Published online Jul 21, 2009. doi: 10.3748/wjg.15.3434
Revised: June 14, 2009
Accepted: June 21, 2009
Published online: July 21, 2009
Metastatic disease from cutaneous melanoma can affect all organs of the body, and varies in its biological behavior and clinical presentation. We present the case of a 58-year-old man who arrived at our clinic with acute abdominal pain, which, after investigation, was diagnosed as acute cholecystitis. The patient underwent laparotomy and cholecystectomy. Two years ago, he underwent surgical removal of a primary cutaneous melanoma on his right upper back. Pathological examination revealed the presence of malignant melanoma with a metastatic lesion of the gallbladder.
- Citation: Vernadakis S, Rallis G, Danias N, Serafimidis C, Christodoulou E, Troullinakis M, Legakis N, Peros G. Metastatic melanoma of the gallbladder: An unusual clinical presentation of acute cholecystitis. World J Gastroenterol 2009; 15(27): 3434-3436
- URL: https://www.wjgnet.com/1007-9327/full/v15/i27/3434.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3434
Malignant cutaneous melanoma can metastasize to virtually any organ. The most common sites of metastatic disease, apart from the regional lymph nodes, are the lungs, liver, brain and colon[12]. Metastases of the biliary tract are rare. Autopsy studies have shown that gallbladder metastases are found in 15% of patients with metastatic melanoma of the gastrointestinal tract, but are reported rarely during the lifetime of the patient because involvement of the gallbladder seldom produces symptoms[3].
We report a case of metastatic melanoma of the gallbladder in a patient who developed symptomatic acute cholecystitis. The patient underwent explorative laparotomy and open cholecystectomy. Despite appropriate therapy, the diagnosis of this condition portends a poor prognosis, with very few patients surviving more than 12 mo[45].
A 58-year-old man was admitted to our clinic with abdominal pain in his right upper quadrant, and associated nausea and vomiting. On examination, he was febrile and there was tenderness upon palpation of the right upper abdominal quadrant and the epigastrium. Laboratory tests were within normal limits (white blood cell count 10 600/mm3, normal liver enzymes and normal serum amylase). an ultrasound scan demonstrated sludge in the gallbladder and thickening of the gallbladder wall, without dilatation of the intra- and extrahepatic biliary tree. The patient had undergone a Billroth II operation via median laparotomy for gastric ulcer disease 15 years previously.
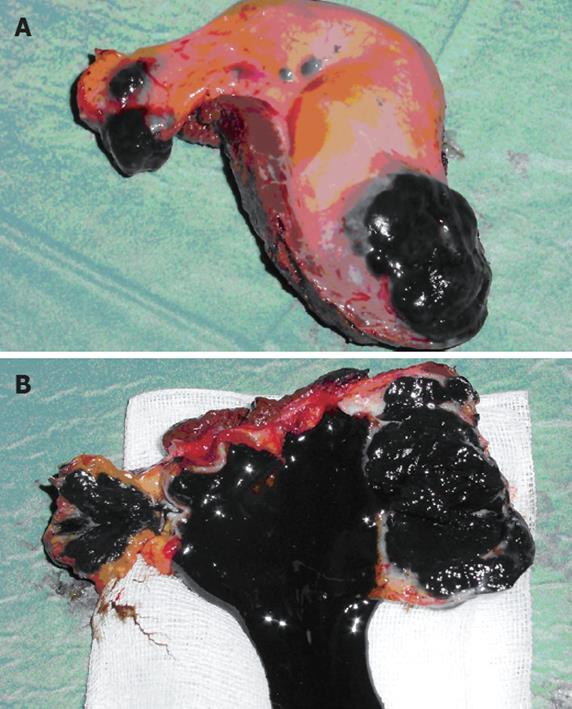
A presumptive diagnosis of acute cholecystitis was made. An exploratory laparotomy and cholecystectomy was performed. Intraoperatively, the gallbladder was shown macroscopically to be acutely inflamed and thickened. Examination of the surgical specimen showed an ulcerated black mass in the fundus and the cystic duct of the gallbladder, and the lumen was filled with black sludge and necrotic material (Figure 1).
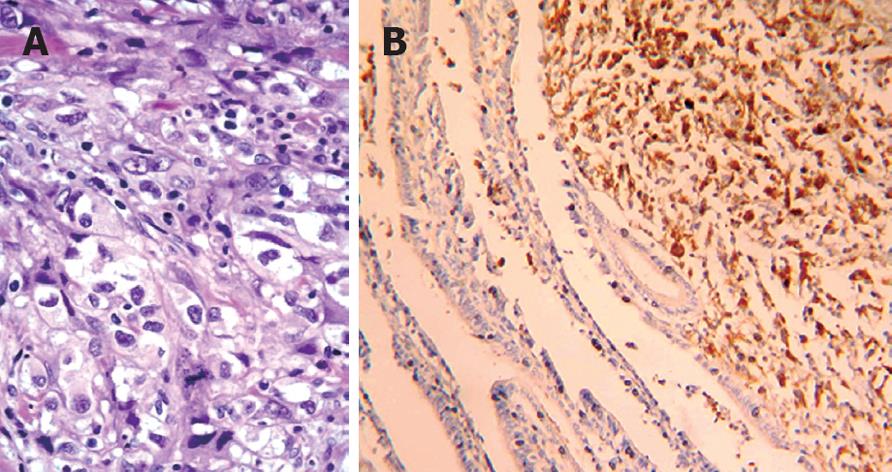
Pathological examination revealed acute cholecystitis and the presence of a malignant tumor invading through the gallbladder wall, with extensive invasion of lymphatic vessels and fat adipose tissue around the gallbladder. Its morphologic morphological appearance and immunohistological profile[6], corresponded to a metastatic focus of a cutaneous malignant melanoma with high cellular polymorphism and melanin production (Figure 2).
The patient had undergone surgical resection of a malignant melanoma (superficial spreading type, Clark anatomical level III, with a depth of infiltration of 3.8 mm by Breslow) 2 years ago on his right upper back. Adjuvant immunological therapy with interferon was performed at that time.
On postoperative day 7, the patient was discharged home in good condition. Four weeks later, his course was complicated by the development of increasing neurological symptoms. On computer tomography investigation, he was found to have brain metastases. The primary tumor was probably the cutaneous malignant melanoma. He received two courses of combination chemotherapy with a dacarbazine (DTIC) regimen, but the disease continued to progress. He received two cycles of chemotherapy with temozolomide, with no success.
The patient died of widespread disease 5 mo after the initial diagnosis of the metastatic disease.
Metastases of cutaneous melanoma to the gallbladder and biliary tree are uncommon and usually clinically asymptomatic. The most common sites of distant metastases are the skin, lungs, liver and brain[278]. According to autopsy results, such asymptomatic metastases to the gallbladder and bile ducts occur in 4%-20% of patients with metastatic melanoma[389], and constitute up to 50% of the metastatic neoplasms of these organs[24].
Despite these statistical data, it is rare for metastatic gallbladder melanoma to be present with symptoms during the patient’s life time[489], as demonstrated by the discrepancy in published case reports and the rate of detection at autopsy[34810].
The most common presentation of symptomatic metastatic disease to the gallbladder is acute cholecystitis followed by jaundice caused by obstruction of the common bile duct[48]. Also hematobilia and biliary fistulae may develop[4811].
The examination of choice for the assessment of gallbladder metastatic tumors is ultrasound scanning, an examination with high specificity and sensitivity[10]. In particular, the use of color Doppler ultrasound can support a preoperative differential diagnosis of malignancy, because of the presence of pathological blood flow within the lesion in patients with a past medical history of cutaneous malignant melanoma[2412].
The role of surgical treatment of metastatic melanoma of the gallbladder remains unclear because of the lack of experience[2]. Goals of therapy include palliation of symptoms, minimization of complications and improvement in overall survival. Recently, cholecystectomy has been indicated for patients with isolated, resectable gallbladder metastases, and can achieve longer survival[24]. As an alternative to open surgery, the laparoscopic approach has arisen as a minimally invasive option[589].
From the literature, the prognosis of metastatic melanoma to the gallbladder is dismal, with a median survival of 6-9 mo[248] and long-term disease-free survival is achieved in only 1%-2% of patients[2]. Once involvement of the gallbladder is documented, it is probable that the metastases are widespread.
Despite the advances made in chemotherapy[13] and immunotherapy[1415] for metastatic melanoma, the results are still poor and disappointing[248].
In conclusion, we report this case to make clear the necessity of meticulous investigation of lesions of the hepatobiliary system when the patient has a past history of cutaneous malignant melanoma. The best chance for achieving the longest survival is active screening and early detection. In addition, diligence and vigilance are required to ensure that patients adhere to current screening guidelines. Active follow-up is necessary to identify metachronous lesions early.
| 1. | Meyers MO, Frey DJ, Levine EA. Pancreaticoduodenectomy for melanoma metastatic to the duodenum: a case report and review of the literature. Am Surg. 1998;64:1174-1176. |
| 2. | Gogas J, Mantas D, Gogas H, Kouskos E, Markopoulos C, Vgenopoulou S. Metastatic melanoma in the gallbladder: report of a case. Surg Today. 2003;33:135-137. |
| 3. | Das Gupta TK, Brasfield RD. Metastatic melanoma : a clinicopathologic study. Cancer. 1964;17:1323-1339. |
| 4. | Dong XD, DeMatos P, Prieto VG, Seigler HF. Melanoma of the gallbladder: a review of cases seen at Duke University Medical Center. Cancer. 1999;85:32-39. |
| 5. | Kohler U, Jacobi T, Sebastian G, Nagel M. [Laparoscopic cholecystectomy in isolated gallbladder metastasis of malignant melanoma]. Chirurg. 2000;71:1517-1520. |
| 6. | Gassler N, Banafsche N, Quentmeier A, Otto HF, Helmke BM. [Secondary malignant melanoma of the gallbladder. A contribution to the differential diagnosis of pigmented lesions of the gallbladder]. Pathologe. 2004;25:155-159. |
| 7. | Balch CM, Houghton AM. Diagnosis of metastatic melanoma at distant sites. Cutaneous Melanoma. 2nd ed. Philadelphia: Lippincott 1992; 439-467. |
| 8. | Langley RG, Bailey EM, Sober AJ. Acute cholecystitis from metastatic melanoma to the gall-bladder in a patient with a low-risk melanoma. Br J Dermatol. 1997;136:279-282. |
| 9. | Cellerino P, Corsi F, Morandi E, Foschi D, Trabucchi E. Metastatic melanoma of the gallbladder. Eur J Surg Oncol. 2000;26:815-816. |
| 10. | Goldin EG. Malignant melanoma metastatic to the gallbladder. Case report and review of the literature. Am Surg. 1990;56:369-373. |
| 11. | Daunt N, King DM. Metastatic melanoma in the biliary tree. Br J Radiol. 1982;55:873-874. |
| 12. | Avila NA, Shawker TH, Fraker D. Color-flow Doppler ultrasonography in metastatic melanoma of the gallbladder. J Clin Ultrasound. 1994;22:342-347. |
| 13. | Del Prete SA, Maurer LH, O’Donnell J, Forcier RJ, LeMarbre P. Combination chemotherapy with cisplatin, carmustine, dacarbazine, and tamoxifen in metastatic melanoma. Cancer Treat Rep. 1984;68:1403-1405. |
| 14. | Atkins MB. Immunotherapy and experimental approaches for metastatic melanoma. Hematol Oncol Clin North Am. 1998;12:877-902, viii. |
| 15. | Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105-2116. |