Published online Jul 21, 2009. doi: 10.3748/wjg.15.3367
Revised: June 6, 2009
Accepted: June 13, 2009
Published online: July 21, 2009
AIM: To determine whether magnetic resonance imaging (MRI) can be used to categorize small bowel Crohn’s disease (SB CD) into groups that correlate with response to medical therapy and surgical pathology.
METHODS: Data was collected from all patients with MRI evidence of SB CD without significant colonic disease over a 32-mo period. Two radiologists, blinded to clinical findings, evaluated each MRI and grouped them based on bowel wall thickness and wall enhancement. These categories were: (1) “fibrosis”, (2) “mild segmental hyper-enhancement and mild wall thickening”, (3) “mild segmental hyper-enhancement and marked wall thickening”, (4) “marked segmental transmural hyper-enhancement”. Patient response to additional medical therapy post-MRI was prospectively determined at 8-wk. Non-responders underwent endoscopy and were offered therapeutic endoscopy or surgery. Surgical pathology was assessed against the MRI category.
RESULTS: Fifty-five patients were included. Females and category “2” patients were more likely, and patients with luminal narrowing and hold-up less likely, to respond to medical therapy (P < 0.05). Seventeen patients underwent surgery. The surgical pathological findings of fibrosis and the severity of inflammation correlated with the MRI category in all cases.
CONCLUSION: Our findings suggest that SB CD can be grouped by the MRI findings and that these groups are associated with patients more likely to respond to continued medical therapy. The MRI categories also correlated with the presence and level of intestinal inflammation and fibrosis on surgical pathology, and may be of prognostic use in the management of CD patients.
- Citation: Lawrance IC, Welman CJ, Shipman P, Murray K. Correlation of MRI-determined small bowel Crohn’s disease categories with medical response and surgical pathology. World J Gastroenterol 2009; 15(27): 3367-3375
- URL: https://www.wjgnet.com/1007-9327/full/v15/i27/3367.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3367
Crohn’s disease (CD) is a life-long, chronic, relapsing condition. Morbidity is high and its incidence and prevalence are increasing worldwide[1]. Despite the development of new medical therapies, there is little evidence that they alter the natural history of CD. Due to the transmural nature of CD, fibrosis, stenosis and obstruction often result[2], with fibrosis and stricture formation still the most common reason for intestinal resection.
CD frequently involves the terminal ileum (TI) so investigation of the small bowel (SB) is of the utmost importance. Unfortunately, TI assessment by endoscopy is not optimal, with a significant proportion of colonoscopic examinations failing to reach the cecum[3]. The mainstay of SB investigation has been SB enterography or SB enteroclysis (SBE) that requires placement of a nasojejunal tube (NJT). More recently CT enteroclysis (CTE), wireless capsule enteroscopy (WCE), double balloon enteroscopy, MR SB enteroclysis (MRE) and MR enterography have become available. Conventional radiology (SBE and SB enterography) only has a sensitivity of between 23% and 80% for the detection of typical CD radiological lesions[4–6]. It delivers ionising radiation and is suboptimal for the assessment of extra-intestinal involvement and complications. WCE on the other hand allows for excellent visualization of the SB mucosa, but its specificity is lower than other methods[6], it does not clearly localise the lesions, histology cannot be taken, and there is a small but definite capsule retention rate contraindicating its use in SB strictures[7].
CTE has been well described[8], has good sensitivity (71%-95%) and impressive specificity (90%-98%) and is superior to conventional enteroclysis[9]. The use of ionising radiation in CTE in a generally young patient cohort, however, is not ideal. CTE also lacks functional information, has poor fluoroscopic control of SB filling and suboptimal soft tissue contrast[610–12]. MRI also has high sensitivity and specificity in the evaluation of CD[13] and while it does not provide as consistently good mucosal detail as conventional enteroclysis, it has a strong correlation with pathologic findings and does not use ionising radiation[14–16]. MRI also demonstrates good soft tissue contrast, subtle degrees of contrast enhancement and conveys functional information, while potentially differentiating active inflammation from fibrosis in preliminary studies[1617]. A retrospective paper correlating CTE findings with surgical pathology in 36 patients accurately detected strictures, fistulas, abscesses and inflammatory masses in 94% of patients[18]. CTE was deemed to provide accurate preoperative diagnosis for SB CD. As MRI potentially has the ability to differentiate inflammation from fibrosis, the question arises as to whether MR imaging could be used to predict patient response to continued medical therapy and the need for surgery?
The hypothesis of this study was that SB enhancement, bowel wall thickness and other findings on MRI could categorise CD patients and that these categories could be used to identify patients who were more likely to respond to continuing medical therapy. It was also hypothesised that these categories would correlate with the presence and level of intestinal inflammation and fibrosis identified on surgical pathology.
All subjects were patients of the Centre for Inflammatory Bowel Diseases, Fremantle Hospital; a specialist inflammatory bowel disease (IBD) unit in a 450-bed tertiary institution. All patients had previously been confirmed as having CD on previous endoscopic examination with histological confirmation. Data was collected from all CD patients with SB disease undergoing MRI enterography or MRE over a 32-mo period. Any patient with a contraindication to the intravenous use of gadodiamide was excluded. Response to medical therapy was determined prospectively.
All patients drank only clear fluids for 6 h prior to their MRI and were nil by mouth for 2 h. Patients either underwent MRE or MR enterography. Patients who underwent an MR enterography (including those patients where placement of the NJT failed for technical reasons or patient refusal) drank 1000 mL of the bowel contrast agent over 20 min. For an MRE, an NJT (Bilbao-Dotter, Cook, Australia) was placed under fluoroscopy. The bowel contrast agent used was a polyethylene glycol-water solution (Glycoprep-C, Pharmatel Fresenius Kabi, Australia), which was injected manually through the NJT (60 mL/min increasing to 120-150 mL/min). A total of 800-2000 mL was usually required to distend the SB to the TI. This varied depending on previous bowel resections.
Patients were imaged supine using a 1.5T MRI system (Avanto SQ, Siemens Medical Solutions, Erlangen, Germany) with a surface array coil providing compression. In patients with an ileostomy, the stoma bag was emptied and a sponge placed between the surface coil and stoma.
A scout image was acquired to ensure adequate coverage and assess retrograde filling of the stomach. SB filling was dynamically assessed using a coronal 150 mm thick single slab T2-weighted (HASTE) fat saturated sequence acquired repeatedly without breath-holding to monitor for retrograde stomach filling and SB distension. These images were combined into a cine loop and used to assess stenotic lesions. If there was doubt as to TI contrast filling, a single breath-hold coronal T2-weighted sequence (HASTE) with a 6 mm slice thickness and 30% gap was obtained.
To reduce bowel peristalsis and prolong SB distension, 10 mg intravenous hyoscine butylbromide (Buscopan, Boehringer Ingleheim, Australia) was given if there were no contraindications. Once there was adequate SB filling, a coronal pre-contrast T1-weighted 3D gradient echo (VIBE) with a 2 mm slice thickness was obtained. Intravenous gadodiamide (Omniscan, Amersham, Australia) was injected (0.2 mL/kg) with post-contrast imaging commencing at 60 s. Post-contrast VIBE sequences were obtained in the coronal and axial planes. The axial plane required 2 overlapping sections covering the upper and lower abdomen.
Further imaging included a coronal steady-state free precession sequence (trueFISP) with a 6 mm slice thickness and 30% gap obtained with and without fat saturation, axial steady-state free precession sequence (trueFISP) with a 6 mm slice thickness and 30% gap obtained without fat saturation in 2 blocks of the upper and lower abdomen, coronal T2-weighted half Fourier single shot turbo spin echo (HASTE) sequence with 6 mm slice thickness and 30% gap, and axial T2-weighted HASTE sequence with a 5 mm slice thickness and 30% gap was obtained in 2 blocks of the upper and lower abdomen. If a site of pathology was identified at an overlap point on the axial images, then a further set of targeted axial trueFISP and HASTE images were obtained.
For MR enterography, an initial single breath-hold coronal T2-weighted sequence (HASTE) with a 6 mm slice thickness and 30% gap was obtained. If there was adequate filling of the TI, buscopan was injected and routine imaging performed as above. With suboptimal filling of the TI, but sufficient bowel contrast proximally, reassessment occurred at 5-min intervals for 15 min. If there was still inadequate filling of SB loops, the patient drank a further 500 mL of contrast.
Each MRI was evaluated by consensus of two radiologists (CW and PS) with experience in both gastrointestinal and MR imaging blinded to clinical findings. Image analysis was performed using a standardised worksheet. Diseased bowel was identified as abnormal bowel wall thickening and abnormal transmural or mucosal enhancement. Bowel wall thickness was assessed as normal (< 3 mm), mildly abnormal (3-6 mm) or markedly abnormal (> 6 mm)[19]. Mucosal enhancement was contrast enhancement localized to the inner layer of the intestinal wall and transmural enhancement was homogenous contrast enhancement of the whole wall. The degree of pathological bowel wall contrast enhancement was assessed as none, mild (less than renal cortical enhancement) or marked (more than renal cortical enhancement) and classified as mucosal, transmural enhancement or both. The degree of enhancement was measured relative to the renal cortex on the coronal acquisition, and axial views were used for problem solving and cross-referencing of bowel loops.
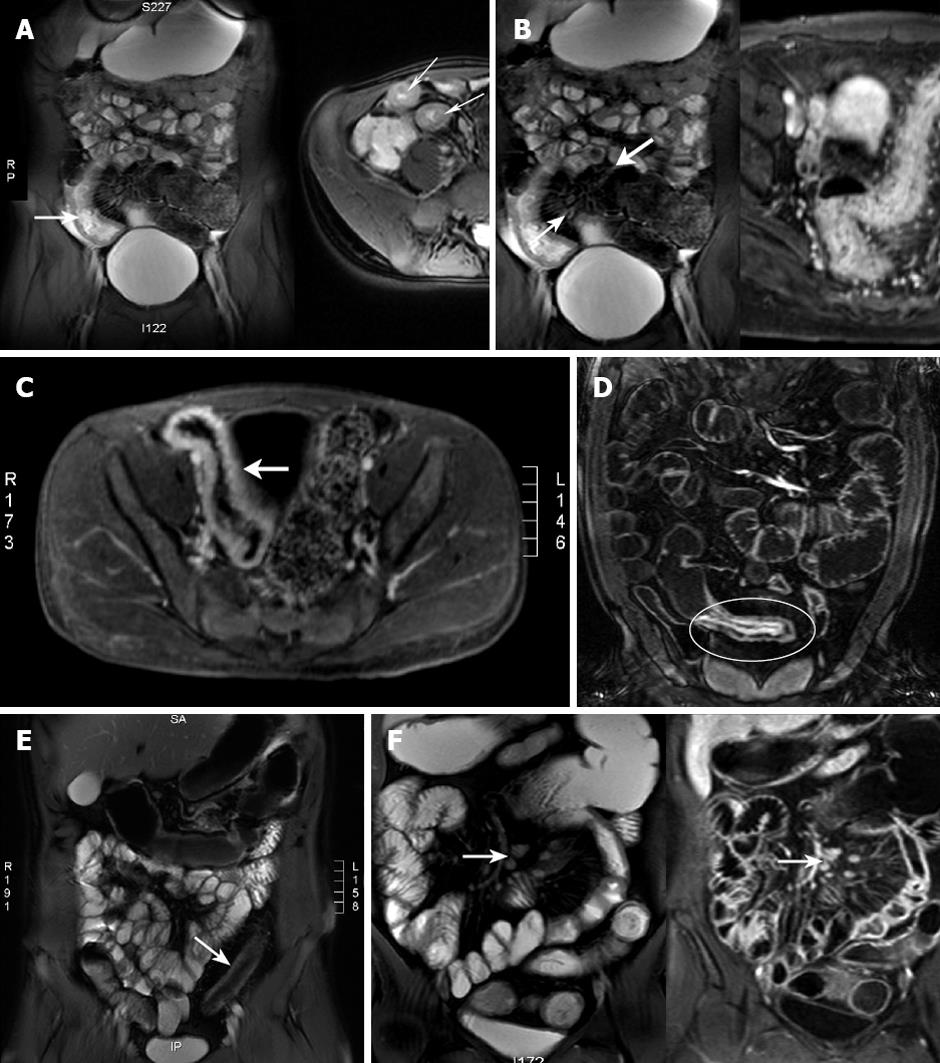
Mesenteric hyperemia, fibrofatty proliferation, enlarged local lymph nodes and/or abnormally enhancing lymph nodes were assessed (Figure 1). Mesenteric nodes < 5 mm in short axis were considered physiological. Larger nodes, especially if clustered and contrast enhancing, were considered pathological[20]. The number of regional nodes and length of diseased segment were noted. Disease complications, including fistulas or abscesses, were recorded, as was free fluid, and stenoses with or without functional obstruction. Signs of functional obstruction were pre-stenotic dilatation and delayed contrast progression on the dynamic HASTE series.
Based on the SB MRI findings, the CD patients were grouped into one of four categories; (1) “fibrosis”, (2) “mild segmental hyper-enhancement and mild wall thickening”, (3) “mild segmental hyper-enhancement and marked wall thickening”, and (4) “marked segmental transmural hyper-enhancement” (Table 1). Studies were classified as “fibrosis” if there was bowel wall thickening without contrast enhancement. The diagnostic confidence of this was increased if the wall thickening had reduced signal intensity on T1- and T2-weighted images and if this region remained of constant calibre. “Marked segmental transmural hyper-enhancement” was diagnosed if there was marked transmural enhancement with increased bowel wall thickness.
| Category 1 | Category 2 | Category 3 | Category 4 | |
| Mucosal enhancement | Nil | Mild | Mild | Mild or marked |
| Transmural enhancement | Nil | Nil or mild | Nil or mild | Marked |
| Wall thickness (mm) | > 3 | < 6 | > 6 | > 3 |
“Mild segmental hyper-enhancement and mild wall thickening” was present if mucosal and/or transmural contrast enhancement was mild with only mild bowel wall thickening (3-6 mm). “Mild segmental hyper-enhancement and marked wall thickening” was present if there was mild mucosal and/or transmural contrast enhancement, but the bowel wall was > 6 mm.
Response to continued medical therapy was assessed prospectively. CD was diagnosed in accordance with previously established international criteria[21] exclusive of infective enterocolitis, Behçet’s disease and microscopic colitis. Response and remission to medical therapy was assessed by the Harvey Bradshaw index (HBI)[22]. A response was a reduction in the HBI of ≥ 3 and remission was defined as a HBI < 5. For patients who had undergone colectomy, physician assessment with endoscopy or imaging was used to assess response/remission. A response was determined after a further 8 wk of medical therapy. If medical therapy failed, then endoscopic or surgical options were offered. Correlation between the MRI findings and response to medical therapy was assessed, and for patients undergoing surgical resection the correlation between surgical pathology and the MRI was also assessed.
Specialist histopathologists reported on the macroscopic appearance and histology of the surgical specimens. They graded the degree of inflammation based on the bowel wall thickness, mesentery appearance, mesenteric lymph nodes, mucosal ulceration and the density and extent of both the acute and chronic inflammatory cell infiltrates, loss of goblet cells, crypt abscesses and architectural distortion. “Mild inflammatory infiltrates with scattered glandular architectural distortion and crypt branching” was termed mild inflammation. “Mucosal ulceration with marked glandular changes, dilated complex crypts, a transmural mixed cellular infiltrate and reactive changes in the mesentery” was termed severe. Fibrosis was determined by the presence of a stricture, the bowel wall thickness and assessment of collagen deposition in the bowel wall.
Logistic regression was undertaken by a statistician (KM) and assessed wall enhancement, degree of enhancement, bowel wall thickness, abscess, fibro-fatty proliferation, fistula, free fluid, length of disease, mesenteric hyperemia, luminal narrowing with and without prestenotic dilatation, regional lymph nodes and enhancement, sex, and age. Variable selection was undertaken retaining effects significant at the 0.05 level, and the significant effects and odds ratios are presented, along with 95% confidence intervals.
Data was collected over a 32-mo period on 55 CD patients who had abnormal SB-MRI findings consistent with SB CD without significant active colonic disease. Three of these patients were asymptomatic despite the abnormal MRI findings and were not included in any further analysis. Of the remaining 52 patients (Table 2), 38.5% were male (20/52) with an average age at diagnosis of 38.5 years (range 14-79 years). Forty-two patients (81%) had disease confined to the small intestine (one patient only suffered from proximal SB disease - L4) and 10 (19%) suffered from ileocolonic CD. Of these 10 patients, 6 had previously undergone a colectomy or proctocolectomy and had ongoing active disease confined to the SB. The remaining 4 patients had ileal inflammation and colonic inflammation confined to the cecum or right colon. A further 14 patients had previously undergone surgery for their CD. Thirteen underwent a terminal ileal resection and a limited right hemicolectomy, with 3 of these patients each having had a further 2 resections of the neoterminal ileum for stricturing disease. The final patient had 3 resections of the mid SB for stenosing CD.
| CD (n = 52) | |
| Gender: male | 38.5% (20/52) |
| Age at diagnosis (yr) | 38.9 ± 16.0 (14-79) |
| A1 ≤ 16 | 11.5% (6/52) |
| A2 17-40 | 55.8% (29/52) |
| A3 > 40 | 32.7% (17/52) |
| Crohn’s disease | |
| L1 Terminal ileum | 78.8% (41/52) |
| L2 Colon | 0% (0/52) |
| L3 Ileocolonic | 19.2% (10/52) |
| L4 Upper GI | 7.7% (4/52) |
| P Perianal | 9.6% (5/52) |
| B1 Non-stricturing | |
| Non-penetrating | 75.0% (39/52) |
| B2 Stricturing | 19.2% (10/52) |
| B3 Penetrating | 5.8% (3/52) |
| Colectomy | 11.5% (6/52) |
| Terminal ileal resection | 25.0% (13/52) |
| Proximal SB resection | 1.9% (1/52) |
Utilising a combination of the maximal SB wall thickness and level of mucosal/mural enhancement, patients were allocated into 1 of 4 categories (Table 1). All the patients in category “4” also demonstrated 2 or more extra-intestinal changes of inflammation (i.e. mesenteric hyperemia, fatty proliferation, enlarged regional lymph nodes, pathological lymph node enhancement or free fluid) indicating a more severe level of inflammation. MRI detection of bowel wall enhancement and its degree, level of bowel wall thickness, as well as the presence of an abscess, fibro-fatty proliferation, mesenteric hyperemia, a fistula, free fluid or enlarged regional lymph nodes are detailed in Table 3. The length of bowel involved and the presence of luminal narrowing with and without prestenotic dilatation, patient age and sex are also presented.
| MRI group (n) | Male (%) | Age (yr) | Bowel length involved (cm) | Wall thickness (mm) | Degree of SB wall enhancement | Mucosal enhancement (%) | Transmural enhancement (%) | Mesenterichyperaemia (%) | Fibrofatty proliferation (%) | Regional lymph nodes (%) | Free fluid (%) | Narrowed lumen no hold-up (%) | Prestenotic dilation ± holdup (%) | Fistula (%) |
| 1 (7) | 28.6 | 37.9 ± 9.4 | 6.3 ± 7.9 | < 3 0% | None 100% | 0 | 0 | 0 | 14.3 | 0 | 14.3 | 57.1 | 14.3 | 0 |
| (23-54) | (1-25) | 3-6 71.4% | Mild 0% | |||||||||||
| > 6 28.6% | Marked 0% | |||||||||||||
| 2 (9) | 22.2 | 32.8 ± 9.2 | 13.8 ±12.3 | < 3 11.1% | None 0% | 100 | 88.9 | 33.3 | 33.3 | 66.6 | 33.3 | 44.4 | 0 | 22.2 |
| (17-78) | (3-30) | 3-6 88.9% | Mild 100% | |||||||||||
| > 6 0% | Marked 0% | |||||||||||||
| 3 (16) | 43.8 | 40.1 ± 16.2 | 16.2 ± 12.0 | < 3 0% | None 0% | 93.8 | 43.8 | 50.0 | 62.5 | 37.5 | 18.8 | 18.8 | 62.5 | 25.0 |
| (14-79) | (2-50) | 3-6 0% | Mild 100% | |||||||||||
| > 6 100% | Marked 0% | |||||||||||||
| 4 (20) | 45.0 | 41 ± 19.2 | 19.2 ± 11.6 | < 3 0% | None 0% | 100 | 90.0 | 75.0 | 75.0 | 85.0 | 35.0 | 35.0 | 35.0 | 20.0 |
| (14-49) | (2-45) | 3-6 10% | Mild 0% | |||||||||||
| > 6 90% | Marked 100% |
The medical therapies received by the patients included the use of steroids (prednisone, budesonide), 5-aminosalicylic acid (5ASA) azathioprine/6-mercaptopurine (AZA/6MP), methotrexate (MTX), antibiotics and anti-TNF alpha therapy (Table 4). Medical therapy was not standardized between patients, but was tailored to the individual patient. Twenty-seven of the 52 patients responded to medical therapy and 2 of these patients responded to conservative non-IBD related medical therapy. One patient refused to take any medical therapy prior to an endoscopic examination. If any patient failed to respond to the continued medical therapy, an endoscopic examination was offered to determine whether there was ongoing active inflammatory disease or if the inflammation was controlled and any stricturing was amenable to endoscopic dilatation.
| MRI group | Steroids | 5ASA | Antibiotics | AZA/6MP | MTX | Anti-TNF α | Response |
| 1 (n = 7) | 57.10 | 57.10 | 28.50 | 85.70 | 0.00 | 42.90 | 14.30 |
| 2 (n = 9) | 55.50 | 88.90 | 0.00 | 22.20 | 33.30 | 44.40 | 100.00 |
| 3 (n = 16) | 81.30 | 68.80 | 6.30 | 50.00 | 0.00 | 12.50 | 43.80 |
| 4 (n = 20) | 70.00 | 70.00 | 5.00 | 40.00 | 0.00 | 35.00 | 50.00 |
| Responders (n = 27) | 63.00 | 63.00 | 11.10 | 44.40 | 3.70 | 25.90 | N/A |
| Non-responders (n = 25) | 76.00 | 80.00 | 4.00 | 48.00 | 8.00 | 36.00 | N/A |
Twenty-five patients failed to respond to medical therapy (2/4 patients with ileal and right sided colonic disease, 3/6 with active SB disease post colectomy and 20/42 with SB disease). No significant differences were detected between the medical therapy received and the response/remission rates overall, or between any of the MRI categories (Table 4), suggesting that despite non-standardized medical therapy, the differences in the medical therapies were not primarily responsible for differences observed in the response rates.
Six patients had previously undergone total colectomy, so clinical response after a further 8 wk of medical therapy was determined by physician assessment and either endoscopic or radiological assessment. Of the remaining 46 patients, the average HBI was 8.7 (range 6-20) prior to medical therapy. Logistic regression identified that females were more likely to respond to medical therapy than males (OR = 5.46, 95% CI = 1.295-23.00, P = 0.021). Patients with intestinal narrowing and prestenotic dilatation with or without hold up were less likely to respond to medical therapy (OR = 7.85, 95% CI 1.73-35.6, P = 0.008). This was primarily due to the lack of response in patients with luminal narrowing and hold up, who were less likely to respond to medical therapy than either patients without luminal narrowing, or those with narrowing and no hold up (P < 0.0001 and P = 0.007 respectively, Table 5). Luminal narrowing was observed in 44.4% (4/9), 81.3% (13/16) and 70% (14/20) of patients in category “2”“3” and “4” respectively, while narrowing also occurred in 71.4% (5/7) of category “1” patients, suggesting that luminal narrowing can occur without the presence of inflammation detected by MRI. No significant differences were detected between response rates and age, the presence of mesenteric hyperemia, fibrofatty proliferation, enlarged local lymph nodes, nodal enhancement, free fluid, length of involved segment, or the presence of fistulas or an abscess.
| MRI group | No narrowed lumen | Narrowed lumen no hold up | Narrowed lumen with hold up |
| 1 | Response 0% (0/2) | Response 25% (1/4) | Response 0% (0/1) |
| n = 7 | No response 100% (2/2) | No response 75% (3/4) | No response 100% (1/1) |
| 2 | Response 100% (5/5) | Response 100% (4/4) | Response N/A |
| n = 9 | No response 0% (0/5) | No response 0% (0/4) | No response N/A |
| 3 | Response 66.7% (2/3) | Response 66.7% (2/3) | Response 40% (4/10) |
| n = 16 | No response 33.3% (1/3) | No response 33.3% (1/3) | No response 60% (6/10) |
| 4 | Response 83.3% (5/6) | Response 57.1% (4/7) | Response 14.3% (1/7) |
| n = 20 | No response 16.7% (1/6) | No response 42.9% (3/7) | No response 85.7% (6/7) |
| Total | Response 75.0% (12/16) | Response 61.1% (11/18) | Response 27.8% (5/18) |
| n = 52 | No response 25.0% (4/16) | No response 38.9% (7/18) | No response 72.2% (7/18) |
No statistical difference was detected in response to continued medical therapy related to bowel wall thickness (Figure 1A, ≤ 6 mm vs > 6 mm). Patients with mild or marked SB intestinal wall enhancement, however, were more likely to respond than those with SB wall abnormalities without enhancement (OR 33.0, 95% CI 2.56-426.7 and OR 15.0, 95% CI 1.22-184, respectively). There was no difference in patient response related to enhancement localized to the mucosal or mural layers compared to enhancement in both layers.
Regarding a separate analysis, controlling for sex, patients in category “2” were more likely to respond to medical therapy when compared to any of the other categories (OR 39.69, P = 0.0014 compared with category “1”; OR 11.56, P = 0.023 compared with category “3”; OR 8.98, P = 0.040 compared with category “4”) and more likely to go into remission compared to patients in category “1” (P = 0.021), with border-line significance for category “3” (P = 0.050), but not when compared to category “4” (P = 0.24). This lack of significance was most likely secondary to low patient numbers. There were no other statistically significant differences between the other 3 groups. Controlling for the category type, females were still more likely to respond than males (OR = 5.91, P = 0.0071).
Twenty-five patients failed to respond to medical therapy and were offered colonoscopic examination to determine whether there was ongoing active inflammatory disease or if there was intestinal stricturing without inflammation that would be amenable to endoscopic dilatation. Six (85.7%) of 7 patients failed medical therapy in category “1”; 1 patient refused colonoscopy and 1 went to surgery due to uncontrolled intestinal bleeding. The remaining 4 patients demonstrated no active colonic or terminal ileal inflammation at colonoscopy, but 3 had an ileal/anastomotic stricture that was endoscopically dilated with resolution of their symptoms. All category “2” patients responded to continued medical therapy. Nine of 16 (56.3%) category “3” patients failed to respond to continued medical therapy. Two of these patients demonstrated no inflammation at colonoscopy and underwent dilatation of an ileal/anastomotic stricture with resolution of their symptoms. A further 4 patients demonstrated inflammation of the ileum alone without a stricture identified and 3 patients did not demonstrate inflammation or stricture in either the colon or terminal ileum. These patients had been noted to have proximal SB CD on MRI. Ten of 20 patients (50%) in category “4” failed to respond to medical therapy. Six patients underwent colonscopy and all demonstrated severe ileal inflammation. Among those remaining, two patients elected to go straight to surgery without a colonoscopic examination, one was not physically fit for an endoscopic procedure and one died from other causes unrelated to his IBD.
Seventeen patients went to surgery (16 after failure of medical therapy and 1 who relapsed after responding to medical therapy; Table 6). Specialist histopathologists assessed the macroscopic appearance and histology of the surgical specimens. The 2 patients in category “1”demonstrated fibrosis macroscopically and microscopically with only minimal or no inflammation detected histopathologically. In conjunction with the endoscopic assessment, 5 of the 6 non-responders in category “1” did not demonstrate histological or endoscopic inflammation, consistent with the MRI findings of fibrosis without active inflammation. The single category “2” patient undergoing surgery demonstrated no fibrosis macroscopically or histologically. Macroscopic and histological fibrosis, however, was present in all 7 category “3” patients undergoing surgery. This MRI category included those patients considered to have mild inflammation but intestinal fibrosis on MRI, and the findings of the surgical pathology indicated that this classification highly correlated with intestinal fibrosis. Of the 8 patients with mild segmental hyper-enhancement on MRI (categories “2” and “3”), 7 demonstrated mild and 1 moderate SB inflammation histologically. Of the 7 category “4” patients undergoing surgery, 3 demonstrated fibrosis macroscopically and histologically. All 7 demonstrated florid SB inflammation. In one patient, the pathology described severe inflammation and fistulas, in combination with submucosal fibrosis without macroscopic fibrosis. All fistulas (n = 4) identified by surgical pathology had previously been identified on SB MRI.
| No. | Sex | Age | MRI assessment | Surgical pathology | |||
| Group | Fistula | Inflammation | Fibrosis | Fistula | |||
| 1 | F | 23 | 1 | No | Minimal | Yes | No |
| 2 | M | 45 | 1 | No | No | Yes | No |
| 3 | F | 39 | 2 | Yes | Mild | No | Yes |
| 4 | M | 17 | 3 | No | Mild | Yes | No |
| 5 | F | 59 | 3 | No | Mild | Yes | No |
| 6 | F | 31 | 3 | No | Mild | Yes | No |
| 7 | M | 18 | 3 | No | Moderate | Yes | No |
| 8 | M | 24 | 3 | Yes | Mild | Yes | Yes |
| 9 | F | 37 | 3 | No | Mild | Yes | No |
| 10 | M | 41 | 3 | No | Mild | Yes | No |
| 11 | F | 62 | 4 | No | Florid | Yes | No |
| 12 | M | 19 | 4 | No | Florid | Yes | No |
| 13 | M | 61 | 4 | No | Florid | Yes | No |
| 14 | F | 21 | 4 | Yes | Florid | No | Yes |
| 15 | M | 43 | 4 | Yes | Florid | Yes | Yes |
| 16 | M | 14 | 4 | No | Florid | No | No |
| 17 | F | 56 | 4 | No | Florid | No | No |
In summary, the surgical pathology confirmed the MRI assessment as to the presence or absence of fibrosis in all the category “1”, “2” and “3” patients (10/10) while confirming the presence of moderate to severe intestinal inflammation with or without fibrosis in all 7 of the category “4” patients. This suggests that the MRI categories are accurate in the classification of both inflammation severity and the presence of fibrosis.
CD is a transmural disease that may result in obstructive symptoms secondary to intestinal inflammation and/or fibrosis. Fibrosis is not effectively treated by pharmaceutical agents and the presence of fibrotic strictures is one reason behind the high rate of surgical intervention[23]. Radiological investigations have undergone a revolution with both CTE and MRE demonstrating impressive specificity and sensitivity in the assessment of SB CD. CTE, however, delivers ionising radiation with concerns that even a single abdominal CT may increase the life-time risk of malignancy[24]. This risk is even greater in the younger population[25]. MR, however, provides good soft tissue contrast that may differentiate between intestinal inflammation and fibrosis, and appears to be superior to CT scanning[1617]. For long-term safety issues, as well as potentially better diagnostic capabilities, MR examination of the SB would appear to be preferable to CT.
TI attenuation is associated with histologically active CD on CTE[26]. There are limited studies that compare MRI findings with SB inflammation, but they suggest that contrast enhancement intensity correlates with intestinal inflammation[27]. The level of bowel wall thickening on CTE, however, was not an independent risk factor for disease activity once attenuation was considered, similar to our MRI findings. MRI data that combines both bowel wall thickening and signal intensity has previously demonstrated greater accuracy in assessing disease severity[2627]. In view of these observations patients in our study were classified on the basis of intestinal wall enhancement and bowel wall thickening on MRI.
Our findings suggest that the SB MRI findings can be used to categorise CD patients, and that these categories correlate with patient response to ongoing medical therapy and the histological findings at surgery. No significant differences were detected between the medical therapy regime used and the response/remission rates overall, or between any of the MRI categories, suggesting that the medical therapy was not primarily responsible for differences observed in the response rates. Overall females were more likely to respond to medical therapy than males. This may potentially be impacted by 7 of the 9 patients in category “2” being female, but reasons behind this observation are unclear. Controlling for sex, however, patients in category “2” were still more likely to respond to continued medical therapy than any of the other categories.
Macroscopic/histological fibrosis was present on surgical pathology in all the category “1” and “3” patients undergoing surgery. All category “4” patients undergoing surgery demonstrated florid inflammation on the surgical pathology. In contrast, no category “2” or “3” patients demonstrated florid inflammation on surgical pathology with all but one having only mild inflammation. These findings may explain why category “2” patients were more likely to respond to medical therapy as they only had mild inflammation and were yet to develop fibrotic changes, unlike patients with marked wall thickening which is associated with fibrosis and/or florid inflammation.
A further finding from this work was that patients with intestinal narrowing and prestenotic dilatation were less likely to respond to medical therapy. It has been suggested that narrowing can be differentiated by CTE into inflammatory and fibrostenotic lesions[28]. In our study it was noted that there was a similar percentage of patients with luminal narrowing with severe inflammation (70%, 14/20) compared to patients with fibrosis and mild inflammation (81.3%, 13/16) or fibrosis and no inflammation (71.4%, 5/7). Patients with narrowing and inflammation, however, were more likely to have narrowing with hold up (47.2% 17/36) than patients without inflammation (14.3%, 1/7), suggesting that MRI may also be able to differentiate inflammatory from fibrostenotic lesions.
MRI findings of marked wall thickening without bowel wall enhancement were observed to be negatively associated with a response to medical therapy, while minimal bowel wall thickening with mild wall enhancement predicted a good response to continued medical therapy. As patient MR categories based on SB wall thickness and luminal wall enhancement appear to correlate with the response to continuing medical therapy as well as with the surgical pathology, our findings would support the use of SB MRI as a diagnostic and prognostic tool in the management of patients with SB CD.
Small bowel (SB) involvement is common in Crohn’s disease (CD). The study aimed to determine whether magnetic resonance imaging (MRI) could predict response to medical therapy and correlate with surgical pathology in these patients.
Utilising a combination of the maximal SB wall thickness and level of mucosal/mural enhancement detected on MRI, patients were able to be grouped into 1 of 4 categories; (1) “fibrosis”, (2) “mild segmental hyper-enhancement and mild wall thickening”, (3) “mild segmental hyper-enhancement and marked wall thickening”, and (4) “marked segmental transmural hyper-enhancement”. This categorization was then used to determine a prognostic role for SB MRI in the management of CD.
Category “2” patients were more likely to respond to medical therapy than any other category, as were females, while patients with intestinal narrowing and prestenotic dilatation with or without hold up were less likely to respond. The presence of marked wall thickening without bowel wall enhancement was negatively associated with a medical response. Surgical pathology confirmed the MRI assessment of inflammation severity and presence of fibrosis.
The proposed MR categories appeared to correlate with response to continuing medical therapy and the surgical pathology, thereby supporting the use of SB MRI as a diagnostic and prognostic tool in the management of patients with SB CD.
CD is a chronic inflammatory condition of the intestine that frequently involves the terminal ileum with narrowing and fibrosis. Harvey Bradshaw index (HBI) is a means of measuring a clinical response in CD with a reduction in the HBI of ≥ 3 being a clinical response and remission defined as a HBI < 5 terminal ileum. MRI is a cross sectional imaging technique that does not utilize radiation and provides excellent tissue differentiation.
This manuscript can be accepted. This paper has new useful information for the readers of World J Gastroenterol.
| 1. | Munkholm P, Langholz E, Nielsen OH, Kreiner S, Binder V. Incidence and prevalence of Crohn's disease in the county of Copenhagen, 1962-87: a sixfold increase in incidence. Scand J Gastroenterol. 1992;27:609-614. |
| 2. | Harper PH, Fazio VW, Lavery IC, Jagelman DG, Weakley FL, Farmer RG, Easley KA. The long-term outcome in Crohn’s disease. Dis Colon Rectum. 1987;30:174-179. |
| 3. | Brown AL, Skehan SJ, Greaney T, Rawlinson J, Somers S, Stevenson GW. Value of double-contrast barium enema performed immediately after incomplete colonoscopy. AJR Am J Roentgenol. 2001;176:943-945. |
| 4. | Marmo R, Rotondano G, Piscopo R, Bianco MA, Siani A, Catalano O, Cipolletta L. Capsule endoscopy versus enteroclysis in the detection of small-bowel involvement in Crohn’s disease: a prospective trial. Clin Gastroenterol Hepatol. 2005;3:772-776. |
| 5. | Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn’s disease. Am J Gastroenterol. 2006;101:954-964. |
| 6. | Solem CA, Loftus EV Jr, Fletcher JG, Baron TH, Gostout CJ, Petersen BT, Tremaine WJ, Egan LJ, Faubion WA, Schroeder KW. Small-bowel imaging in Crohn’s disease: a prospective, blinded, 4-way comparison trial. Gastrointest Endosc. 2008;68:255-266. |
| 7. | Cheon JH, Kim YS, Lee IS, Chang DK, Ryu JK, Lee KJ, Moon JS, Park CH, Kim JO, Shim KN. Can we predict spontaneous capsule passage after retention? A nationwide study to evaluate the incidence and clinical outcomes of capsule retention. Endoscopy. 2007;39:1046-1052. |
| 8. | Gore RM, Balthazar EJ, Ghahremani GG, Miller FH. CT features of ulcerative colitis and Crohn’s disease. AJR Am J Roentgenol. 1996;167:3-15. |
| 9. | Doerfler OC, Ruppert-Kohlmayr AJ, Reittner P, Hinterleitner T, Petritsch W, Szolar DH. Helical CT of the small bowel with an alternative oral contrast material in patients with Crohn disease. Abdom Imaging. 2003;28:313-318. |
| 10. | Casola G, vanSonnenberg E, Neff CC, Saba RM, Withers C, Emarine CW. Abscesses in Crohn disease: percutaneous drainage. Radiology. 1987;163:19-22. |
| 11. | Schreyer AG, Seitz J, Feuerbach S, Rogler G, Herfarth H. Modern imaging using computer tomography and magnetic resonance imaging for inflammatory bowel disease (IBD) AU1. Inflamm Bowel Dis. 2004;10:45-54. |
| 12. | Mako EK, Mester AR, Tarjan Z, Karlinger K, Toth G. Enteroclysis and spiral CT examination in diagnosis and evaluation of small bowel Crohn's disease. Eur J Radiol. 2000;35:168-175. |
| 13. | Mackalski BA, Bernstein CN. New diagnostic imaging tools for inflammatory bowel disease. Gut. 2006;55:733-741. |
| 14. | Gourtsoyiannis N, Papanikolaou N, Grammatikakis J, Papamastorakis G, Prassopoulos P, Roussomoustakaki M. Assessment of Crohn’s disease activity in the small bowel with MR and conventional enteroclysis: preliminary results. Eur Radiol. 2004;14:1017-1024. |
| 15. | Gourtsoyiannis NC, Grammatikakis J, Papamastorakis G, Koutroumbakis J, Prassopoulos P, Rousomoustakaki M, Papanikolaou N. Imaging of small intestinal Crohn’s disease: comparison between MR enteroclysis and conventional enteroclysis. Eur Radiol. 2006;16:1915-1925. |
| 16. | Masselli G, Casciani E, Polettini E, Lanciotti S, Bertini L, Gualdi G. Assessment of Crohn’s disease in the small bowel: Prospective comparison of magnetic resonance enteroclysis with conventional enteroclysis. Eur Radiol. 2006;16:2817-2827. |
| 17. | Bernstein CN, Greenberg H, Boult I, Chubey S, Leblanc C, Ryner L. A prospective comparison study of MRI versus small bowel follow-through in recurrent Crohn’s disease. Am J Gastroenterol. 2005;100:2493-2502. |
| 18. | Vogel J, da Luz Moreira A, Baker M, Hammel J, Einstein D, Stocchi L, Fazio V. CT enterography for Crohn’s disease: accurate preoperative diagnostic imaging. Dis Colon Rectum. 2007;50:1761-1769. |
| 19. | Florie J, Wasser MN, Arts-Cieslik K, Akkerman EM, Siersema PD, Stoker J. Dynamic contrast-enhanced MRI of the bowel wall for assessment of disease activity in Crohn’s disease. AJR Am J Roentgenol. 2006;186:1384-1392. |
| 20. | Koh DM, Miao Y, Chinn RJ, Amin Z, Zeegen R, Westaby D, Healy JC. MR imaging evaluation of the activity of Crohn’s disease. AJR Am J Roentgenol. 2001;177:1325-1332. |
| 21. | Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989;170:2-6; discussion 16-19. |
| 23. | Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn’s disease. Relationship between the clinical pattern and prognosis. Gastroenterology. 1985;88:1818-1825. |
| 24. | Brenner DJ, Elliston CD. Estimated radiation risks potentially associated with full-body CT screening. Radiology. 2004;232:735-738. |
| 25. | Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001;176:289-296. |
| 26. | Bodily KD, Fletcher JG, Solem CA, Johnson CD, Fidler JL, Barlow JM, Bruesewitz MR, McCollough CH, Sandborn WJ, Loftus EV Jr. Crohn Disease: mural attenuation and thickness at contrast-enhanced CT Enterography--correlation with endoscopic and histologic findings of inflammation. Radiology. 2006;238:505-516. |
| 27. | Low RN, Sebrechts CP, Politoske DA, Bennett MT, Flores S, Snyder RJ, Pressman JH. Crohn disease with endoscopic correlation: single-shot fast spin-echo and gadolinium-enhanced fat-suppressed spoiled gradient-echo MR imaging. Radiology. 2002;222:652-660. |
| 28. | Chiorean MV, Sandrasegaran K, Saxena R, Maglinte DD, Nakeeb A, Johnson CS. Correlation of CT enteroclysis with surgical pathology in Crohn’s disease. Am J Gastroenterol. 2007;102:2541-2550. |