Published online Jul 28, 2009. doi: 10.3748/wjg.15.3462
Revised: June 24, 2009
Accepted: July 1, 2009
Published online: July 28, 2009
Alcoholic liver disease (ALD) and hepatitis C virus (HCV) infection represent, either alone or in combination, more than two thirds of all patients with liver disease in the Western world. This review discusses the epidemiology and combined impact of ALD and HCV on the progression of liver disease. ALD and HCV affect the progression of liver disease to liver cirrhosis and hepatocellular carcinoma (HCC) in a synergistic manner. Thus, the risk for HCC increases five times with a daily alcohol consumption of 80 g; in the presence of HCV it is increased 20-fold, and a combination of both risk factors leads to a more than 100-fold risk for HCC development. Alcohol consumption also decreases the response to interferon treatment which is probably due to a lack of compliance than a direct effect on HCV replication. Several molecular mechanisms are discussed that could explain the synergistic interaction of alcohol and HCV on disease progression. They include modulation of the immune response and apoptosis, increased oxidative stress via induction of CYP2E1 and the hepatic accumulation of iron. Thus, both HCV and alcohol independently cause hepatic iron accumulation in > 50% of patients probably due to suppression of the liver-secreted systemic iron hormone hepcidin. A better understanding of hepcidin regulation could help in developing novel therapeutic approaches to treat the chronic disease in the future. For now, it can be generally concluded that HCV-infected patients should abstain from alcohol and alcoholics should be encouraged to participate in detoxification programs.
- Citation: Mueller S, Millonig G, Seitz HK. Alcoholic liver disease and hepatitis C: A frequently underestimated combination. World J Gastroenterol 2009; 15(28): 3462-3471
- URL: https://www.wjgnet.com/1007-9327/full/v15/i28/3462.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3462
Together, alcoholic liver disease (ALD) and chronic hepatitis C virus (HCV) infection are the most frequent chronic liver diseases in the Western world. In addition, they frequently coexist in the same individual. While both diseases alone have a similar progression sequence leading to cirrhosis in circa 15% of patients within 10-20 years, their coexistence dramatically enhances disease progression in a so-called synergistic manner. This synergism affects both fibrosis progression and the development of hepatocellular carcinoma (HCC). The basic molecular mechanisms of this synergism are far from being understood but may include increased production of reactive oxygen species (ROS) and deposition of iron. In the present article, we review and discuss the epidemiology of ALD and HCV infection, the synergistic impact of combined alcohol and HCV on the progression of liver disease, viral replication and response to anti-HCV treatment. We finally analyze potentially underlying mechanisms that may explain the interaction between alcohol and HCV and offer novel molecular strategies for future therapeutic interventions.
Chronic alcohol consumption causes approximately 50% of the chronic liver disease burden in Germany and the death of more than 18 000 inhabitants per year[1]. In the US alcohol is also responsible for more than 50% of liver related deaths, and ALD is a major health care cost expenditure, accounting for nearly $3 billion annually[2]. At present, the country with the fastest increase in alcohol associated health problems is the Peoples Republic of China with an annual per capita increase in alcohol consumption of 400% and more in some geographic regions[3]. The exact number of alcohol related deaths is difficult to obtain due to inaccurate reporting of ethanol use. Since patients with compensated liver cirrhosis may often die by causes not obviously related to liver disease e.g. infectious complications, official mortality tables most likely underestimate the true prevalence of ALD. If the relationship between alcohol intake and prevalence of ALD is examined on a population basis, the risk of developing ALD starts at 20-30 g ethanol per day. Liver cirrhosis develops only in 10%-20% of people consuming more than 80 g of ethanol daily[2]. Approximately 5% of the whole population in the US meet diagnostic criteria for alcoholism[4]. In Germany, more than 17.8% of the population > 18 years drink more than 20-30 g of alcohol per day[5], and a comparable number of 5% show high risk drinking behavior (> 80 g/d)[5].
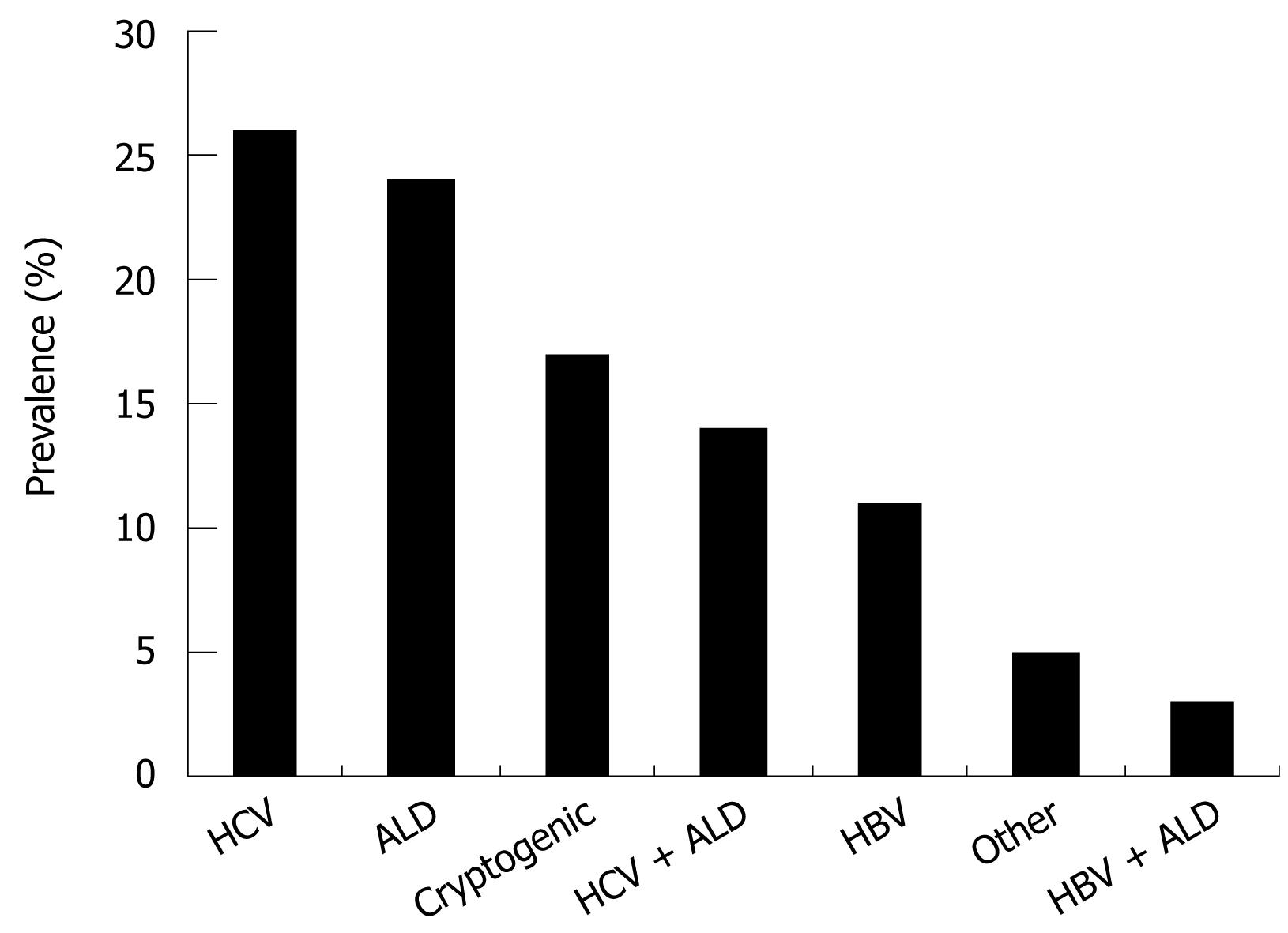
In contrast to ALD, the prevalence of HCV is easier to determine based on serological studies. The worldwide seroprevalence of HCV antibodies is estimated to be 3% with marked geographic variations from 1% in North America to 10% in North Africa[6]. The prevalence is higher in males than in females (2.5% vs 1.2%) and is highest in the 30-49 years old age group[7]. Taken together, there is an estimated prevalence of high risk drinking and HCV of 1%-5% in the Western world. According to recent data from the Center of Disease Control and Prevention, the prevalence of HCV and ALD is relatively similar at 26% and 24%, respectively. Although there is a selection bias, these prevalence data are somehow reflected by large transplant centers. In our transplant center at the University of Heidelberg, liver cirrhosis due to HCV and ALD are leading causes for liver transplantation accounting for 32% and 24%, respectively, of all liver transplantations. In summary, HCV and ALD represent either alone or in combination, more than two thirds of all patients with liver disease in the Western world[8] (Figure 1).
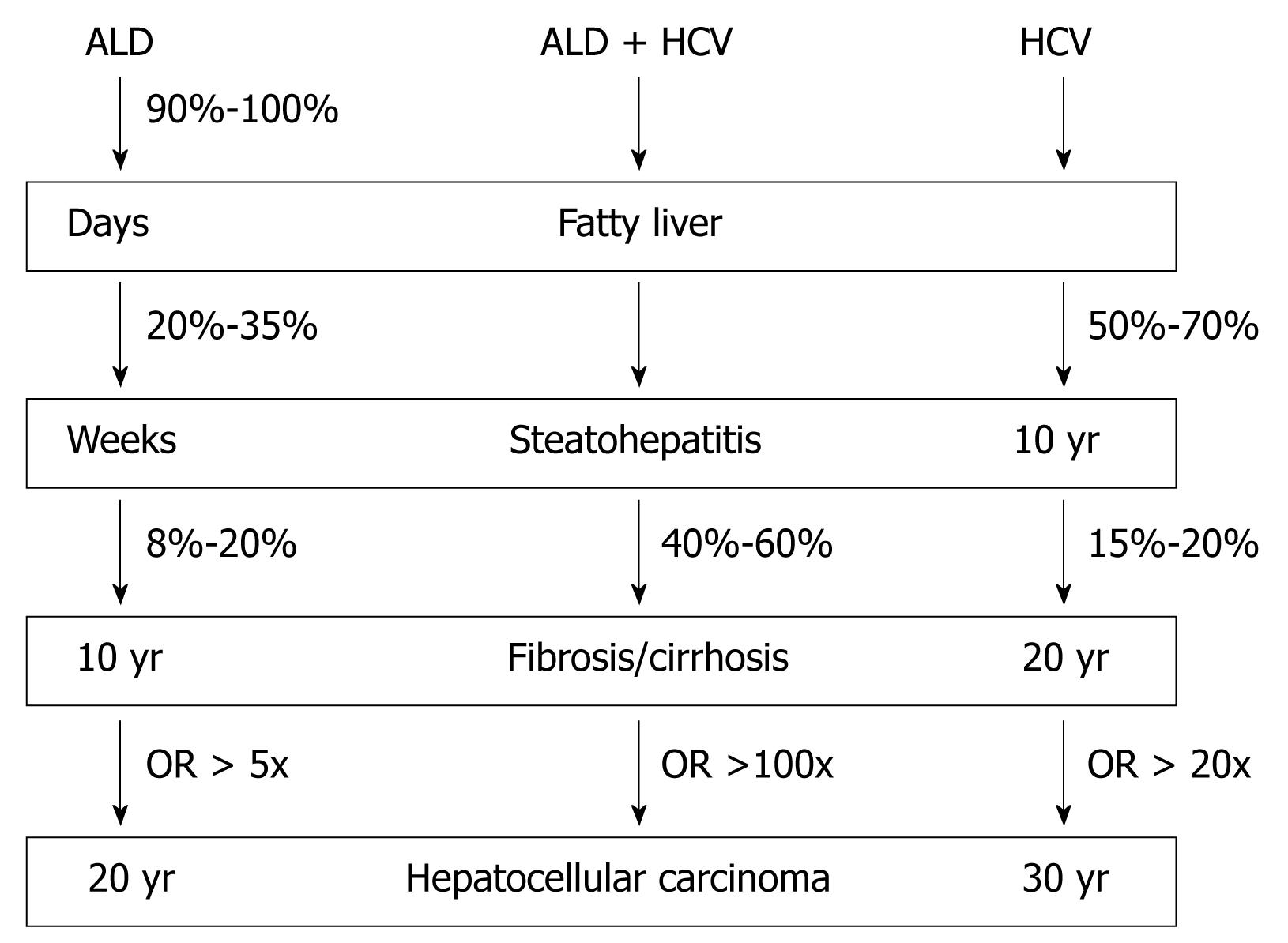
ALD is the most important organ manifestation of chronic alcohol consumption. Ninety percent to one hundred percent of heavy drinkers develop alcoholic fatty liver. Ten percent to thirty-five percent of them develop alcoholic steatohepatitis and 8%-20% develop alcoholic liver cirrhosis within 10-20 years[9]. The natural course of ALD and HCV is given in Figure 2. Due to better treatment options for complications of liver cirrhosis e.g. variceal bleeding, the prevalence of HCC is increasing with an annual risk of 1%-2%. HCC represents the most common cause of death in patients with ALD. HCV shows a similar progression pattern. In a US study, the mean interval between HCV infection, chronic hepatitis, cirrhosis and HCC was circa 10, 20 and 30 years, respectively[10]. A large cohort study with long-term follow up showed that 75% of HCV-infected patients develop persistent infection while severe progressive liver disease occurred in 15%-20%[11].
Factors that contribute to progression of ALD and HCV are summarized in Table 1. For ALD, these include the amount of alcohol consumed over a life time[1213], drinking patterns, and nutritional status. Both malnutrition and obesity are associated with an increased risk for alcoholic cirrhosis[14–16]. This is especially relevant with the endemic occurrence of non-alcoholic fatty liver disease (NAFLD) in the Western World due to obesity and being overweight associated with diabetes mellitus type II and peripheral insulin resistance. Co-medication of certain drugs together with ethanol may also harm the liver by increased conversion to toxic metabolites due to induced enzyme systems. This is well known for acetaminophen[17], methotrexate[18] and the tuberculostatic drug isoniazid[17] but also occurs with retinoids such as β-carotin and vitamin A[19]. Males and females show different courses of ALD and HCV. While females are more susceptible to alcoholic damage, they progress slower in chronic HCV infection. Other important factors that contribute to disease progression in ALD are co-morbidities such as HBV, hemochromatosis, and Wilson’s disease. Factors associated with HCV progression are co-infection with HBV, HIV, schistosomiasis or conditions of immunosuppression. Finally, iron accumulation has been recognized both in ALD and HCV as an independent risk factor for the development of HCC. Pathological hepatocellular iron deposits are found in more than 50% of patients with either HCV or ALD. Underlying mechanisms and potential therapeutic options are still under investigation.
| HCV | ALD |
| Male gender | Female gender |
| Amount and duration of alcohol consumption | |
| Continuous drinking (vs sporadic drinking) | |
| Overweight/malnutrition | |
| Hepatic iron deposition age > 40 | Hepatic iron deposition |
| Immunosuppression | Vitamin A, co-medication |
Chronic alcoholics have an increased prevalence of HCV infection, increasing with the severity of the ALD. Takase et al[20] showed that HCV prevalence demonstrated by anti-HCV positivity increases with the severity of ALD, having a prevalence of approximately 5% in alcoholic fibrosis, almost 40% in alcoholic cirrhosis and almost in 80% in HCC due to alcohol. This could be due to the lifestyle of chronic alcoholics, since many of them are also intravenous drug abusers, which is a high risk for HCV infection. It could also be due to the immunosuppressive effect of alcohol decreasing the HCV-clearance rate after infection since it has been shown that alcohol suppresses the function of various immune components including natural killer cells, neutrophils, monocytes and others[21].
A great number of studies emphasize the fact that alcoholics respond poorly to interferon therapy. More than ten years ago, Mochida et al[22] showed that almost 30% of non-alcoholics responded biochemically and virologically to interferon therapy compared to less than 10% of alcoholics. The question remained open whether this is due to a direct inhibitory effect of alcohol on interferon response or due to poor compliance of these patients. Pessione et al[23] studied serum HCV RNA in HCV patients with increasing alcohol intake (reported in gram per week). In this study a significant dose-dependent increase in serum HCV RNA was noted starting from 70 g alcohol per week. In line with this observation, a decrease of alcohol consumption prior treatment of hepatitis C significantly reduced viral load. In addition, Cromie et al[24] showed that viral load decreased highly significantly within 4 mo when patients cut down on alcohol consumption from 39-100 g/d to 0-50 g/d. More recent data, however, clearly suggest that the poor response of alcoholics towards interferon therapy is more likely due to reduced compliance. In this study, the recorded alcohol consumption during the months before HCV treatment was associated with an increased rate of therapy drop out (3% vs 26%, P = 0.002)[25] while the response rate was comparable (25% vs 23%) after correction for this confounding factor. In conclusion, poor compliance of alcoholics is probably the major cause for poor antiviral response to HCV therapy.
A huge number of studies have shown that concomitant alcohol consumption in the presence of HCV increases progression of fibrosis[2326–54]. This means that fibrosis occurs at an earlier time point and its development is accelerated. A summary of selected studies on alcohol consumption and fibrosis progression is given in Table 2. Thus, it has been shown in more than 2000 HCV patients that fibrosis progression was significant (P < 0.001) if more than 50 g/d alcohol is consumed[26]. Similar results were obtained by Roudot-Thoraval et al[27] with a prevalence of cirrhosis of 35 % vs 18 % (P < 0.001). Pessione showed in more than 200 HCV patients that weekly alcohol consumption correlated significantly with fibrosis score[23]. He also showed that the relative risk for decompensated cirrhosis correlated with alcohol intake. Alcohol-driven fibrogenesis in HCV patients is dose-dependent and starts at less than 30 g/d. Overweight and obese patients as well as type II diabetics are especially sensitive to fibrosis progression[55]. HCV patients with excessive alcohol abuse have a 2-3 fold increased risk of severe liver disease compared with HCV patients without a history of drinking[56]. So far it is still unclear how long a patient has to abstain from alcohol before the negative effect of alcohol is abolished[57]. Alcoholics with HCV infection seem to stop drinking more frequently compared to alcoholics without HCV infection. This is possibly due to a higher awareness in these patients that liver disease can lead to cirrhosis and death without a change in lifestyle[58]. Finally, it has been a continuous debate whether small amounts of alcohol (< 20-30 g/d) alter progression in HCV infection. An answer may come from a Scandinavian study by Westin et al[59]. These authors investigated 78 patients with hepatitis C infection who underwent two liver biopsies in a mean interval of 6.3 years. Alcohol consumption was less than 40 g/d. The authors found progressive fibrosis with (a) increased total alcohol consumption (15.4 kg vs 3.9 kg; P = 0.007), (b) increased daily alcohol consumption (5.7 g vs 2.6 g/d; P = 0.03) and (c) increased frequency of drinking occasions (35 vs 8 d per year; P = 0.006). These results underscore that even small amounts of alcohol may increase fibrosis progression in HCV. Confirmation comes from another prospective study by Hezode et al[60] who showed an impact of mild alcohol consumption on histological activity and fibrosis starting as low as 20 g/d.
| No. of patients | Alcohol consumption | Results | Ref. |
| 2235 | 0 g, < 50 g, > 50 g | > 50 g independent risk factor for fibrosis progression (P < 0.001) | [26] |
| 6664 | > 5/6 drinks (female/male), > 1 year | Higher risk of cirrhosis (35% vs 18 %) | [27] |
| 176 | > 40/60 g (female/male), > 5 years | Faster cirrhosis progression (58 % vs 10 %), 2-3 fold increased risk of developing cirrhosis | [29] |
| 168 | Low < 30, medium 30-80; high > 80 g/d, > 5 J | Alcohol consumption low/medium/high significantly different between non-cirrhotics (58%/27%/16%) and cirrhotics (76%/15%/9%) (P < 0.05) | [28] |
| 234 | Lifetime alcohol consumption | Cirrhotics have greater alcohol consumption than patients with hepatitis (240 g/wk vs 146 g/wk) (P = 0.02) | [30] |
| 233 | 0, 25, 50, 75, 100, > 125 g | Weekly alcohol consumption correlates with serum HCV RNA levels and fibrosis score (P < 0.001) | [23] |
| 702 | 0/175 g/d | HCV increases OR for cirrhosis from 1 to 15 (0 g), 9.2 to 147.2 (175 g) | [141] |
| 1667 | Subgroup: > 260 g/wk vs < 90 g/wk | Risk for cirrhosis increases by 3.6 | [31] |
| 636 | > 80 | RR for cirrhosis: HCV 7.8, HCV + alcohol 31.1 | [32] |
Various studies have shown that there is an increased risk of HCC in patients with HCV and alcohol abuse compared to either HCV or ALD alone[61–68]. Since these studies vary considerably in their definition of alcohol abuse, Table 3 is restricted to comparable studies that tried to identify the independent contribution of HCV and ALD to HCC development. It can be concluded from these data, that a daily uptake of > 80 g alcohol alone increases HCC risk 5-fold while the presence of HCV alone increases HCC 20-fold. A combination of both risk factors increases the risk for HCC development over 100-fold. Thus, HCV and alcohol act truly synergistic on HCC development.
The underlying molecular mechanisms of alcohol and HCV-mediated liver disease are complex and they are still incompletely understood despite intensive efforts over decades.
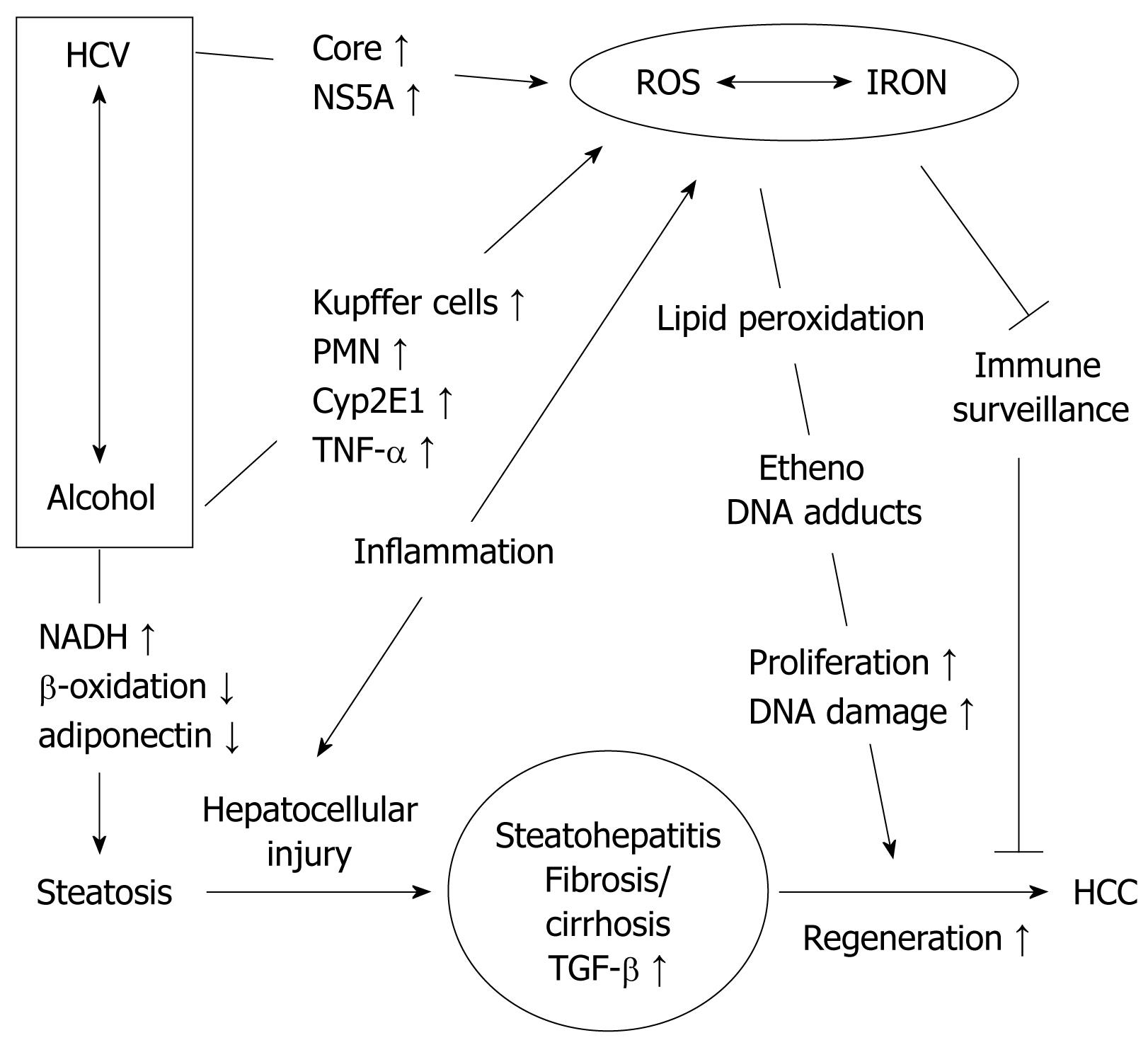
Both alcohol and HCV can reproduce the four sequential hallmarks of liver disease: steatosis, steatohepatitis, fibrosis and HCC. Molecular key features of ethanol and HCV mediated liver damage include direct biochemical consequences of alcohol metabolism such as the production of acetaldehyde, generation of reactive oxygen species (ROS) and oxidative damage, epigenetic modifications such as hypomethylation of histones and modulation of the signaling machinery. Some of these events lead to similar downstream effects such as fatty liver, ROS and iron accumulation but are based on different mechanisms which could well explain the synergistic effects of alcohol and HCV on the liver. Thus, steatosis in HCV is mainly caused by impairment of mitochondria preventing mitochondrial metabolism of fatty acids, while ethanol primarily stimulates lipogenesis. On the other hand, HCV and ethanol both stimulate ROS generation via distinct mechanisms and they both lead to hepatic iron accumulation, one of the most pro-fibrogenic and pro-tumorigenic factors in liver disease. For this reason, ROS generation and iron accumulation are discussed separately below. It should also be mentioned that alcohol may have direct molecular effects on HCV infection since it exerts stimulatory effects on HCV replication probably via signaling pathways[69]. The enhanced quasispecies complexity in the hypervariable region 1 of HCV in alcoholics may be one major cause that sensitizes for faster disease progression[70].
Ethanol biochemically leads to a shift towards NADH which ultimately stimulates lipogenesis. In addition, ethanol is metabolized to the mutagenic metabolite acetaldehyde and during that reaction ROS are produced mainly as a byproduct of CYP2E1. Additional mechanisms include the release of cytokines such as TNF-α, which increases free fatty acid release from adipocytes in the periphery of the liver[71], stimulates lipogenesis in hepatocytes[72], and inhibits β-oxidation of fatty acids[73]. Chronic ethanol consumption also impairs transport and secretion of triglycerides as VLDL[74] which again leads to an increased hepatic fat accumulation. Activation of macrophages by lipopolysaccharides via the toll-like receptor 4 (TLR-4) leads to the production of a variety of inflammatory mediators, such as TNF-α and ROS. HCV also leads to steatosis but in contrast to ALD mainly via a decreased mitochondrial β-oxidation with ultrastructural alterations of hepatocyte mitochondria in more than half of the patients. This means that HCV and alcohol stimulate fat accumulation in the liver via distinct mechanisms. In addition to its role in steatosis, abnormal production of TNF-α is also a critical inflammatory component in the liver induced by chronic ethanol exposure[7576]. Although direct exposure of macrophages in culture can mimic some of the effects of ethanol[7778], there seem to be multiple hepatic and extra-hepatic consequences of ethanol that finally render Kupffer cells more reactive to LPS, leading to generation of ROS and ROS-modulated signal transduction cascades[7980]. The fat regulating hormone adiponectin also seems to be involved in ethanol mediated steatohepatitis[81–83]. Some data indicate that ethanol directly drives fibrosis progression: acetaldehyde is supposed to increase TGF-β1 secretion[84] and both ethanol and acetaldehyde induce accumulation of collagen[85]. Similar findings have been shown for HCV-replicating hepatoma cells[86].
HCC pathogenesis by ethanol seems to require several factors[87] including the presence of cirrhosis, oxidative stress, altered methyl transfer resulting in DNA hypomethylation, and a decrease in retinoic acid. In addition, co-morbidities such as viral hepatitis, diabetes mellitus and obesity are known to accelerate HCC development in patients with ALD. ROS play an important role in hepatocarcinogenesis[8788]. Chronic ethanol consumption results in the generation of ROS via multiple pathways leading to lipid peroxidation (LPO) and LPO-byproducts such as 4-hydroxy-2-nonenal (4-HNE) and malondialdehyde (MDA). These DNA-reactive aldehydes in turn form mutagenic exocyclic DNA adducts including 1, N6-ethenodeoxyadenosine (εdA) and 3, N4-ethenodeoxycytidine[8990].
The generation of ROS seems to be a hallmark of both ALD and HCV[91–95] (Figure 3). While location and mechanisms of their generation differ markedly between ALD and HCV, downstream events of oxidative damage are similar due to the high but rather unspecific reactivity of species such as hydroxyl radicals or lipid peroxidation products. Hepatocyte mitochondria are structurally altered in more than 50% of HCV patients and these conditions are accompanied by a significant depletion of hepatocellular and lymphocyte glutathione (GSH), an increase of oxidized GSH (GSSG) and the lipid peroxidation marker malondialdehyde[91]. ROS are either induced directly by the virus or indirectly through activation of inflammatory cells. HCV core[96] and NS5A[9697] have been implicated in generating ROS via mitochondrial damage and calcium release.
ROS also play an important role in alcohol-induced liver injury and in hepatocarcinogenesis[87–90]. Several enzyme systems are capable of producing ROS including the mitochondrial respiratory chain, the cytosolic enzymes xanthine oxidase and aldehyde oxidase, as well as the microsomal cytochrome P450-dependent mono-oxygenases[88]. One member of the latter system, cytochrome P450 2E1 (CYP2E1), is involved in the major pathway by which ethanol generates oxidative stress. Expression of CYP2E1 has been shown to correlate well with the generation of hydroxyethyl radicals and with LPO products such as 4-HNE and MDA[98]. CYP2E1 is induced by chronic alcohol consumption within a week even at a relatively low ethanol dose (40 g/d), but the degree of CYP2E1 induction shows high variations between individuals[99]. Inhibition of CYP2E1 by chlormethiazole, a specific CYP2E1 inhibitor, improved ALD as shown in the Tsukamoto-French rat model[100]. An increase of oxidative DNA adducts and of mutagenic apurinic and apyrimidinic DNA sites has been found in chronically ethanol-treated wild- type mice but not in mice that lack functional CYP2E1[101] further stressing the importance of CYP2E1 in the generation of DNA damage following ethanol ingestion. Increased levels of Cyp2E1 also potentiate pro-apoptotic effects of TGF-β resulting in increased cell death of hepatocytes[102]. Recently, we have been able to detect etheno-DNA adducts such as εdA in the livers of patients with ALD[89103].
Kupffer cells are also an important source of ROS during ethanol exposure[93] and in response to LPS[104]. NADPH oxidase-dependent production of ROS is implicated in ethanol-induced liver injury since p47phox -/- mice which are deficient in this regulatory subunit of NADPH oxidase are resistant to chronic ethanol-induced injury[105]. Chronic ethanol feeding increases the LPS-stimulated production of ROS by Kupffer cells; this increase is primarily due to an increase in NADPH oxidase activation after chronic ethanol feeding[81]. Recently, Thakur and colleagues have specifically identified NADPH oxidase-derived ROS as an important contributor to increased TLR-4 mediated signal transduction and TNF-α expression in rat Kupffer cells, particularly after chronic ethanol exposure[81].
In contrast to hepatitis B infection, iron deposits are found in more than 50% of patients with HCV infection or chronic ethanol consumption[96106–109]. Even mild to moderate alcohol consumption has recently been shown to increase the prevalence of iron overload[110]. Iron localization has been reported in Kupffer cells[108] as well as in hepatocytes[111–113]. In our experience, iron accumulation is more common in hepatocytes than Kupffer cells in patients with ALD. Increased hepatic iron content is associated with greater mortality from alcoholic cirrhosis, suggesting a pathogenic role of iron in ALD. Genetic hemochromatosis in conjunction with excessive alcohol consumption exacerbates liver injury[100]. It should be mentioned that iron per se is the most profibrogenic and genotoxic factor and 50% of patients with hereditary hemochromatosis develop fibrosis and have a 200-fold increased risk for HCC[114]. On the other hand, immune surveillance can be impaired by iron overload, since it compromises anti-tumor activity of macrophages[115–117].
The underlying mechanisms of iron accumulation observed in ALD and HCV are still poorly understood but seem to involve an inadequate upregulation of the iron hormone hepcidin. Genome wide microarray based screening for candidate genes that could cause iron overload involved several genes not yet linked to iron metabolism[118]. Preliminary data from ALD patients and ethanol-treated mice models suggest that hepatic iron uptake pathways are increased in the liver and potential mechanisms involve an increase of the transferrin receptor (TfR)1 and repression of the systemic iron hormone hepcidin that controls duodenal iron absorption and RES-mediated iron release via the iron exporter ferroportin[119120]. Using novel in vitro and in vivo models[121122], we have recently demonstrated that H2O2 alone increases TfR1 via posttranscriptional and translation mechanisms ultimately leading to cellular accumulation of iron[123124]. These data show that chronic exposure of cells to non toxic levels of H2O2 lead to accumulation of iron via distinct regulatory mechanisms promoting Fenton chemistry. We suggest that increased oxidative stress in the form of H2O2 is an important regulatory factor that causes continuous iron accumulation and may support ALD progression. Valuable information on the direct interaction of HCV with host metabolism has been gained from studies with genetically modified animals, though with some controversial results[125]. Thus mice transgenic for the total open reading frame of HCV under the murine albumin promoter developed steatosis and liver cancer[126127], but this association disappeared in later generations of animals, casting doubt on the earlier conclusions that HCV infection alone (in the absence of cirrhosis and iron overload) drives hepatic carcinogenesis[109128129]. In addition, iron overload induced in mice either through diet[130131] or genetic deletion of the HFE locus[132] did not lead to advanced fibrosis or HCC. In HCV transgenic mice, hepcidin was found to be suppressed despite iron loading. This is unexpected, since hepcidin inhibits cellular iron efflux by inducing internalization of ferroportin[133], an iron exporter that is expressed in macrophages, hepatocytes and intestinal cells. The mechanism by which hepcidin was downregulated in the present model remains elusive, since cytokines such as TNF-α, IL-1β and IL-6 which can upregulate hepcidin levels[134135] were not suppressed. Other important players such as iron regulatory proteins (IRP1 and IRP2) which sense iron but also ROS at the cellular level have not been assessed[136].
Finally, it has also been investigated whether iron directly affects HCV replication. In hepatoma cells iron loading promoted reporter expression under the control of regulatory HCV mRNA stem-loop structures by upregulating expression of the translation initiation factor 3eIF3[137]. In contrast, iron was shown to suppress HCV replication by inactivating the RNA polymerase NS5B[138]. Clinical data indicate that iron status does not significantly influence HCV replication in vivo, since the response to therapy of patients with β-thalassemia was not influenced by the degree of iron accumulation[139], and venesection did not reduce hepatitis C viral load[140]. Taken together, iron accumulation in patients with HCV and ALD is an important progression factor. The underlying mechanisms are being intensively studied in search for novel therapeutic approaches.
ALD and HCV are the most common liver diseases in the Western world either alone or in combination. Coexistence of both diseases has a true synergistic effect on fibrosis progression and HCC development. Thus, a daily consumption of more than 80 g alcohol increases the risk for HCC 5-fold, in the presence of HCV 100-fold while HCV alone increases the risk for HCC 20-fold. Alcohol abusers have an increased prevalence of HCV infection probably due to lifestyle or to immune suppression. Alcoholics also have a decreased response rate to antiviral therapy which is most probably due to poor compliance. There is obviously no safe level of drinking in patients with hepatitis C and it remains unclear how long abstinence is necessary to abolish the negative effect of alcohol on the liver. Potential mechanisms which may explain the synergistic negative effect of alcohol and HCV infection on liver disease include generation of ROS, iron accumulation, steatosis induction, immune modulation, stimulation of HCV replication and direct DNA damage. Abstaining from drinking in HCV patients who do not respond to antiviral treatment is the sole efficient treatment option to date. A better understanding of the underlying molecular mechanisms could help to develop novel targeted treatment options.
| 1. | McCullough AJ, O’Connor JF. Alcoholic liver disease: proposed recommendations for the American College of Gastroenterology. Am J Gastroenterol. 1998;93:2022-2036. |
| 2. | Maher JJ. Alcoholic liver disease. Gastrointestinal and Liver Disease Vol II. Saunders: Philadelphia 2002; 1375-1391. |
| 3. | Cochrane J, Chen H, Conigrave KM, Hao W. Alcohol use in China. Alcohol Alcohol. 2003;38:537-542. |
| 4. | Drinking in the United States: Main findings from the 1992 national longitudinal alcohol epidemiologic survey (NLAES). NIH Publication No 99-3519. 1998;. |
| 5. | Jahrbuch Sucht 2007. Geesthacht: Neuland Verlagsgesellschaft mbH. 2007;. |
| 6. | Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis. 2000;20:1-16. |
| 7. | Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, Kaslow RA, Margolis HS. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556-562. |
| 8. | Singal AK, Anand BS. Mechanisms of synergy between alcohol and hepatitis C virus. J Clin Gastroenterol. 2007;41:761-772. |
| 9. | Seitz HK, Becker P. Alcohol-induced hepatitis: pathophysiology and treatment. Chronic hepatitis: metabolic, cholestatic, viral und autoimmun. Cluver Academic Publisher: Dordrecht-Boston-London 2007; 16-31. |
| 10. | Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463-1466. |
| 11. | Alter HJ, Seeff LB. Recovery, persistence, and sequelae in hepatitis C virus infection: a perspective on long-term outcome. Semin Liver Dis. 2000;20:17-35. |
| 12. | Lelbach WK. Cirrhosis in the alcoholic and its relation to the volume of alcohol abuse. Ann N Y Acad Sci. 1975;252:85-105. |
| 13. | Pequignot G. Les problemes nutritionelles de la societe industrielle. La vie medicale en Canada francais. 1974;3:216-255. |
| 14. | Halsted C. Role of nutrition in the treatment of alcoholic liver disease. Treatment of liver diseases. Masson: Barcelona 1999; 221-232. |
| 15. | Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36:150-155. |
| 16. | Seitz HK, Suter P. Ethanol toxicity and nutritional status. Nutritional toxicology. 2nd ed. Taylor and Francis: London, New York 2002; 122-154. |
| 17. | Lieber CS. Alcohol and the liver: 1994 update. Gastroenterology. 1994;106:1085-1105. |
| 18. | Slattery JT, Nelson SD, Thummel KE. The complex interaction between ethanol and acetaminophen. Clin Pharmacol Ther. 1996;60:241-246. |
| 19. | Leo MA, Kim C, Lowe N, Lieber CS. Interaction of ethanol with beta-carotene: delayed blood clearance and enhanced hepatotoxicity. Hepatology. 1992;15:883-891. |
| 20. | Takase S, Matsuda Y, Sawada M, Takada N, Takada A. Effect of alcohol abuse on HCV replication. Gastroenterol Jpn. 1993;28:322. |
| 21. | Geissler M, Gesien A, Wands JR. Inhibitory effects of chronic ethanol consumption on cellular immune responses to hepatitis C virus core protein are reversed by genetic immunizations augmented with cytokine-expressing plasmids. J Immunol. 1997;159:5107-5113. |
| 22. | Mochida S, Ohnishi K, Matsuo S, Kakihara K, Fujiwara K. Effect of alcohol intake on the efficacy of interferon therapy in patients with chronic hepatitis C as evaluated by multivariate logistic regression analysis. Alcohol Clin Exp Res. 1996;20:371A-377A. |
| 23. | Pessione F, Degos F, Marcellin P, Duchatelle V, Njapoum C, Martinot-Peignoux M, Degott C, Valla D, Erlinger S, Rueff B. Effect of alcohol consumption on serum hepatitis C virus RNA and histological lesions in chronic hepatitis C. Hepatology. 1998;27:1717-1722. |
| 24. | Cromie SL, Jenkins PJ, Bowden DS, Dudley FJ. Chronic hepatitis C: effect of alcohol on hepatitic activity and viral titre. J Hepatol. 1996;25:821-826. |
| 25. | Anand BS, Currie S, Dieperink E, Bini EJ, Shen H, Ho SB, Wright T. Alcohol use and treatment of hepatitis C virus: results of a national multicenter study. Gastroenterology. 2006;130:1607-1616. |
| 26. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. |
| 27. | Roudot-Thoraval F, Bastie A, Pawlotsky JM, Dhumeaux D. Epidemiological factors affecting the severity of hepatitis C virus-related liver disease: a French survey of 6,664 patients. The Study Group for the Prevalence and the Epidemiology of Hepatitis C Virus. Hepatology. 1997;26:485-490. |
| 28. | Serfaty L, Chazouilleres O, Poujol-Robert A, Morand-Joubert L, Dubois C, Chretien Y, Poupon RE, Petit JC, Poupon R. Risk factors for cirrhosis in patients with chronic hepatitis C virus infection: results of a case-control study. Hepatology. 1997;26:776-779. |
| 29. | Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28:805-809. |
| 30. | Ostapowicz G, Watson KJ, Locarnini SA, Desmond PV. Role of alcohol in the progression of liver disease caused by hepatitis C virus infection. Hepatology. 1998;27:1730-1735. |
| 31. | Thomas DL, Astemborski J, Rai RM, Anania FA, Schaeffer M, Galai N, Nolt K, Nelson KE, Strathdee SA, Johnson L. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450-456. |
| 32. | Harris HE, Ramsay ME, Heptonstall J, Soldan K, Eldridge KP. The HCV National Register: towards informing the natural history of hepatitis C infection in the UK. J Viral Hepat. 2000;7:420-427. |
| 33. | Roulot D, Vallet-Pichard A. [Natural history of factors influencing the severity of chronic HCV infection in HIV-HCV coinfected patients]. Gastroenterol Clin Biol. 2007;31:881-886. |
| 34. | Bonnard P, Lescure FX, Amiel C, Guiard-Schmid JB, Callard P, Gharakhanian S, Pialoux G. Documented rapid course of hepatic fibrosis between two biopsies in patients coinfected by HIV and HCV despite high CD4 cell count. J Viral Hepat. 2007;14:806-811. |
| 35. | De Bona A, Galli L, Gallotta G, Guzzo A, Alagna L, Lazzarin A, Uberti-Foppa C. Rate of cirrhosis progression reduced in HIV/HCV co-infected non-responders to anti-HCV therapy. New Microbiol. 2007;30:259-264. |
| 36. | Sulkowski MS, Benhamou Y. Therapeutic issues in HIV/HCV-coinfected patients. J Viral Hepat. 2007;14:371-386. |
| 37. | Franchini M. Is time to treat for HCV all the HIV/HCV co-infected hemophiliacs? Hematology. 2006;11:209-213. |
| 38. | Picciotto FP, Tritto G, Lanza AG, Addario L, De Luca M, Di Costanzo GG, Lampasi F, Tartaglione MT, Marsilia GM, Calise F. Sustained virological response to antiviral therapy reduces mortality in HCV reinfection after liver transplantation. J Hepatol. 2007;46:459-465. |
| 39. | Schiavini M, Angeli E, Mainini A, Zerbi P, Duca PG, Gubertini G, Vago L, Fociani P, Giorgi R, Cargnel A. Risk factors for fibrosis progression in HIV/HCV coinfected patients from a retrospective analysis of liver biopsies in 1985-2002. HIV Med. 2006;7:331-337. |
| 40. | Santana JL, Rodriguez-Medina JR, Rodriguez-Orengo JF. Clinical challenges and controversies in the management of HIV/ HCV-coinfected individuals. P R Health Sci J. 2004;23:35-40. |
| 41. | Monto A, Kakar S, Dove LM, Bostrom A, Miller EL, Wright TL. Contributions to hepatic fibrosis in HIV-HCV coinfected and HCV monoinfected patients. Am J Gastroenterol. 2006;101:1509-1515. |
| 42. | Ruiz-Sancho A, Soriano V. [HIV and HCV coinfection]. Enferm Infecc Microbiol Clin. 2006;24:335-345; quiz 346. |
| 43. | Stroffolini T, Sagnelli E, Mariano A, Craxi A, Almasio P. Characteristics of HCV positive subjects referring to hospitals in Italy: a multicentre prevalence study on 6,999 cases. J Viral Hepat. 2006;13:351-354. |
| 44. | Chen SL, Morgan TR. The natural history of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:47-52. |
| 45. | Park JS, Saraf N, Dieterich DT. HBV plus HCV, HCV plus HIV, HBV plus HIV. Curr Gastroenterol Rep. 2006;8:67-74. |
| 46. | Sola R, Alvarez MA, Balleste B, Montoliu S, Rivera M, Miquel M, Cirera I, Morillas RM, Coll S, Planas R. Probability of liver cancer and survival in HCV-related or alcoholic-decompensated cirrhosis. A study of 377 patients. Liver Int. 2006;26:62-72. |
| 47. | Brau N, Salvatore M, Rios-Bedoya CF, Fernandez-Carbia A, Paronetto F, Rodriguez-Orengo JF, Rodriguez-Torres M. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47-55. |
| 48. | Ratti L, Pozzi M, Bosch J. Pathophysiology of portal hypertension in HCV-related cirrhosis. Putative role of assessment of portal pressure gradient in Peginterferon-treated patients. Dig Liver Dis. 2005;37:886-893. |
| 49. | Colletta C, Smirne C, Fabris C, Toniutto P, Rapetti R, Minisini R, Pirisi M. Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology. 2005;42:838-845. |
| 50. | Dinis-Ribeiro M, Ramalho F, Gloria H, Marinho R, Raimundo M, Serejo F, Velosa J, Carneiro-de-Moura M. Factors associated with the development of cirrhosis in patients with HCV chronic infection. Hepatogastroenterology. 2005;52:176-179. |
| 51. | Dieterich DT, Kontorinis N, Agarwal K. HIV/HCV coinfection in clinical practice. J Int Assoc Physicians AIDS Care (Chic Ill). 2004;3 Suppl 1:S4-S14; quiz S16-S17. |
| 52. | Prakash O, Mason A, Luftig RB, Bautista AP. Hepatitis C virus (HCV) and human immunodeficiency virus type 1 (HIV-1) infections in alcoholics. Front Biosci. 2002;7:e286-e300. |
| 53. | Poynard T, Ratziu V, Benhamou Y, Opolon P, Cacoub P, Bedossa P. Natural history of HCV infection. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:211-228. |
| 54. | Mazzella G, Accogli E, Sottili S, Festi D, Orsini M, Salzetta A, Novelli V, Cipolla A, Fabbri C, Pezzoli A. Alpha interferon treatment may prevent hepatocellular carcinoma in HCV-related liver cirrhosis. J Hepatol. 1996;24:141-147. |
| 55. | Monto A, Patel K, Bostrom A, Pianko S, Pockros P, McHutchison JG, Wright TL. Risks of a range of alcohol intake on hepatitis C-related fibrosis. Hepatology. 2004;39:826-834. |
| 56. | Delarocque-Astagneau E, Roudot-Thoraval F, Campese C, Desenclos JC. The Hepatitis C Surveillance System Steering Committee. Past excessive alcohol consumption: a major determinant of severe liver disease among newly referred hepatitis C virus infected patients in hepatology reference centers, France, 2001. Ann Epidemiol. 2005;15:551-557. |
| 57. | Tabone M, Sidoli L, Laudi C, Pellegrino S, Rocca G, Della Monica P, Fracchia M, Galatola G, Molinaro GC, Arico S. Alcohol abstinence does not offset the strong negative effect of lifetime alcohol consumption on the outcome of interferon therapy. J Viral Hepat. 2002;9:288-294. |
| 58. | Rifai MA, Moles JK, Lehman LP, Van der Linden BJ. Hepatitis C screening and treatment outcomes in patients with substance use/dependence disorders. Psychosomatics. 2006;47:112-121. |
| 59. | Westin J, Lagging LM, Spak F, Aires N, Svensson E, Lindh M, Dhillon AP, Norkrans G, Wejstal R. Moderate alcohol intake increases fibrosis progression in untreated patients with hepatitis C virus infection. J Viral Hepat. 2002;9:235-241. |
| 60. | Hezode C, Lonjon I, Roudot-Thoraval F, Pawlotsky JM, Zafrani ES, Dhumeaux D. Impact of moderate alcohol consumption on histological activity and fibrosis in patients with chronic hepatitis C, and specific influence of steatosis: a prospective study. Aliment Pharmacol Ther. 2003;17:1031-1037. |
| 61. | Matsuda Y, Amuro Y, Higashino K, Hada T, Yamamoto T, Fujikura M, Yamaguchi K, Shimomura S, Iijima H, Nakano T. Relation between markers for viral hepatitis and clinical features of Japanese patients with hepatocellular carcinoma: possible role of alcohol in promoting carcinogenesis. Hepatogastroenterology. 1995;42:151-154. |
| 62. | Kuwana K, Ichida T, Kamimura T, Ohkoshi S, Ogata N, Harada T, Endoh K, Asakura H. Risk factors and the effect of interferon therapy in the development of hepatocellular carcinoma: a multivariate analysis in 343 patients. J Gastroenterol Hepatol. 1997;12:149-155. |
| 63. | Tagger A, Donato F, Ribero ML, Chiesa R, Portera G, Gelatti U, Albertini A, Fasola M, Boffetta P, Nardi G. Case-control study on hepatitis C virus (HCV) as a risk factor for hepatocellular carcinoma: the role of HCV genotypes and the synergism with hepatitis B virus and alcohol. Brescia HCC Study. Int J Cancer. 1999;81:695-699. |
| 64. | Aizawa Y, Shibamoto Y, Takagi I, Zeniya M, Toda G. Analysis of factors affecting the appearance of hepatocellular carcinoma in patients with chronic hepatitis C. A long term follow-up study after histologic diagnosis. Cancer. 2000;89:53-59. |
| 65. | Kwon SY, Ahn MS, Chang HJ. Clinical significance of hepatitis C virus infection to alcoholics with cirrhosis in Korea. J Gastroenterol Hepatol. 2000;15:1282-1286. |
| 66. | Khan KN, Yatsuhashi H. Effect of alcohol consumption on the progression of hepatitis C virus infection and risk of hepatocellular carcinoma in Japanese patients. Alcohol Alcohol. 2000;35:286-295. |
| 67. | Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, Decarli A, Trevisi P, Ribero ML, Martelli C. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323-331. |
| 68. | Hassan MM, Hwang LY, Hatten CJ, Swaim M, Li D, Abbruzzese JL, Beasley P, Patt YZ. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206-1213. |
| 69. | Plumlee CR, Lazaro CA, Fausto N, Polyak SJ. Effect of ethanol on innate antiviral pathways and HCV replication in human liver cells. Virol J. 2005;2:89. |
| 70. | Takahashi K, Takahashi T, Takahashi S, Watanabe K, Boku S, Matsui S, Arai F, Asakura H. Difference in quasispecies of the hypervariable region 1 of hepatitis C virus between alcoholic and non-alcoholic patients. J Gastroenterol Hepatol. 2001;16:416-423. |
| 71. | Hardardottir I, Doerrler W, Feingold KR, Grunfeld C. Cytokines stimulate lipolysis and decrease lipoprotein lipase activity in cultured fat cells by a prostaglandin independent mechanism. Biochem Biophys Res Commun. 1992;186:237-243. |
| 72. | Feingold KR, Grunfeld C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J Clin Invest. 1987;80:184-190. |
| 73. | Nachiappan V, Curtiss D, Corkey BE, Kilpatrick L. Cytokines inhibit fatty acid oxidation in isolated rat hepatocytes: synergy among TNF, IL-6, and IL-1. Shock. 1994;1:123-129. |
| 74. | Navasa M, Gordon DA, Hariharan N, Jamil H, Shigenaga JK, Moser A, Fiers W, Pollock A, Grunfeld C, Feingold KR. Regulation of microsomal triglyceride transfer protein mRNA expression by endotoxin and cytokines. J Lipid Res. 1998;39:1220-1230. |
| 75. | Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605-G611. |
| 76. | Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467-1476. |
| 77. | Kishore R, McMullen MR, Nagy LE. Stabilization of tumor necrosis factor alpha mRNA by chronic ethanol: role of A + U-rich elements and p38 mitogen-activated protein kinase signaling pathway. J Biol Chem. 2001;276:41930-41937. |
| 78. | Shi L, Kishore R, McMullen MR, Nagy LE. Chronic ethanol increases lipopolysaccharide-stimulated Egr-1 expression in RAW 264.7 macrophages: contribution to enhanced tumor necrosis factor alpha production. J Biol Chem. 2002;277:14777-14785. |
| 79. | Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005-L1028. |
| 80. | Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247-254. |
| 81. | Thakur V, Pritchard MT, McMullen MR, Nagy LE. Adiponectin normalizes LPS-stimulated TNF-alpha production by rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2006;290:G998-G1007. |
| 82. | Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91-100. |
| 83. | You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568-577. |
| 84. | Anania FA, Potter JJ, Rennie-Tankersley L, Mezey E. Activation by acetaldehyde of the promoter of the mouse alpha2(I) collagen gene when transfected into rat activated stellate cells. Arch Biochem Biophys. 1996;331:187-193. |
| 85. | Chen A. Acetaldehyde stimulates the activation of latent transforming growth factor-beta1 and induces expression of the type II receptor of the cytokine in rat cultured hepatic stellate cells. Biochem J. 2002;368:683-693. |
| 86. | Schulze-Krebs A, Preimel D, Popov Y, Bartenschlager R, Lohmann V, Pinzani M, Schuppan D. Hepatitis C virus-replicating hepatocytes induce fibrogenic activation of hepatic stellate cells. Gastroenterology. 2005;129:246-258. |
| 87. | Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599-612. |
| 88. | Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc. 2006;65:278-290. |
| 89. | Frank A, Seitz HK, Bartsch H, Frank N, Nair J. Immunohistochemical detection of 1,N6-ethenodeoxyadenosine in nuclei of human liver affected by diseases predisposing to hepato-carcinogenesis. Carcinogenesis. 2004;25:1027-1031. |
| 90. | Wang Y, Millonig G, Nair J, Patsenker E, Stickel F, Mueller S, Bartsch H, Seitz HK. Ethanol-induced cytochrome P4502E1 causes carcinogenic etheno-DNA lesions in alcoholic liver disease. Hepatology. 2009;25:Epub ahead of print. |
| 91. | Barbaro G, Di Lorenzo G, Asti A, Ribersani M, Belloni G, Grisorio B, Filice G, Barbarini G. Hepatocellular mitochondrial alterations in patients with chronic hepatitis C: ultrastructural and biochemical findings. Am J Gastroenterol. 1999;94:2198-2205. |
| 92. | Valgimigli M, Valgimigli L, Trere D, Gaiani S, Pedulli GF, Gramantieri L, Bolondi L. Oxidative stress EPR measurement in human liver by radical-probe technique. Correlation with etiology, histology and cell proliferation. Free Radic Res. 2002;36:939-948. |
| 93. | Wheeler MD, Kono H, Yin M, Nakagami M, Uesugi T, Arteel GE, Gabele E, Rusyn I, Yamashina S, Froh M. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544-1549. |
| 94. | Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778-790. |
| 95. | Hoek JB, Pastorino JG. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol. 2002;27:63-68. |
| 96. | Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366-375. |
| 97. | Gong G, Waris G, Tanveer R, Siddiqui A. Human hepatitis C virus NS5A protein alters intracellular calcium levels, induces oxidative stress, and activates STAT-3 and NF-kappa B. Proc Natl Acad Sci USA. 2001;98:9599-9604. |
| 98. | Whitfield JB, Zhu G, Heath AC, Powell And LW, Martin NG. Effects of alcohol consumption on indices of iron stores and of iron stores on alcohol intake markers. Alcohol Clin Exp Res. 2001;25:1037-1045. |
| 99. | Oneta CM, Lieber CS, Li J, Ruttimann S, Schmid B, Lattmann J, Rosman AS, Seitz HK. Dynamics of cytochrome P4502E1 activity in man: induction by ethanol and disappearance during withdrawal phase. J Hepatol. 2002;36:47-52. |
| 100. | Tavill AS, Qadri AM. Alcohol and iron. Semin Liver Dis. 2004;24:317-325. |
| 101. | Bradford BU, Kono H, Isayama F, Kosyk O, Wheeler MD, Akiyama TE, Bleye L, Krausz KW, Gonzalez FJ, Koop DR. Cytochrome P450 CYP2E1, but not nicotinamide adenine dinucleotide phosphate oxidase, is required for ethanol-induced oxidative DNA damage in rodent liver. Hepatology. 2005;41:336-344. |
| 102. | Zhuge J, Cederbaum AI. Increased toxicity by transforming growth factor-beta 1 in liver cells overexpressing CYP2E1. Free Radic Biol Med. 2006;41:1100-1112. |
| 103. | Gebhardt AC, Lucas D, Menez JF, Seitz HK. Chlormethiazole inhibition of cytochrome P450 2E1 as assessed by chlorzoxazone hydroxylation in humans. Hepatology. 1997;26:957-961. |
| 104. | Spolarics Z. Endotoxemia, pentose cycle, and the oxidant/antioxidant balance in the hepatic sinusoid. J Leukoc Biol. 1998;63:534-541. |
| 105. | Kono H, Rusyn I, Yin M, Gabele E, Yamashina S, Dikalova A, Kadiiska MB, Connor HD, Mason RP, Segal BH. NADPH oxidase-derived free radicals are key oxidants in alcohol-induced liver disease. J Clin Invest. 2000;106:867-872. |
| 106. | Oliver J, Agundez JA, Morales S, Fernandez-Arquero M, Fernandez-Gutierrez B, de la Concha EG, Diaz-Rubio M, Martin J, Ladero JM. Polymorphisms in the transforming growth factor-beta gene (TGF-beta) and the risk of advanced alcoholic liver disease. Liver Int. 2005;25:935-939. |
| 107. | Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248-258. |
| 108. | Farinati F, Cardin R, De Maria N, Della Libera G, Marafin C, Lecis E, Burra P, Floreani A, Cecchetto A, Naccarato R. Iron storage, lipid peroxidation and glutathione turnover in chronic anti-HCV positive hepatitis. J Hepatol. 1995;22:449-456. |
| 109. | Moriya K, Nakagawa K, Santa T, Shintani Y, Fujie H, Miyoshi H, Tsutsumi T, Miyazawa T, Ishibashi K, Horie T. Oxidative stress in the absence of inflammation in a mouse model for hepatitis C virus-associated hepatocarcinogenesis. Cancer Res. 2001;61:4365-4370. |
| 110. | Ioannou GN, Dominitz JA, Weiss NS, Heagerty PJ, Kowdley KV. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology. 2004;126:1293-1301. |
| 111. | Chapoutot C, Esslimani M, Joomaye Z, Ramos J, Perney P, Laurent C, Fabbro-Peray P, Larrey D, Domergue J, Blanc F. Liver iron excess in patients with hepatocellular carcinoma developed on viral C cirrhosis. Gut. 2000;46:711-714. |
| 112. | Smith BC, Gorve J, Guzail MA, Day CP, Daly AK, Burt AD, Bassendine MF. Heterozygosity for hereditary hemochromatosis is associated with more fibrosis in chronic hepatitis C. Hepatology. 1998;27:1695-1699. |
| 113. | Lefkowitch JH, Yee HT, Sweeting J, Green PH, Magun AM. Iron-rich foci in chronic viral hepatitis. Hum Pathol. 1998;29:116-118. |
| 114. | Niederau C, Fischer R, Sonnenberg A, Stremmel W, Trampisch HJ, Strohmeyer G. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N Engl J Med. 1985;313:1256-1262. |
| 115. | Huang H, Fujii H, Sankila A, Mahler-Araujo BM, Matsuda M, Cathomas G, Ohgaki H. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol. 1999;155:1795-1801. |
| 116. | Green R, Esparza I, Schreiber R. Iron inhibits the nonspecific tumoricidal activity of macrophages. A possible contributory mechanism for neoplasia in hemochromatosis. Ann N Y Acad Sci. 1988;526:301-309. |
| 117. | Deugnier Y, Turlin B. Iron and hepatocellular carcinoma. J Gastroenterol Hepatol. 2001;16:491-494. |
| 118. | Hagist S, Sultmann H, Millonig G, Hebling U, Kieslich D, Kuner R, Balaguer S, Seitz HK, Poustka A, Mueller S. In vitro-targeted gene identification in patients with hepatitis C using a genome-wide microarray technology. Hepatology. 2009;49:378-386. |
| 119. | Harrison-Findik DD, Schafer D, Klein E, Timchenko NA, Kulaksiz H, Clemens D, Fein E, Andriopoulos B, Pantopoulos K, Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J Biol Chem. 2006;281:22974-22982. |
| 120. | Kohgo Y, Ohtake T, Ikuta K, Suzuki Y, Hosoki Y, Saito H, Kato J. Iron accumulation in alcoholic liver diseases. Alcohol Clin Exp Res. 2005;29:189S-193S. |
| 121. | Rost D, Welker A, Welker J, Millonig G, Berger I, Autschbach F, Schuppan D, Mueller S. Liver-homing of purified glucose oxidase: a novel in vivo model of physiological hepatic oxidative stress (H2O2). J Hepatol. 2007;46:482-491. |
| 122. | Mueller S. Sensitive and nonenzymatic measurement of hydrogen peroxide in biological systems. Free Radic Biol Med. 2000;29:410-415. |
| 123. | Mueller S, Pantopoulos K, Hubner CA, Stremmel W, Hentze MW. IRP1 activation by extracellular oxidative stress in the perfused rat liver. J Biol Chem. 2001;276:23192-23196. |
| 124. | Andriopoulos B, Hegedusch S, Mangin J, Riedel HD, Hebling U, Wang J, Pantopoulos K, Mueller S. Sustained hydrogen peroxide induces iron uptake by transferrin receptor-1 independent of the iron regulatory protein/iron-responsive element network. J Biol Chem. 2007;282:20301-20308. |
| 125. | Liang TJ, Heller T. Pathogenesis of hepatitis C-associated hepatocellular carcinoma. Gastroenterology. 2004;127:S62-S71. |
| 126. | Lerat H, Honda M, Beard MR, Loesch K, Sun J, Yang Y, Okuda M, Gosert R, Xiao SY, Weinman SA. Steatosis and liver cancer in transgenic mice expressing the structural and nonstructural proteins of hepatitis C virus. Gastroenterology. 2002;122:352-365. |
| 127. | Disson O, Haouzi D, Desagher S, Loesch K, Hahne M, Kremer EJ, Jacquet C, Lemon SM, Hibner U, Lerat H. Impaired clearance of virus-infected hepatocytes in transgenic mice expressing the hepatitis C virus polyprotein. Gastroenterology. 2004;126:859-872. |
| 128. | Moriya K, Yotsuyanagi H, Shintani Y, Fujie H, Ishibashi K, Matsuura Y, Miyamura T, Koike K. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J Gen Virol. 1997;78:1527-1531. |
| 129. | Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065-1067. |
| 130. | Pigeon C, Turlin B, Iancu TC, Leroyer P, Le Lan J, Deugnier Y, Brissot P, Loreal O. Carbonyl-iron supplementation induces hepatocyte nuclear changes in BALB/CJ male mice. J Hepatol. 1999;30:926-934. |
| 131. | Carthew P, Dorman BM, Edwards RE, Francis JE, Smith AG. A unique rodent model for both the cardiotoxic and hepatotoxic effects of prolonged iron overload. Lab Invest. 1993;69:217-222. |
| 132. | Zhou XY, Tomatsu S, Fleming RE, Parkkila S, Waheed A, Jiang J, Fei Y, Brunt EM, Ruddy DA, Prass CE. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proc Natl Acad Sci USA. 1998;95:2492-2497. |
| 133. | Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090-2093. |
| 134. | Laftah AH, Sharma N, Brookes MJ, McKie AT, Simpson RJ, Iqbal TH, Tselepis C. Tumour necrosis factor alpha causes hypoferraemia and reduced intestinal iron absorption in mice. Biochem J. 2006;397:61-67. |
| 135. | Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271-1276. |
| 136. | Mueller S. Iron regulatory protein 1 as a sensor of reactive oxygen species. Biofactors. 2005;24:171-181. |
| 137. | Theurl I, Zoller H, Obrist P, Datz C, Bachmann F, Elliott RM, Weiss G. Iron regulates hepatitis C virus translation via stimulation of expression of translation initiation factor 3. J Infect Dis. 2004;190:819-825. |
| 138. | Fillebeen C, Caltagirone A, Martelli A, Moulis JM, Pantopoulos K. IRP1 Ser-711 is a phosphorylation site, critical for regulation of RNA-binding and aconitase activities. Biochem J. 2005;388:143-150. |
| 139. | Sievert W, Pianko S, Warner S, Bowden S, Simpson I, Bowden D, Locarnini S. Hepatic iron overload does not prevent a sustained virological response to interferon-alpha therapy: a long term follow-up study in hepatitis C-infected patients with beta thalassemia major. Am J Gastroenterol. 2002;97:982-987. |
| 140. | Kato J, Kobune M, Nakamura T, Kuroiwa G, Takada K, Takimoto R, Sato Y, Fujikawa K, Takahashi M, Takayama T. Normalization of elevated hepatic 8-hydroxy-2’-deoxyguanosine levels in chronic hepatitis C patients by phlebotomy and low iron diet. Cancer Res. 2001;61:8697-8702. |
| 141. | Corrao G, Arico S. Independent and combined action of hepatitis C virus infection and alcohol consumption on the risk of symptomatic liver cirrhosis. Hepatology. 1998;27:914-919. |