlotte F, Bonyhay L, Poynard T. Fibrogenic impact of high serum glucose in chronic hepatitis C. J Hepatol. 2003;39:1049-1055. |
| 67. | Romero-Gómez M, Del Mar Viloria M, Andrade RJ, Salmerón J, Diago M, Fernández-Rodríguez CM, Corpas R, Cruz M, Grande L, Vázquez L. Insulin resistance impairs sustained response rate to peginterferon plus ribavirin in chronic hepatitis C patients. Gastroenterology. 2005;128:636-641. |
| 68. | Mbow ML, Sarisky RT. What is disrupting IFN-alpha's antiviral activity? Trends Biotechnol. 2004;22:395-399. |
| 69. | Kawaguchi T, Yoshida T, Harada M, Hisamoto T, Nagao Y, Ide T, Taniguchi E, Kumemura H, Hanada S, Maeyama M. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165:1499-1508. |
| 70. | Pazienza V, Clément S, Pugnale P, Conzelman S, Foti M, Mangia A, Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164-1171. |
| 71. | Knobler H, Zhornicky T, Sandler A, Haran N, Ashur Y, Schattner A. Tumor necrosis factor-alpha-induced insulin resistance may mediate the hepatitis C virus-diabetes association. Am J Gastroenterol. 2003;98:2751-2756. |
| 72. | Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Tsukamoto K, Kimura S, Moriya K, Koike K. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840-848. |
| 73. | Walsh MJ, Jonsson JR, Richardson MM, Lipka GM, Purdie DM, Clouston AD, Powell EE. Non-response to antiviral therapy is associated with obesity and increased hepatic expression of suppressor of cytokine signalling 3 (SOCS-3) in patients with chronic hepatitis C, viral genotype 1. Gut. 2006;55:529-535. |
| 74. | Bernsmeier C, Duong FH, Christen V, Pugnale P, Negro F, Terracciano L, Heim MH. Virus-induced over-expression of protein phosphatase 2A inhibits insulin signalling in chronic hepatitis C. J Hepatol. 2008;49:429-440. |
| 75. | Nathan DM, Angus PW, Gibson PR. Hepatitis B and C virus infections and anti-tumor necrosis factor-alpha therapy: guidelines for clinical approach. J Gastroenterol Hepatol. 2006;21:1366-1371. |
| 76. | Romero-Gomez M, Diago M, Andrade RJ, Calleja JL, Salmeron J, Fernandez-Rodriguez CM, Solŕ R, Herrerias JM, Garcia-Samaniego J, Moreno-Otero R. Metformin with peginterferon alfa-2a and ribavirin in the treatment of naïve genotype 1 chronic hepatitis C patients with insulin resistance (TRIC-1): final results of a randomized and double-blinded trial. Hepatology. 2008;48:380A. |
| 77. | Nagashima K, Lopez C, Donovan D, Ngai C, Fontanez N, Bensadoun A, Fruchart-Najib J, Holleran S, Cohn JS, Ramakrishnan R. Effects of the PPARgamma agonist pioglitazone on lipoprotein metabolism in patients with type 2 diabetes mellitus. J Clin Invest. 2005;115:1323-1332. |
| 78. | Negro F. Peroxisome proliferator-activated receptors and hepatitis C virus-induced insulin resistance. PPAR Res. 2009;2009:483485. |
| 79. | Frederiksen KS, Wulf EM, Wassermann K, Sauerberg P, Fleckner J. Identification of hepatic transcriptional changes in insulin-resistant rats treated with peroxisome proliferator activated receptor-alpha agonists. J Mol Endocrinol. 2003;30:317-329. |
| 80. | Jay MA, Ren J. Peroxisome proliferator-activated receptor (PPAR) in metabolic syndrome and type 2 diabetes mellitus. Curr Diabetes Rev. 2007;3:33-39. |
| 81. | Li X, Hansen PA, Xi L, Chandraratna RA, Burant CF. Distinct mechanisms of glucose lowering by specific agonists for peroxisomal proliferator activated receptor gamma and retinoic acid X receptors. J Biol Chem. 2005;280:38317-38327. |
| 82. | Welch JS, Ricote M, Akiyama TE, Gonzalez FJ, Glass CK. PPARgamma and PPARdelta negatively regulate specific subsets of lipopolysaccharide and IFN-gamma target genes in macrophages. Proc Natl Acad Sci USA. 2003;100:6712-6717. |
| 83. | Henson P. Suppression of macrophage inflammatory responses by PPARs. Proc Natl Acad Sci USA. 2003;100:6295-6296. |
| 84. | Conjeevaram H, Burant CF, McKenna , Harsh D, Kang H, Das AK, Everett L, White D, Lok ASF. A randomized, double-blind, placebo-controlled study of PPAR-gamma agonist pioglitazone given in combination with peginterferon and ribavirin in patients with genotype-1 chronic hepatitis C. Hepatology. 2008;48:384A. |
| 85. | Elgouhari HM, Cesario KB, Lopez R, Zein NN. Pioglitazone improves early virologic kinetic response to PEG IFN/RBV combination therapy in hepatitis C genotype 1 naïve pts. Hepatology. 2008;48:383A. |
| 86. | de Gottardi A, Pazienza V, Pugnale P, Bruttin F, Rubbia-Brandt L, Juge-Aubry CE, Meier CA, Hadengue A, Negro F. Peroxisome proliferator-activated receptor-alpha and -gamma mRNA levels are reduced in chronic hepatitis C with steatosis and genotype 3 infection. Aliment Pharmacol Ther. 2006;23:107-114. |
| 87. | Takada N, Sanda T, Okamoto H, Yang JP, Asamitsu K, Sarol L, Kimura G, Uranishi H, Tetsuka T, Okamoto T. RelA-associated inhibitor blocks transcription of human immunodeficiency virus type 1 by inhibiting NF-kappaB and Sp1 actions. J Virol. 2002;76:8019-8030. |
| 88. | Fujita N, Kaito M, Kai M, Sugimoto R, Tanaka H, Horiike S, Konishi M, Iwasa M, Watanabe S, Adachi Y. Effects of bezafibrate in patients with chronic hepatitis C virus infection: combination with interferon and ribavirin. J Viral Hepat. 2006;13:441-448. |
| 89. | Chang KO, George DW. Bile acids promote the expression of hepatitis C virus in replicon-harboring cells. J Virol. 2007;81:9633-9640. |
| 90. | Podevin P, Rosmorduc O, Conti F, Calmus Y, Meier PJ, Poupon R. Bile acids modulate the interferon signalling pathway. Hepatology. 1999;29:1840-1847. |
| 91. | Li X, Huang J, Yi P, Bambara RA, Hilf R, Muyan M. Single-chain estrogen receptors (ERs) reveal that the ERalpha/beta heterodimer emulates functions of the ERalpha dimer in genomic estrogen signaling pathways. Mol Cell Biol. 2004;24:7681-7694. |
| 92. | Watashi K, Inoue D, Hijikata M, Goto K, Aly HH, Shimotohno K. Anti-hepatitis C virus activity of tamoxifen reveals the functional association of estrogen receptor with viral RNA polymerase NS5B. J Biol Chem. 2007;282:32765-73272. |
| 93. | Anderson VR, Curran MP. Nitazoxanide: a review of its use in the treatment of gastrointestinal infections. Drugs. 2007;67:1947-1967. |
| 94. | Korba BE, Montero AB, Farrar K, Gaye K, Mukerjee S, Ayers MS, Rossignol JF. Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antiviral Res. 2008;77:56-63. |
| 95. | Elazar M, Liu M, McKenna S, Liu P, Gehrig EA, Elfert A, Puglisi J, Rossignol JF, Glenn JS. Nitazoxanide (NTZ) is an inducer of eIF2a and PKR phosphorylation [abstract]. Hepatology. 2008;48:1151A. |
| 96. | Korba BE, Elazar M, Lui P, Rossignol JF, Glenn JS. Potential for hepatitis C virus resistance to nitazoxanide or tizoxanide. Antimicrob Agents Chemother. 2008;52:4069-4071. |
| 97. | Rossignol JF, Elfert A, El-Gohary Y, Keeffe EB. Improved virologic response in chronic hepatitis C genotype 4 treated with nitazoxanide, peginterferon, and ribavirin. Gastroenterology. 2009;136:856-862. |