Published online Jul 21, 2009. doi: 10.3748/wjg.15.3329
Revised: June 16, 2009
Accepted: June 23, 2009
Published online: July 21, 2009
Bile acids are implicated as etiologic agents in cancer of the gastrointestinal (GI) tract, including cancer of the esophagus, stomach, small intestine, liver, biliary tract, pancreas and colon/rectum. Deleterious effects of bile acid exposure, likely related to carcinogenesis, include: induction of reactive oxygen and reactive nitrogen species; induction of DNA damage; stimulation of mutation; induction of apoptosis in the short term, and selection for apoptosis resistance in the long term. These deleterious effects have, so far, been reported most consistently in relation to esophageal and colorectal cancer, but also to some extent in relation to cancer of other organs. In addition, evidence is reviewed for an association of increased bile acid exposure with cancer risk in human populations, in specific human genetic conditions, and in animal experiments. A model for the role of bile acids in GI carcinogenesis is presented from a Darwinian perspective that offers an explanation for how the observed effects of bile acids on cells contribute to cancer development.
- Citation: Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol 2009; 15(27): 3329-3340
- URL: https://www.wjgnet.com/1007-9327/full/v15/i27/3329.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3329
Although it was proposed that bile acids are carcinogens as early as 1939 and 1940, there was little evidence at that early time that bile acids act as carcinogens in the gastrointestinal (GI) tract (reviewed in[1]). Since then, however, evidence has accumulated that exposure of cells of the GI tract to repeated high physiologic levels of bile acids is an important risk factor for GI cancer. Here we review the substantial evidence, much of it obtained in the last few years, for a role of bile acids in cancers of the esophagus, stomach, small intestine, liver, biliary tract, pancreas and colon/rectum. High exposure to bile acids may occur in a number of settings, but, most importantly, is prevalent among individuals who have a high dietary fat intake[2]. A rapid effect on cells of high bile acid exposure is the generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS). Increased production of ROS/RNS, can lead to increased DNA damage and then increased mutation. The production of ROS/RNS following bile acid exposure likely occurs through multiple pathways involving disruptions of the cell membrane and mitochondria[1]. For each organ of the GI tract, we review evidence, where available, on deleterious effects of bile acids, including the induction of ROS/RNS, induction of DNA damage, mutation and apoptosis, and the development of reduced apoptosis capability upon chronic exposure. Reduced ability to undergo apoptosis is important because apoptosis is a beneficial process that rids the body of cells with unrepaired DNA damage that can cause mutation. Reduced apoptosis capability has been linked to increased mutagenesis[3–5]. We also review epidemiologic evidence and results of animal experiments indicating that long-term exposure to elevated levels of bile acids increases GI cancer risk.
The annual world-wide number of deaths due to cancer is about 7.6 million, and among these about 2.8 million (36%) are due to cancers of the GI tract[6]. A recent prospective study was carried out on red and processed meat in relation to cancer incidence in a cohort of approximately half a million men and women[7]. Individuals in the highest quintile of red meat intake, compared with those in the lowest, had a statistically significant elevated risk of esophageal, colorectal and liver cancer. Also, for processed meat, the risk of colorectal cancer was elevated. Both types of meat are sources of saturated fat and iron, which have independently been associated with carcinogenesis. In addition, processed meats contain nitrates and nitrites, precursors of N-nitroso mutagenic compounds.
The estimated yearly number of deaths world-wide from esophageal cancer is 300 034 for men and 142 228 for women[6], making it the sixth leading cause of cancer deaths among men and women combined. There are two principal histologic types of esophageal cancer, adenocarcinoma and squamous cell carcinoma. In the United States, the incidence of adenocarcinoma has increased four-fold between 1973 and 2002, whereas squamous cell carcinoma has declined 30% over the same period, making adenocarcinoma the predominant form of esophageal cancer[8]. Barrett’s metaplasia of the esophagus is an important predisposing condition for the development of esophageal adenocarcinoma[9]. Barrett’s esophagus (BE) is a metaplastic lesion of the distal esophagus, characterized by the replacement of the normal squamous epithelium by columnar intestinal epithelium containing goblet cells. BE is associated with increased duodeno-gastro-esophageal reflux[1011], which causes increased exposure of the esophagus to bile acids from the duodenum and acidity (gastric acidity) from the stomach. Individuals with esophageal adenocarcinoma experience even greater exposure to bile than persons with uncomplicated BE[12]. Expression of bile acid transporter proteins is increased in BE tissues, suggesting that the development of BE metaplasia may be an adaptation to protect cells from bile acids[13]. Thus progression to BE and to adenocarcinoma may be strongly influenced by bile acid exposure. As discussed next, evidence indicates that short-term exposure of esophageal cells to bile acids induces oxidative stress, DNA damage, mutation and apoptosis; and among surviving cells selects over the long-run for resistance to apoptosis and ultimately cancer.
Five studies have shown that bile acids cause increased production of ROS in esophageal cells, including those from BE metaplasia. A cocktail of five bile acids designed to mimic the bile acids present in gastroesophageal reflux was used to test whether reflux induces ROS[14]. The five bile acids were glycocholic acid (GCA), taurocholic acid (TCA), glycodeoxycholic acid (GDCA), glycochenodeoxycholic acid (GCDCA) and deoxycholic acid (DCA). This cocktail induced ROS in biopsies from human BE metaplastic tissue. The bile acid cocktail also induced ROS in cultured SV40-transformed squamous esophageal epithelial cells (HET1-A). DCA induced ROS in cultured human esophageal adenocarcinoma cells (OE33) and squamous cell carcinoma cells (KYSE-30)[15]. GCDCA in acidic media induced ROS in cultured esophageal squamous cell lines derived from patients with gastroesophageal reflux disease (GERD) with BE, or without BE[16]. When mice were fed a zinc deficient diet containing a DCA supplement, ROS production was increased and BE-like lesions developed[17].
Six studies showed that bile acids induce DNA damage in esophageal cells (Table 1), and five of these reported evidence for oxidative DNA damage.
| Cells/tissues | Bile acids that induce DNA damage | Assay for damage | Ref. |
| Cultured SV40-transformed, squamous esophageal epithelial cells (HET1-A) and Barrett’s associated adenocarcinoma cells (FLO-1) | DCA; also cocktail containing GCA, TCA, TCDCA | Comet assay1 for strand breaks | [18] |
| Cultured SV40-transformed, squamous esophageal epithelial cells (HET1-A) | DCA | Comet assay for strand breaks; evidence for oxidative mechanism involving nitric oxide | [19] |
| Cultured human adenocarcinoma cells (OE33) | DCA | Micronuclei assay; induction of micronuclei by DCA, reduced by antioxidants | [1520] |
| Biopsies from human Barrett’s esophageal metaplastic tissue | Cocktail containing DCA, GCA, TCA, GDCA, GCDCA | 8-OHdG, an oxidized form of the DNA base guanine; assayed by IHC | [14] |
| Mouse model of esophagitis and Barrett’s esophagus | DCA (as dietary supplement; also zinc deficiency) | 8-OHdG assayed by IHC | [17] |
The findings that bile acids induce DNA damage suggest that bile acids may also increase the frequency of mutation, since replication of a damaged DNA template strand often results in a replication error and thus a mutation.
Esophagoduodenostomies were performed on Big Blue F1 lacI transgenic rats to surgically increase duodeno-gastro-esophageal reflux[21]. The frequency of lacI mutant cells proved to be significantly higher in the esophageal mucosa of the surgically altered rats than in the unaltered control rats, indicating that components of refluxate, such as bile acids, increase mutation. Forty-six percent of the mutant cells were altered at CpG dinucleotide sites, and the majority of these mutations (61%) were C to T or G to A transitions. This pattern of mutation is similar to that in human esophageal adenocarcinoma, suggesting that reflux is not only mutagenic, but also carcinogenic. Consistent with these findings, it was found that DCA treatment of cultured esophageal cells cause an increase in the frequency of GC to AT mutations in the p53 gene[15]. In addition, increased duodeno-gastro-esophageal reflux was observed to increase mutagenesis using a surgical model in Big Blue mice (rather than rats)[22].
Bile acids induce apoptosis in esophageal cells, perhaps through the mediation of damaging ROS. DCA induced apoptosis in esophageal biopsies from normal human squamous epithelium[23]. Also, five different bile acids [GCDCA, GDCA, TCA, taurochenodeoxycholic acid (TCDCA) and taurodeoxycholic acid (TDCA)] individually, and also in a mixture, induced apoptosis of cultured human normal esophageal mucosal epithelial cells[24].
Although a short-term effect of high bile acid exposure is induction of apoptosis, a longer-term effect of repeated high exposure to apoptosis-inducing agents, such as bile acids, appears to be selection for apoptosis resistant cells. When tissue samples from patients with normal esophagus, esophagitis, BE lesions and adenocarcinomas were studied for apoptosis capability, it was found that apoptosis is inhibited early in the dysplasia-carcinoma sequence of BE by over-expression of the anti-apoptotic protein, Bcl-2[25], presumably as a result of chronic gastroesophageal reflux containing bile acids. BE cells have high levels of the anti-apoptotic proteins IL-6, Bcl-xL and Mcl-1[26]. Studies of tissues obtained from patient biopsies, indicated that BE cells are resistant to apoptosis induction by DCA compared to esophageal squamous epithelium and normal colon epithelium[23]. Reduced apoptosis competence may arise by mutation in genes encoding proteins necessary for apoptosis. Since cells resistant to apoptosis have a growth advantage in the presence of agents that ordinarily induce apoptosis, such as bile acids, these cells will tend to proliferate to form a field of apoptosis resistant cells[27]. Within such a defective field, repeated encounters with bile acids in reflux would cause further DNA damage. Such DNA damage, leading to further mutation, may give rise to malignancy.
Considerable evidence indicates an association of bile acid exposure with esophageal cancer. In rats, reflux of duodenal or gastro-duodenal contents, that include bile acids, induced esophageal carcinoma in the absence of exogenous carcinogen[28]. Rat surgical models with increased duodenal reflux into the esophagus, but without added carcinogen, caused esophagitis, BE-like lesions and adenocarcinomas[29–32]. Persons with BE were found to have increased duodenoesophageal reflux and increased exposure to bile acids in their refluxate, suggesting that the BE premalignant lesion is linked to bile acid exposure[1011]. In a rat duodenal-contents reflux model, a high animal-fat intake changed the bile acid composition of bile juice and increased the development of BE and esophageal adenocarcinoma[33].
In summary, evidence indicates that, in esophageal cells and tissues, bile acids have the short-term effect of inducing oxidative stress, oxidative DNA damage, mutation and apoptosis. Over a longer period, bile acids are implicated in the development of apoptosis resistance and eventually the development of adenocarcinoma.
The estimated yearly number of deaths world-wide from gastric cancer is 511 549 for men and 288 681 for women[7], making it the second leading cause of cancer deaths among men and women combined. Infection by the bacterium Helicobacter pylori is the major etiologic risk factor in gastric carcinogenesis. However, gastroesophageal reflux appears to have an important role in the development of gastric cardia adenocarcinoma[3435] which may have an etiology similar to that of esophageal adenocarcinoma[34].
Exposure of cultured gastric carcinoma cells (St23123) to TCDCA increased production of ROS[36]. DCA induced apoptosis in cultured human gastric epithelial cells[37]. In rats, TCA increased stomach tumorigenesis induced by the carcinogen N-methyl-N’-nitro-N-nitrosoguanidine[38]. Carcinoma in the gastric stump (generated in rats by surgical gastrectomy) was increased by dietary fat intake and increased bile acid output[39]. Gastric adenocarcinomas were found to develop in a rat surgical model of duodenal reflux[40]. Gastroesophageal reflux in humans is implicated in adenocarcinoma of the gastric cardia[343541]. Thus, elevated bile acid exposure is associated with increased ROS, induction of apoptosis and increased development of cancer of the gastric cardia.
Small intestinal cancer is relatively infrequent compared to other cancers of the GI tract. In the United States, only 0.2% of all cancer deaths are due to cancer of the small intestine. Elevated risk of carcinoid tumor of the small intestine is associated with saturated fat intake[42], consistent with an etiologic role of bile acids. Fifty-three percent of adenocarcinomas of the small intestine arise in the duodenum, although the length of the duodenum is only 4% of the entire length of the small intestine. In addition, 57% of these duodenal cancers arise in the 6-7 cm segment that includes the outlet (Ampulla of Vater) of the common bile duct where bile (including bile acids) and pancreatic secretions empty into the small intestine[43]. Most adenomas and carcinomas of the small intestine and extrahepatic bile ducts arise in the region of the Papilla of Vater (which includes the Ampulla of Vater)[44]. Patients who have undergone a cholecystectomy are at increased risk of cancer of the small intestine, a risk that declines with increasing distance from the common bile duct[45]. These findings indicate that exposure to high levels of bile might be the underlying cause of carcinomas of the small intestine.
Individuals with familial adenomatous polyposis (FAP) have an increased risk of developing adenomas and cancer of the small and large intestine. In the small intestine, these lesions arise mostly near the outlet of the common bile duct, where their distribution parallels bile acid exposure[4647]. In a mouse model of FAP (Apcmin/+), higher dietary fat intake was associated with an increase in small intestinal tumors[48]. Administration of CDCA in this FAP mouse model increased duodenal tumors, suggesting that unconjugated bile acids contribute to periampullary tumor formation in the setting of an Apcmin/+ genotype[49].
The farnesoid X receptor (FXR) is a member of the nuclear receptor superfamily, and bile acids are endogenous ligands of FXR. FXR is necessary for maintaining bile acid homeostasis, and activation of FXR induces the expression of ileal bile acid binding protein (IBAB) and ileal bile acid transporters. In Apcmin/+ mice, FXR deficiency led to an increase in the size of small intestine adenocarcinomas[50]. Taken together, these results indicate that bile acids play a central role in cancer of the small intestine.
The estimated yearly number of deaths world-wide from liver cancer is 474 215 for men and 205 656 for women[6], making it the third leading cause of cancer deaths among men and women combined. The majority of liver cancers world-wide arise as a result of chronic infection by hepatitis B or C virus, or from exposure to aflatoxin B1, a carcinogenic food contaminant. Excessive alcohol consumption is another risk factor. However, the risk of hepatocellular carcinoma is elevated in individuals with late stage primary biliary cirrhosis, a possible autoimmune disease[51]. Liver cancer can also arise in children with a defect in the bile acid export pump[52]. Thus bile acids are implicated in at least some cases of liver cancer.
Several studies have shown that bile acids induce ROS in cells of the liver. TCDCA induced ROS in isolated rat hepatocytes[5354]. ROS were also induced in rat hepatocytes by GCDCA[55–57] and by DCA[58]. Taurolithocholate-3-sulfate induced ROS both in rat hepatocytes and a human hepatoma cell line (Huh7)[59].
Treatment of human hepatoma cells (HepG2) with DCA activated the gadd153 promoter[60]. This promoter is activated by DNA damage, suggesting that DCA induces DNA damage in hepatoma cells.
DCA is a promoter of preneoplastic lesions (hyperplastic nodules) in hepatocellular carcinogenesis[6162]. Evidence has also been presented that DCA, given as a dietary supplement in rats, possess initiating activity for hepatocarcinogenesis[63]. At least 12 studies have shown that bile acids induce apoptosis in liver cells. These are listed in Table 2. Apoptosis induced in liver cells by hydrophobic bile acids is likely caused by oxidative stress[59].
| Cells/tissues | Bile acid(s) that induced apoptosis | Ref. |
| Isolated rat hepatocytes | GDCA | [6465] |
| GCDCA | [5566] | |
| GCDCA, GCA | [57] | |
| GCDCA | [67] | |
| Isolated rat and mouse hepatocytes | DCA | [68] |
| Liver tissue sections from rats fed DCA, and cultured human hepatocellular carcinoma cells (HuH-7) | DCA | [58] |
| Cultured rat hepatocyes (McNtcp.24 cells) | GCDC | [6970] |
| Cultured human hepatocellular carcinoma cells (HuH-7) | GCDCA | [71] |
| Rat hepatocytes and human hepatoma carcinoma cells (HuH-7) | Taurolithocholate-3-sulfate | [59] |
Four studies indicated that bile acid-induced apoptosis in liver cells is mediated by ROS. A lazaroid antioxidant (U83836E) inhibited induction of apoptosis in isolated rat hepatocytes[55]. The antioxidants α-tocopherol, ebselen or idebenone (a coenzyme Q analogue) inhibited apoptosis of isolated rat hepatocytes by GCDCA and GCA[57]. Also in isolated rat hepatocytes, the antioxidants β-carotene and α-tocopherol inhibited GCDCA induced apoptosis[67]. LCA and CDCA activated the antioxidant responsive element Nrf2 in human hepatoma-derived cells (HepG2), mouse hepatoma-derived cells (Hepa1c1c7) and primary human hepatocytes[72]. Nrf2 activation inhibits apoptosis, and the target genes of activated Nrf2 include the genes that encode the rate-limiting enzyme in glutathione biosynthesis and thioredoxin reductase 1. The general finding that induction of apoptosis in liver cells by bile acids can be reduced by anti-oxidants implies that this induction is mediated by ROS.
The bile salt export pump conveys bile acids from the hepatocyte cytoplasm into bile canaliculi. Mutations in the ABCB11 gene cause a deficiency in the bile salt export pump, leading to intrahepatic accumulation of toxic bile salts. Children with such mutations have an increased incidence of hepatocellular carcinoma[5273]. Mice lacking the farnesoid X receptor, which controls the synthesis and export of bile acids, have increased hepatic bile acids. These mice have a high incidence of liver tumors[7475]. Such findings led to the suggestion that in cholestatic liver disease, chronic exposure to bile acids may play an important role in hepatocellular carcinogenesis[51].
Cholangiocarcinoma (CC) is an adenocarcinoma that arises from the bile duct epithelium. The CCs that occur within the liver are referred to as intrahepatic CCs. Those that occur at the confluence of the left and right hepatic duct are termed hilar CCs. The CCs that arise between the hepatic hilum and the duodenal papilla (or Ampulla of Vater) are called extra hepatic CCs[76].
The gallbladder and bile duct are exposed to high concentrations of bile acids. The bile acids excreted from the liver into the gall bladder are at a concentration of approximately 100 mmol/L[77]. The lifetime risk for developing cholangiocarcinoma in patients with primary sclerosing cholangitis is estimated at 7%-13%[78], and it was suggested that chronic exposure to bile acids may play an important role in cholangiocellular carcinogenesis[51]. Two children with progressive familial intrahepatic cholestasis and cholangiocarcinoma were found to have an absence of bile salt export pump expression and mutations in the ABCB11 gene[79]. Loss of a functional bile salt export pump may cause cholangiocarcinoma through intracellular accumulation of bile acids. Incubation of immortalized mouse cholangiocytes with GCDC resulted in the generation of ROS and an increase the percentage of cells with oxidative DNA damage (8-OHdG), suggesting that the a long-term effect of excessive exposure of the biliary tract to GCDC may be carcinogenesis[80].
The estimated yearly number of deaths world-wide from pancreatic cancer is 137 206 for men and 122 185 for women[6], making it the eighth leading cause of cancer deaths among men and women combined. Most adenocarcinomas of the pancreas occur in the head of the gland, which is in close proximity to bile[81]. In a hamster surgical model, bile reflux into the pancreatic duct was shown to induce development of intraductal papillary carcinomas of the pancreas[82], suggesting that bile acid may be an etiologic agent in pancreatic cancer. Consistent with this idea, epidemiological studies found a positive correlation between ingestion of a western style high fat diet and the incidence of pancreatic cancer[83–85]. Treatment of human pancreatic cancer cell lines with bile acids (CDCA, DCA or TCDCA) induced cyclooxygenase-2 (COX-2) expression[81]. Since COX-2 is overexpressed in human pancreatic adenocarcinomas, these results also suggest a possible role for bile acids in pancreatic carcinogenesis.
The estimated yearly number of deaths world-wide from cancer of the colon and rectum is 318 798 for men and 284 169 for women[6], making it the fourth leading cause of cancer deaths among men and women combined. Although both inherited mutations, environmental factors (e.g. smoking) and dietary factors are involved in colorectal cancer development, sporadic colorectal cancer appears to be caused predominantly by dietary factors.
The association of risk of colorectal cancer and consumption of red meat and processed meat was assessed in a meta-analysis of 15 prospective studies on red meat and 14 studies on processed meat[86]. The results showed consistent associations between high consumption of red and of processed meat and risk of colorectal cancer. In another recent study, a dose-dependent positive association between saturated fat intake and localized colorectal cancer was found in women, but not in men[87]. In earlier work, a positive association between dietary fat consumption and cancer incidence was reported[88–93]. Dietary total fat intake and saturated fat intake, but not polyunsaturated fat intake, are positively associated with colon cancer incidence[94]. In cancer prone Apcmin/+ mice, a high fat diet results in a significant increase in tumors[48]. A Western-style diet, containing elevated lipids and decreased calcium and vitamin D, induced colonic tumors in normal CB7Bl/6 mice[95–97]. Taken together, these studies implicate dietary fat (primarily from red and processed meat) in the etiology of human colorectal cancer.
Dietary intake of high-fat and high-beef foods results in a significantly higher excretion of fecal secondary bile acids, mainly DCA and LCA[98]. Presumably the increase in DCA and LCA reflects increased production of bile acids in order to emulsify the increased level of dietary fat. Epidemiologic studies have also found that fecal bile acid concentrations are increased in populations with a high incidence of colorectal cancer[99–106]. The most significant bile acids with respect to human colorectal cancer appear to be the secondary bile acids, DCA and LCA[99].
Although repeated exposure of the colorectal epithelium to high physiological concentrations of bile acids appears to be the major etiologic factor in colorectal carcinogenesis, other factors may also be significant. Intake of dietary heme iron is associated with increased risk of colorectal cancer[107], suggesting that iron catalyzed formation of ROS may play a role. The risk of colorectal cancer is also increased by smoking[108]. Bile acids and nicotine from smoking can interact synergistically in colon cells to increase oxidative stress and DNA damage[109].
Twelve studies have reported that bile acids induce production of ROS or RNS in colon cells (Table 3).
| Cells/tissues | Bile acid(s) that induced ROS/RNS | Ref. |
| Human colon surgical resections | DCA (RNS) | [110] |
| Cultured human adenocarcinoma cells (CACO-2) | DCA, LCA (ROS) | [111] |
| Cultured human adenocarcinoma cells (HT-29) | DCA, LCA (ROS) | [112] |
| DCA (ROS) | [36113] | |
| DCA (RNS) | [114] | |
| Cultured human adenocarcinoma cells (HCT116) | DCA (ROS) | [109115116] |
| DCA (RNS) | [117] | |
| Rat colonic mucosa | DCA (ROS) | [118] |
| Mouse colonic mucosa | DCA (ROS, RNS) | [119] |
Fourteen studies showed that bile acids induce DNA damage in colon cells (Table 4), of which a component is likely oxidative DNA damage. Defective repair of oxidative DNA damage is linked to increased risk of colon cancer. The base excision repair pathway deals with oxidative damages in DNA caused by ROS. 8-OHdG is a major oxidative damage in DNA that can mispair with adenine causing G:C to T:A transversion mutations, unless the mispair is corrected. MUTYH is a mammalian DNA glycosylase that initiates base excision repair by excising adenine opposite 8-OHdG. Genetic defects in MUTYH cause multiple polyps[120] and greatly increased risk of colorectal cancer[121] in humans.
| Cells/tissues | Bile acid(s) | Assay for DNA damage | Ref. |
| Isolated mouse colon crypt cells | LCA | Nucleoid sedimentation for strand breaks | [122] |
| Isolated human and rat colon cells LCA | LCA | Comet assay for strand breaks | [123] |
| Isolated rat colon cells | DCA | Immunostaining for poly (ADP-ribose) an indicator of DNA damage | [124] |
| Freshly isolated normal human colonocytes | DCA, CDCA | Comet assay for strand breaks | [125] |
| Cultured human adenocarcinoma cells (HT-29) | DCA, CDCA | Comet assay for strand breaks and modified comet assay for oxidative DNA damage | |
| Cultured human adenocarcinoma cells (HT-29) | DCA, LCA | Comet assay for strand breaks | [112126] |
| Cultured human adenocarcinoma cells (CACO-2) | DCA, LCA | Comet assay for oxidative DNA damage | [111] |
| Cultured human colon adenocarcinoma cells (HCT-116 & HCT-15) | DCA | Comet assay for strand breaks | [127] |
| Cultured human colon adenocarcinoma cells (HCT-116 & HT-29) | DCA | Comet assay for strand breaks | [128] |
| Cultured human colon adenocarcinoma cells (HCT-116) | DCA | Induction of the DNA repair protein BRCA-1 | [129] |
| Induction of DNA damage inducible gene GADD153 | [130] | ||
| Comet assay | [116] | ||
| Cultured human colon adenocarcinoma cells (HCT-116 and HCT-15) | DCA | Induction of DNA damage inducible genes GADD34, GADD45, GADD153 | [131] |
| Colon samples from mouse dietary colitis model | DCA | Oxidative DNA damage: 8-OHdG assayed by immunohistochemistry | [119] |
The numerous studies showing that bile acids induce DNA damage in colon cells suggest that bile acids may also induce mutation and genomic instability. In a model system for inducing tumors in the rat using the carcinogen azoxymethane, DCA not only increased the incidence of colon tumors, but also increased the incidence of tumors with K-ras point mutations[132], suggesting that DCA may induce K-ras mutations. Hydrophobic bile acids cause aneuploidy and micronuclei formation, indicators of genomic instability, in a variety of cell types including colon epithelial cells[133]. Persistent exposure of cultured colon epithelial cells to DCA results in alterations in expression of chromosomal maintenance/mitosis-related genes that might give rise to the observed genomic instability[133].
The 27 studies listed in Table 5 indicate that hydrophobic bile acids induce apoptosis in colon cells. Exposure of colon epithelial cells to DCA causes induction of growth arrest and DNA damage-inducible genes GADD34, GADD45 and GADD153, probably in response to the DNA damage caused by DCA[131]. DCA induced expression of GADD153 is essential for DCA induction of apoptosis[130]. These findings suggest that induction of DNA damage by DCA results in apoptosis. Induction of apoptosis by DCA may protect against the survival of cells with damaged template DNA that upon replication might undergo mutation leading to cancer[134].
| Cells/tissues | Bile acid(s) that induced apoptosis | Ref. |
| Biopsies from normal human colonic mucosa | DCA | [135–139] |
| Colon adenoma cell lines (AA/C1 and RG/C2), and carcinoma cell line (PC/JW/F1) | DCA | [140] |
| Cultured human adenocarcinoma cells (HT-29 and CaCo-2) | DCA | [141142] |
| Cultured human adenocarcinoma cells (HCT-116) | DCA, CDCA | [130143–146] |
| DCA | [116147–149] | |
| Cultured human adenocarcinoma cells (HT-29) | DCA | [114] |
| Cultured human adenocarcinoma cells (HT-29 and HCT-116) | DCA | [150] |
| DCA | [128] | |
| DCA, LCA, CDCA | [151] | |
| Cultured human adenocarcinoma cells (HT-29) and human fetal colonic mucosal cells (FHC) | DCA, LCA, CA, CDCA | [152] |
| Cultured human adenocarcinoma cells (HT-29, SW480, SW620) | DCA, CDCA | [153154] |
| Cultured human adenocarcinoma cells [HCT-116 (p53+) and HCT-15 (p53-)] | DCA | [127] |
| Cultured human adenocarcinoma cells (HCT-116SA apoptosis-sensitive and HCT-116RB, HCT-116RC and HCT-116RD apoptosis resistant) | DCA | [155] |
| Human colonic mucosal samples from surgical resections | DCA | [156] |
Repeated long-term exposure of colonic epithelial cells to high physiologic concentrations of bile acids appears to select for cells that are resistant to induction of apoptosis by bile acids. Such apoptosis-resistant cells might arise and clonally expand through the processes of mutation (or epimutation) and natural selection. Several studies of colon cancer patients have shown that epithelial cells in areas of the colonic mucosa that do not contain the cancer itself have increased resistance to induction of apoptosis by DCA[115135137–139]. The expression of anti-apoptotic protein Bcl-xL is elevated in the colorectal mucosa adjacent to colorectal adenocarcinomas[157]. These findings suggest that tumors may often arise in a field of apoptosis-resistant epithelial cells. A variant of ileal bile acid binding protein (IBABP), termed IBABP-L, is upregulated in colorectal cancer and is necessary for survival of HCT116 colon cancer cells in the presence of physiologic levels of hydrophobic bile acid[158]. This finding suggests that IBABP-L is a key factor in the development of resistance to bile acids in colon cancer cells. Furthermore, repeated long-term exposure of HCT-116 human colonic epithelial cells in culture to sublethal concentrations of DCA selects for cells that have further increased resistance to DCA-induced apoptosis[159]. These observations suggest a link between development of resistance to bile acid-induced apoptosis and colon cancer.
In summary, evidence indicates that, in colonic epithelial cells and tissues, bile acids have the short-term effect of inducing oxidative stress that causes DNA damage leading to mutation and apoptosis. Over a longer period, repeated exposure to high levels of bile acid may select for the development of apoptosis resistant fields of cells and eventually to the development of adenocarcinoma.
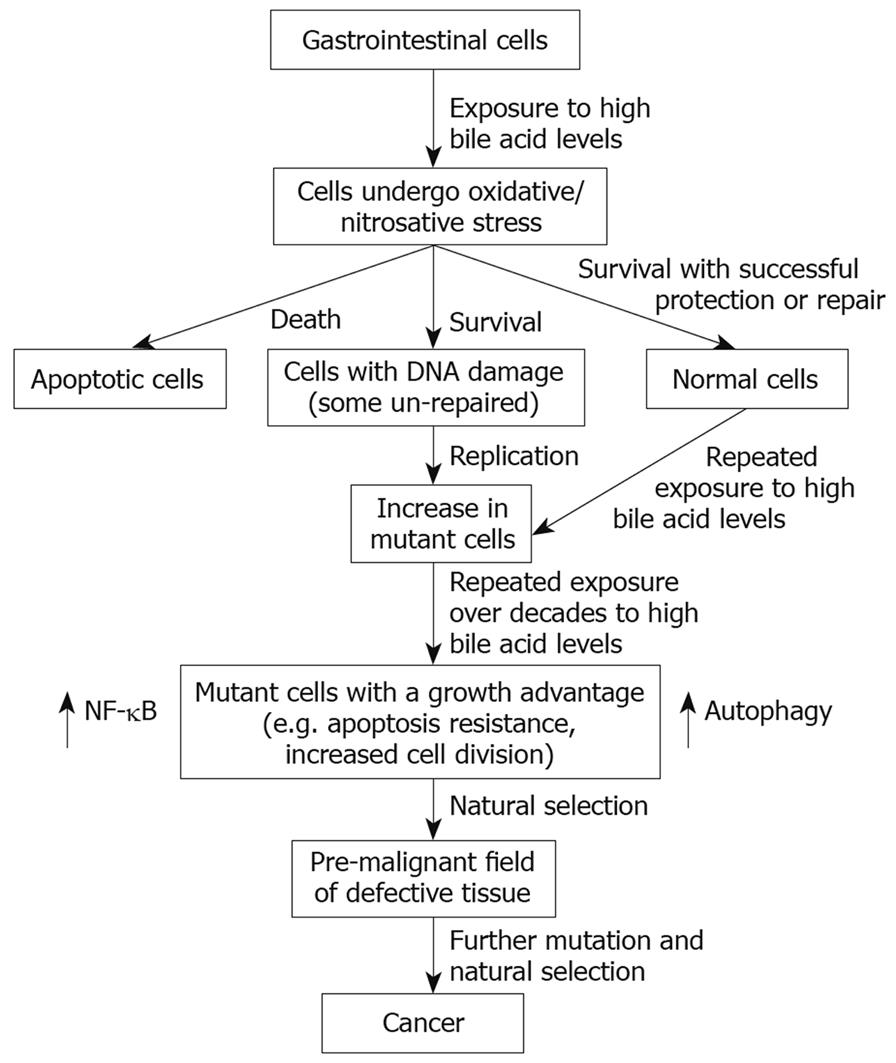
We have emphasized, above, the role of bile acids in inducing ROS/RNS and DNA damage in cells of the GI tract. These stresses, if excessive, can overwhelm cellular defenses resulting in cell death[139160–163]. However, we have also shown that bile acids can activate two major cell survival pathways, NF-κB[115124] and autophagy[164] (Figure 1). Both of the pathways are known to be activated by ROS[165166]. Results from our laboratory indicate that the activation of both pathways by DCA can be attenuated by the use of antioxidants[113115124164]. We have also shown that the NF-κB and autophagy pathways contribute to the stable apoptosis resistance that characterizes cell lines persistently exposed to DCA[159164]. The sensitization to DOC-induced cell death after interfering with these pathways was documented using antisense oligonucleotides against the p65 subunit of NF-κB[159] and pharmacologically through the use of 3-methyladenine[164], an inhibitor of autophagy.
The induction of persistent DNA damage in apoptosis-resistant cells is a dangerous situation that can lead to further mutation and ultimately cancer (Figure 1). An increase in Bcl-2 (an anti-apoptotic protein), for example, may also downregulate Ku DNA binding activity, thereby further amplifying genomic instability through interference with the non-homologous end-joining pathway of DNA repair[167]. The cross-talk between anti-apoptotic proteins and DNA repair proteins is a current area of investigation.
Recently, it has become apparent that nuclear bile acid receptors FXR, VDR and PXR/SXR play an important role in protecting against carcinogenic effects of bile acids. FXR, a member of the nuclear receptor superfamily, responds to bile acids as physiological ligands[168–170]. FXR has a key role in activating pathways that maintain bile acid homeostasis[50]. FXR protects against intestinal tumorigenesis, possibly by a mechanism involving induction of apoptosis[50171].
The vitamin D receptor (VDR) functions as a receptor for the secondary bile acid lithocholic acid, and has a key role in activating a pathway that detoxifies lithocholic acid[172]. Similarly, the human xenobiotic receptor SXR (steroid xenobiotic receptor) and its rodent homolog PXR (pregnane X receptor) are bile acid receptors that, when activated, induce a response that detoxifies bile acids[173174]. PXR promotes bile acid detoxification by activating bile acid metabolizing enzymes and transporters. In both human colon cancer cells and normal mouse colon epithelium PXR/SXR protects against bile acid induced apoptosis[149].
In Figure 1, we suggest a possible general pathway for bile acid induced carcinogenesis based on evidence reviewed above. An immediate effect on cells of the GI tract to exposure to a high physiologic level of bile acids is the induction of ROS/RNS. This can lead to DNA damage and apoptosis in some cells. Among surviving cells, some may remain normal by successfully employing protective and repair mechanisms. Other surviving cells, however, may retain unrepaired DNA damage. When such cells undergo DNA replication using a damaged strand as template, mutations will likely arise. Over years of frequently repeated exposure to high levels of bile acids many mutations will occur, and some of these mutations may provide a growth advantage to the cell in which they occur. The growth advantage may involve apoptosis resistance, and increased and/or aberrant proliferation. Such cells will tend to expand clonally at the expense of neighboring cells to form a field of defective cells. Further repeated exposure to high levels of bile acids will lead to additional mutations. Should some of these mutations arise within a defective field and also provide additional growth advantages, a secondary field will spread within the first field by natural selection. Repetition of this “mutation-and-selection” process over many years, perhaps decades, will lead to a pre-malignant field and eventually to cancer.
| 1. | Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589:47-65. |
| 2. | Reddy BS. Diet and excretion of bile acids. Cancer Res. 1981;41:3766-3768. |
| 3. | Cherbonnel-Lasserre C, Gauny S, Kronenberg A. Suppression of apoptosis by Bcl-2 or Bcl-xL promotes susceptibility to mutagenesis. Oncogene. 1996;13:1489-1497. |
| 4. | Cherbonnel-Lasserre C, Dosanjh MK. Suppression of apoptosis by overexpression of Bcl-2 or Bcl-xL promotes survival and mutagenesis after oxidative damage. Biochimie. 1997;79:613-617. |
| 5. | Saintigny Y, Dumay A, Lambert S, Lopez BS. A novel role for the Bcl-2 protein family: specific suppression of the RAD51 recombination pathway. EMBO J. 2001;20:2596-2607. |
| 6. | Garcia M, Jemal A, Ward EM, Center MM, Hao Y, Siegel RL, Thun MJ. Global Cancer Facts & Figures 2007. Atlanta, GA: American Cancer Society 2007; 25-26. |
| 7. | Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4:e325. |
| 8. | Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2-9. |
| 9. | Falk GW. Barrett's esophagus. Gastroenterology. 2002;122:1569-1591. |
| 10. | Nehra D, Howell P, Williams CP, Pye JK, Beynon J. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut. 1999;44:598-602. |
| 11. | Menges M, Müller M, Zeitz M. Increased acid and bile reflux in Barrett's esophagus compared to reflux esophagitis, and effect of proton pump inhibitor therapy. Am J Gastroenterol. 2001;96:331-337. |
| 12. | Stein HJ, Kauer WK, Feussner H, Siewert JR. Bile reflux in benign and malignant Barrett's esophagus: effect of medical acid suppression and nissen fundoplication. J Gastrointest Surg. 1998;2:333-341. |
| 13. | Dvorak K, Watts GS, Ramsey L, Holubec H, Payne CM, Bernstein C, Jenkins GJ, Sampliner RE, Prasad A, Garewal HS. Expression of bile acid transporting proteins in Barrett's esophagus and esophageal adenocarcinoma. Am J Gastroenterol. 2009;104:302-309. |
| 14. | Dvorak K, Payne CM, Chavarria M, Ramsey L, Dvorakova B, Bernstein H, Holubec H, Sampliner RE, Guy N, Condon A. Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett's oesophagus. Gut. 2007;56:763-771. |
| 15. | Jenkins GJ, D'Souza FR, Suzen SH, Eltahir ZS, James SA, Parry JM, Griffiths PA, Baxter JN. Deoxycholic acid at neutral and acid pH, is genotoxic to oesophageal cells through the induction of ROS: The potential role of anti-oxidants in Barrett's oesophagus. Carcinogenesis. 2007;28:136-142. |
| 16. | Feagins LA, Zhang HY, Zhang X, Hormi-Carver K, Thomas T, Terada LS, Spechler SJ, Souza RF. Mechanisms of oxidant production in esophageal squamous cell and Barrett's cell lines. Am J Physiol Gastrointest Liver Physiol. 2008;294:G411-G417. |
| 17. | Guy NC, Garewal H, Holubec H, Bernstein H, Payne CM, Bernstein C, Bhattacharyya AK, Dvorak K. A novel dietary-related model of esophagitis and Barrett's esophagus, a premalignant lesion. Nutr Cancer. 2007;59:217-227. |
| 18. | Jolly AJ, Wild CP, Hardie LJ. Acid and bile salts induce DNA damage in human oesophageal cell lines. Mutagenesis. 2004;19:319-324. |
| 19. | Jolly AJ, Wild CP, Hardie LJ. Sodium deoxycholate causes nitric oxide mediated DNA damage in oesophageal cells. Free Radic Res. 2009;43:234-240. |
| 20. | Jenkins GJ, Cronin J, Alhamdani A, Rawat N, D'Souza F, Thomas T, Eltahir Z, Griffiths AP, Baxter JN. The bile acid deoxycholic acid has a non-linear dose response for DNA damage and possibly NF-kappaB activation in oesophageal cells, with a mechanism of action involving ROS. Mutagenesis. 2008;23:399-405. |
| 21. | Theisen J, Peters JH, Fein M, Hughes M, Hagen JA, Demeester SR, Demeester TR, Laird PW. The mutagenic potential of duodenoesophageal reflux. Ann Surg. 2005;241:63-68. |
| 22. | Fein M, Peters JH, DeMeester TR. Carcinogenesis in reflux disease--in search for bile-specific effects. Microsurgery. 2007;27:647-650. |
| 23. | Dvorakova K, Payne CM, Ramsey L, Bernstein H, Holubec H, Chavarria M, Bernstein C, Sampliner RE, Riley C, Prasad A. Apoptosis resistance in Barrett's esophagus: ex vivo bioassay of live stressed tissues. Am J Gastroenterol. 2005;100:424-431. |
| 24. | Zhang R, Gong J, Wang H, Wang L. Bile salts inhibit growth and induce apoptosis of culture human normal esophageal mucosal epithelial cells. World J Gastroenterol. 2005;11:6466-6471. |
| 25. | Katada N, Hinder RA, Smyrk TC, Hirabayashi N, Perdikis G, Lund RJ, Woodward T, Klingler PJ. Apoptosis is inhibited early in the dysplasia-carcinoma sequence of Barrett esophagus. Arch Surg. 1997;132:728-733. |
| 26. | Dvorakova K, Payne CM, Ramsey L, Holubec H, Sampliner R, Dominguez J, Dvorak B, Bernstein H, Bernstein C, Prasad A. Increased expression and secretion of interleukin-6 in patients with Barrett's esophagus. Clin Cancer Res. 2004;10:2020-2028. |
| 27. | Bernstein C, Bernstein H, Payne CM, Dvorak K, Garewal H. Field defects in progression to gastrointestinal tract cancers. Cancer Lett. 2008;260:1-10. |
| 28. | Miwa K, Sahara H, Segawa M, Kinami S, Sato T, Miyazaki I, Hattori T. Reflux of duodenal or gastro-duodenal contents induces esophageal carcinoma in rats. Int J Cancer. 1996;67:269-274. |
| 29. | Goldstein SR, Yang GY, Curtis SK, Reuhl KR, Liu BC, Mirvish SS, Newmark HL, Yang CS. Development of esophageal metaplasia and adenocarcinoma in a rat surgical model without the use of a carcinogen. Carcinogenesis. 1997;18:2265-2270. |
| 30. | Fein M, Peters JH, Chandrasoma P, Ireland AP, Oberg S, Ritter MP, Bremner CG, Hagen JA, DeMeester TR. Duodenoesophageal reflux induces esophageal adenocarcinoma without exogenous carcinogen. J Gastrointest Surg. 1998;2:260-268. |
| 31. | Chen X, Yang G, Ding WY, Bondoc F, Curtis SK, Yang CS. An esophagogastroduodenal anastomosis model for esophageal adenocarcinogenesis in rats and enhancement by iron overload. Carcinogenesis. 1999;20:1801-1808. |
| 32. | Su Y, Chen X, Klein M, Fang M, Wang S, Yang CS, Goyal RK. Phenotype of columnar-lined esophagus in rats with esophagogastroduodenal anastomosis: similarity to human Barrett's esophagus. Lab Invest. 2004;84:753-765. |
| 33. | Chen KH, Mukaisho K, Sugihara H, Araki Y, Yamamoto G, Hattori T. High animal-fat intake changes the bile-acid composition of bile juice and enhances the development of Barrett's esophagus and esophageal adenocarcinoma in a rat duodenal-contents reflux model. Cancer Sci. 2007;98:1683-1688. |
| 34. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. |
| 35. | Ye W, Chow WH, Lagergren J, Yin L, Nyrén O. Risk of adenocarcinomas of the esophagus and gastric cardia in patients with gastroesophageal reflux diseases and after antireflux surgery. Gastroenterology. 2001;121:1286-1293. |
| 36. | Lechner S, Müller-Ladner U, Schlottmann K, Jung B, McClelland M, Rüschoff J, Welsh J, Schölmerich J, Kullmann F. Bile acids mimic oxidative stress induced upregulation of thioredoxin reductase in colon cancer cell lines. Carcinogenesis. 2002;23:1281-1288. |
| 37. | Redlak MJ, Dennis MS, Miller TA. Apoptosis is a major mechanism of deoxycholate-induced gastric mucosal cell death. Am J Physiol Gastrointest Liver Physiol. 2003;285:G870-G879. |
| 38. | Kobori O, Shimizu T, Maeda M, Atomi Y, Watanabe J, Shoji M, Morioka Y. Enhancing effect of bile and bile acid on stomach tumorigenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine in Wistar rats. J Natl Cancer Inst. 1984;73:853-861. |
| 39. | Miwa K, Kinami S, Miyazaki I, Hattori T. Positive association between dietary fat intake and risk of gastric stump carcinoma in rats. Carcinogenesis. 1996;17:1885-1889. |
| 40. | Mukaisho K, Miwa K, Kumagai H, Bamba M, Sugihara H, Hattori T. Gastric carcinogenesis by duodenal reflux through gut regenerative cell lineage. Dig Dis Sci. 2003;48:2153-2158. |
| 41. | Dixon MF, Mapstone NP, Neville PM, Moayyedi P, Axon AT. Bile reflux gastritis and intestinal metaplasia at the cardia. Gut. 2002;51:351-355. |
| 42. | Cross AJ, Leitzmann MF, Subar AF, Thompson FE, Hollenbeck AR, Schatzkin A. A prospective study of meat and fat intake in relation to small intestinal cancer. Cancer Res. 2008;68:9274-9279. |
| 43. | Ross RK, Hartnett NM, Bernstein L, Henderson BE. Epidemiology of adenocarcinomas of the small intestine: is bile a small bowel carcinogen? Br J Cancer. 1991;63:143-145. |
| 44. | Fischer HP, Zhou H. Pathogenesis of carcinoma of the papilla of Vater. J Hepatobiliary Pancreat Surg. 2004;11:301-309. |
| 45. | Lagergren J, Ye W, Ekbom A. Intestinal cancer after cholecystectomy: is bile involved in carcinogenesis? Gastroenterology. 2001;121:542-547. |
| 46. | Jagelman DG, DeCosse JJ, Bussey HJ. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet. 1988;1:1149-1151. |
| 47. | Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783-785. |
| 48. | Wasan HS, Novelli M, Bee J, Bodmer WF. Dietary fat influences on polyp phenotype in multiple intestinal neoplasia mice. Proc Natl Acad Sci USA. 1997;94:3308-3313. |
| 49. | Mahmoud NN, Dannenberg AJ, Bilinski RT, Mestre JR, Chadburn A, Churchill M, Martucci C, Bertagnolli MM. Administration of an unconjugated bile acid increases duodenal tumors in a murine model of familial adenomatous polyposis. Carcinogenesis. 1999;20:299-303. |
| 50. | Maran RR, Thomas A, Roth M, Sheng Z, Esterly N, Pinson D, Gao X, Zhang Y, Ganapathy V, Gonzalez FJ. Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J Pharmacol Exp Ther. 2009;328:469-477. |
| 52. | Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, Bull LN, Pawlikowska L, Bilezikçi B, Ozçay F. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44:478-486. |
| 53. | Sokol RJ, Winklhofer-Roob BM, Devereaux MW, McKim JM Jr. Generation of hydroperoxides in isolated rat hepatocytes and hepatic mitochondria exposed to hydrophobic bile acids. Gastroenterology. 1995;109:1249-1256. |
| 54. | Sokol RJ, McKim JM Jr, Goff MC, Ruyle SZ, Devereaux MW, Han D, Packer L, Everson G. Vitamin E reduces oxidant injury to mitochondria and the hepatotoxicity of taurochenodeoxycholic acid in the rat. Gastroenterology. 1998;114:164-174. |
| 55. | Patel T, Gores GJ. Inhibition of bile-salt-induced hepatocyte apoptosis by the antioxidant lazaroid U83836E. Toxicol Appl Pharmacol. 1997;142:116-122. |
| 56. | Sokol RJ, Straka MS, Dahl R, Devereaux MW, Yerushalmi B, Gumpricht E, Elkins N, Everson G. Role of oxidant stress in the permeability transition induced in rat hepatic mitochondria by hydrophobic bile acids. Pediatr Res. 2001;49:519-531. |
| 57. | Yerushalmi B, Dahl R, Devereaux MW, Gumpricht E, Sokol RJ. Bile acid-induced rat hepatocyte apoptosis is inhibited by antioxidants and blockers of the mitochondrial permeability transition. Hepatology. 2001;33:616-626. |
| 58. | Rodrigues CM, Fan G, Ma X, Kren BT, Steer CJ. A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest. 1998;101:2790-2799. |
| 59. | Reinehr R, Becker S, Keitel V, Eberle A, Grether-Beck S, Häussinger D. Bile salt-induced apoptosis involves NADPH oxidase isoform activation. Gastroenterology. 2005;129:2009-2031. |
| 60. | Bernstein H, Payne CM, Bernstein C, Schneider J, Beard SE, Crowley CL. Activation of the promoters of genes associated with DNA damage, oxidative stress, ER stress and protein malfolding by the bile salt, deoxycholate. Toxicol Lett. 1999;108:37-46. |
| 61. | Cameron RG, Imaida K, Tsuda H, Ito N. Promotive effects of steroids and bile acids on hepatocarcinogenesis initiated by diethylnitrosamine. Cancer Res. 1982;42:2426-2428. |
| 62. | Ohtaki Y, Hida T, Hiramatsu K, Kanitani M, Ohshima T, Nomura M, Wakita H, Aburada M, Miyamoto KI. Deoxycholic acid as an endogenous risk factor for hepatocarcinogenesis and effects of gomisin A, a lignan component of Schizandra fruits. Anticancer Res. 1996;16:751-755. |
| 63. | Kitazawa S, Denda A, Tsutsumi M, Tsujiuchi T, Hasegawa K, Tamura K, Maruyama H, Konishi Y. Enhanced preneoplastic liver lesion development under 'selection pressure' conditions after administration of deoxycholic or lithocholic acid in the initiation phase in rats. Carcinogenesis. 1990;11:1323-1328. |
| 64. | Patel T, Bronk SF, Gores GJ. Increases of intracellular magnesium promote glycodeoxycholate-induced apoptosis in rat hepatocytes. J Clin Invest. 1994;94:2183-2192. |
| 65. | Kwo P, Patel T, Bronk SF, Gores GJ. Nuclear serine protease activity contributes to bile acid-induced apoptosis in hepatocytes. Am J Physiol. 1995;268:G613-G621. |
| 66. | Jones BA, Rao YP, Stravitz RT, Gores GJ. Bile salt-induced apoptosis of hepatocytes involves activation of protein kinase C. Am J Physiol. 1997;272:G1109-G1115. |
| 67. | Gumpricht E, Dahl R, Devereaux MW, Sokol RJ. Beta-carotene prevents bile acid-induced cytotoxicity in the rat hepatocyte: Evidence for an antioxidant and anti-apoptotic role of beta-carotene in vitro. Pediatr Res. 2004;55:814-821. |
| 68. | Qiao L, Studer E, Leach K, McKinstry R, Gupta S, Decker R, Kukreja R, Valerie K, Nagarkatti P, El Deiry W. Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen-activated protein kinase-signaling module enhances DCA-induced apoptosis. Mol Biol Cell. 2001;12:2629-2645. |
| 69. | Faubion WA, Guicciardi ME, Miyoshi H, Bronk SF, Roberts PJ, Svingen PA, Kaufmann SH, Gores GJ. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137-145. |
| 70. | Sodeman T, Bronk SF, Roberts PJ, Miyoshi H, Gores GJ. Bile salts mediate hepatocyte apoptosis by increasing cell surface trafficking of Fas. Am J Physiol Gastrointest Liver Physiol. 2000;278:G992-G999. |
| 71. | Higuchi H, Bronk SF, Takikawa Y, Werneburg N, Takimoto R, El-Deiry W, Gores GJ. The bile acid glycochenodeoxycholate induces trail-receptor 2/DR5 expression and apoptosis. J Biol Chem. 2001;276:38610-38618. |
| 72. | Tan KP, Yang M, Ito S. Activation of nuclear factor (erythroid-2 like) factor 2 by toxic bile acids provokes adaptive defense responses to enhance cell survival at the emergence of oxidative stress. Mol Pharmacol. 2007;72:1380-1390. |
| 73. | Strautnieks SS, Byrne JA, Pawlikowska L, Cebecauerová D, Rayner A, Dutton L, Meier Y, Antoniou A, Stieger B, Arnell H. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology. 2008;134:1203-1214. |
| 74. | Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940-946. |
| 75. | Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863-867. |
| 76. | Goodman ZD. Neoplasms of the liver. Mod Pathol. 2007;20 Suppl 1:S49-S60. |
| 77. | Perwaiz S, Tuchweber B, Mignault D, Gilat T, Yousef IM. Determination of bile acids in biological fluids by liquid chromatography-electrospray tandem mass spectrometry. J Lipid Res. 2001;42:114-119. |
| 78. | Lazaridis KN, Gores GJ. Primary sclerosing cholangitis and cholangiocarcinoma. Semin Liver Dis. 2006;26:42-51. |
| 79. | Scheimann AO, Strautnieks SS, Knisely AS, Byrne JA, Thompson RJ, Finegold MJ. Mutations in bile salt export pump (ABCB11) in two children with progressive familial intrahepatic cholestasis and cholangiocarcinoma. J Pediatr. 2007;150:556-559. |
| 80. | Komichi D, Tazuma S, Nishioka T, Hyogo H, Chayama K. Glycochenodeoxycholate plays a carcinogenic role in immortalized mouse cholangiocytes via oxidative DNA damage. Free Radic Biol Med. 2005;39:1418-1427. |
| 81. | Tucker ON, Dannenberg AJ, Yang EK, Fahey TJ. Bile acids induce cyclooxygenase-2 expression in human pancreatic cancer cell lines. Carcinogenesis. 2004;25:419-423. |
| 82. | Adachi T, Tajima Y, Kuroki T, Mishima T, Kitasato A, Fukuda K, Tsutsumi R, Kanematsu T. Bile-reflux into the pancreatic ducts is associated with the development of intraductal papillary carcinoma in hamsters. J Surg Res. 2006;136:106-111. |
| 83. | Ghadirian P, Simard A, Baillargeon J, Maisonneuve P, Boyle P. Nutritional factors and pancreatic cancer in the francophone community in Montréal, Canada. Int J Cancer. 1991;47:1-6. |
| 84. | Binstock M, Krakow D, Stamler J, Reiff J, Persky V, Liu K, Moss D. Coffee and pancreatic cancer: an analysis of international mortality data. Am J Epidemiol. 1983;118:630-640. |
| 85. | Wynder EL, Mabuchi K, Maruchi N, Fortner JG. Epidemiology of cancer of the pancreas. J Natl Cancer Inst. 1973;50:645-667. |
| 86. | Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119:2657-2664. |
| 87. | Butler LM, Wang R, Koh WP, Stern MC, Yuan JM, Yu MC. Marine n-3 and saturated fatty acids in relation to risk of colorectal cancer in Singapore Chinese: a prospective study. Int J Cancer. 2009;124:678-686. |
| 88. | Drasar BS, Irving D. Environmental factors and cancer of the colon and breast. Br J Cancer. 1973;27:167-172. |
| 89. | Knox EG. Foods and diseases. Br J Prev Soc Med. 1977;31:71-80. |
| 90. | Miller AB, Howe GR, Jain M, Craib KJ, Harrison L. Food items and food groups as risk factors in a case-control study of diet and colo-rectal cancer. Int J Cancer. 1983;32:155-161. |
| 91. | McKeown-Eyssen GE, Bright-See E. Dietary factors in colon cancer: international relationships. Nutr Cancer. 1984;6:160-170. |
| 93. | Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med. 1990;323:1664-1672. |
| 94. | Hursting SD, Thornquist M, Henderson MM. Types of dietary fat and the incidence of cancer at five sites. Prev Med. 1990;19:242-253. |
| 95. | Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22:1871-1875. |
| 96. | Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30:88-92. |
| 97. | Yang K, Kurihara N, Fan K, Newmark H, Rigas B, Bancroft L, Corner G, Livote E, Lesser M, Edelmann W. Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res. 2008;68:7803-7810. |
| 98. | Reddy BS, Hanson D, Mangat S, Mathews L, Sbaschnig M, Sharma C, Simi B. Effect of high-fat, high-beef diet and of mode of cooking of beef in the diet on fecal bacterial enzymes and fecal bile acids and neutral sterols. J Nutr. 1980;110:1880-1887. |
| 100. | Cheah PY. Hypotheses for the etiology of colorectal cancer--an overview. Nutr Cancer. 1990;14:5-13. |
| 101. | Hill MJ, Drasar BS, Hawksworth G, Aries V, Crowther JS, Williams RE. Bacteria and aetiology of cancer of large bowel. Lancet. 1971;1:95-100. |
| 102. | Hill MJ, Taylor AJ, Thompson MH, Wait R. Fecal steroids and urinary volatile phenols in four Scandinavian populations. Nutr Cancer. 1982;4:67-73. |
| 103. | Crowther JS, Drasar BS, Hill MJ, Maclennan R, Magnin D, Peach S, Teoh-chan CH. Faecal steroids and bacteria and large bowel cancer in Hong Kong by socio-economic groups. Br J Cancer. 1976;34:191-198. |
| 104. | Reddy BS, Wynder EL. Large-bowel carcinogenesis: fecal constituents of populations with diverse incidence rates of colon cancer. J Natl Cancer Inst. 1973;50:1437-1442. |
| 105. | Reddy BS, Hedges AR, Laakso K, Wynder EL. Metabolic epidemiology of large bowel cancer: fecal bulk and constituents of high-risk North American and low-risk Finnish population. Cancer. 1978;42:2832-2838. |
| 106. | Jensen OM, MacLennan R, Wahrendorf J. Diet, bowel function, fecal characteristics, and large bowel cancer in Denmark and Finland. Nutr Cancer. 1982;4:5-19. |
| 107. | Lee DH, Anderson KE, Harnack LJ, Folsom AR, Jacobs DR Jr. Heme iron, zinc, alcohol consumption, and colon cancer: Iowa Women's Health Study. J Natl Cancer Inst. 2004;96:403-407. |
| 108. | Giovannucci E, Martínez ME. Tobacco, colorectal cancer, and adenomas: a review of the evidence. J Natl Cancer Inst. 1996;88:1717-1730. |
| 109. | Crowley-Weber CL, Dvorakova K, Crowley C, Bernstein H, Bernstein C, Garewal H, Payne CM. Nicotine increases oxidative stress, activates NF-kappaB and GRP78, induces apoptosis and sensitizes cells to genotoxic/xenobiotic stresses by a multiple stress inducer, deoxycholate: relevance to colon carcinogenesis. Chem Biol Interact. 2003;145:53-66. |
| 110. | Casellas F, Mourelle M, Papo M, Guarner F, Antolin M, Armengol JR, Malagelada JR. Bile acid induced colonic irritation stimulates intracolonic nitric oxide release in humans. Gut. 1996;38:719-723. |
| 111. | Venturi M, Hambly RJ, Glinghammar B, Rafter JJ, Rowland IR. Genotoxic activity in human faecal water and the role of bile acids: a study using the alkaline comet assay. Carcinogenesis. 1997;18:2353-2359. |
| 112. | Booth LA, Gilmore IT, Bilton RF. Secondary bile acid induced DNA damage in HT29 cells: are free radicals involved? Free Radic Res. 1997;26:135-144. |
| 113. | Washo-Stultz D, Crowley-Weber CL, Dvorakova K, Bernstein C, Bernstein H, Kunke K, Waltmire CN, Garewal H, Payne CM. Role of mitochondrial complexes I and II, reactive oxygen species and arachidonic acid metabolism in deoxycholate-induced apoptosis. Cancer Lett. 2002;177:129-144. |
| 114. | Washo-Stultz D, Hoglen N, Bernstein H, Bernstein C, Payne CM. Role of nitric oxide and peroxynitrite in bile salt-induced apoptosis: relevance to colon carcinogenesis. Nutr Cancer. 1999;35:180-188. |
| 115. | Payne CM, Weber C, Crowley-Skillicorn C, Dvorak K, Bernstein H, Bernstein C, Holubec H, Dvorakova B, Garewal H. Deoxycholate induces mitochondrial oxidative stress and activates NF-kappaB through multiple mechanisms in HCT-116 colon epithelial cells. Carcinogenesis. 2007;28:215-222. |
| 116. | Longpre JM, Loo G. Protection of human colon epithelial cells against deoxycholate by rottlerin. Apoptosis. 2008;13:1162-1171. |
| 117. | Dall'Agnol M, Bernstein C, Bernstein H, Garewal H, Payne CM. Identification of S-nitrosylated proteins after chronic exposure of colon epithelial cells to deoxycholate. Proteomics. 2006;6:1654-1662. |
| 118. | Craven PA, Pfanstiel J, DeRubertis FR. Role of reactive oxygen in bile salt stimulation of colonic epithelial proliferation. J Clin Invest. 1986;77:850-859. |
| 119. | Bernstein H, Holubec H, Bernstein C, Ignatenko N, Gerner E, Dvorak K, Besselsen D, Ramsey L, Dall'Agnol M, Blohm-Mangone KA. Unique dietary-related mouse model of colitis. Inflamm Bowel Dis. 2006;12:278-293. |
| 120. | Wang L, Baudhuin LM, Boardman LA, Steenblock KJ, Petersen GM, Halling KC, French AJ, Johnson RA, Burgart LJ, Rabe K. MYH mutations in patients with attenuated and classic polyposis and with young-onset colorectal cancer without polyps. Gastroenterology. 2004;127:9-16. |
| 121. | Jenkins MA, Croitoru ME, Monga N, Cleary SP, Cotterchio M, Hopper JL, Gallinger S. Risk of colorectal cancer in monoallelic and biallelic carriers of MYH mutations: a population-based case-family study. Cancer Epidemiol Biomarkers Prev. 2006;15:312-314. |
| 122. | Kulkarni MS, Yielding KL. DNA damage and repair in epithelial (mucous) cells and crypt cells from isolated colon. Chem Biol Interact. 1985;52:311-318. |
| 123. | Pool-Zobel BL, Leucht U. Induction of DNA damage by risk factors of colon cancer in human colon cells derived from biopsies. Mutat Res. 1997;375:105-115. |
| 124. | Payne CM, Crowley C, Washo-Stultz D, Briehl M, Bernstein H, Bernstein C, Beard S, Holubec H, Warneke J. The stress-response proteins poly(ADP-ribose) polymerase and NF-kappaB protect against bile salt-induced apoptosis. Cell Death Differ. 1998;5:623-636. |
| 125. | Rosignoli P, Fabiani R, De Bartolomeo A, Fuccelli R, Pelli MA, Morozzi G. Genotoxic effect of bile acids on human normal and tumour colon cells and protection by dietary antioxidants and butyrate. Eur J Nutr. 2008;47:301-309. |
| 126. | Booth LA, Bilton RF. Genotoxic potential of the secondary bile acids: a role for reactive oxygen species. DNA and free radicals: Techniques, mechanisms and applications. London: OICA International 1998; 161-177. |
| 127. | Powolny A, Xu J, Loo G. Deoxycholate induces DNA damage and apoptosis in human colon epithelial cells expressing either mutant or wild-type p53. Int J Biochem Cell Biol. 2001;33:193-203. |
| 128. | Glinghammar B, Inoue H, Rafter JJ. Deoxycholic acid causes DNA damage in colonic cells with subsequent induction of caspases, COX-2 promoter activity and the transcription factors NF-kB and AP-1. Carcinogenesis. 2002;23:839-845. |
| 129. | Romagnolo DF, Chirnomas RB, Ku J, Jeffy BD, Payne CM, Holubec H, Ramsey L, Bernstein H, Bernstein C, Kunke K. Deoxycholate, an endogenous tumor promoter and DNA damaging agent, modulates BRCA-1 expression in apoptosis-sensitive epithelial cells: loss of BRCA-1 expression in colonic adenocarcinomas. Nutr Cancer. 2003;46:82-92. |
| 130. | Qiao D, Im E, Qi W, Martinez JD. Activator protein-1 and CCAAT/enhancer-binding protein mediated GADD153 expression is involved in deoxycholic acid-induced apoptosis. Biochim Biophys Acta. 2002;1583:108-116. |
| 131. | Scott DW, Mutamba S, Hopkins RG, Loo G. Increased GADD gene expression in human colon epithelial cells exposed to deoxycholate. J Cell Physiol. 2005;202:295-303. |
| 132. | Narahara H, Tatsuta M, Iishi H, Baba M, Uedo N, Sakai N, Yano H, Ishiguro S. K-ras point mutation is associated with enhancement by deoxycholic acid of colon carcinogenesis induced by azoxymethane, but not with its attenuation by all-trans-retinoic acid. Int J Cancer. 2000;88:157-161. |
| 133. | Payne CM, Bernstein C, Dvorak K, Bernstein H. Hydrophobic bile acids, genomic instability, Darwinian selection, and colon carcinogenesis. Clinical and Experimental Gastroenterology. 2008;1:19-47. |
| 134. | Bernstein C, Bernstein H, Payne CM, Garewal H. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat Res. 2002;511:145-178. |
| 135. | Payne CM, Bernstein H, Bernstein C, Garewal H. Role of apoptosis in biology and pathology: resistance to apoptosis in colon carcinogenesis. Ultrastruct Pathol. 1995;19:221-248. |
| 136. | Samaha HS, Bernstein C, Payne CM, Garewal HS, Sampliner RE, Bernstein H. Bile salt induction of apoptosis in goblet cells of the normal human colonic mucosa: relevance to colon cancer. Acta Microsc. 1995;4:43-58. |
| 137. | Garewal H, Bernstein H, Bernstein C, Sampliner R, Payne C. Reduced bile acid-induced apoptosis in "normal" colorectal mucosa: a potential biological marker for cancer risk. Cancer Res. 1996;56:1480-1483. |
| 138. | Bernstein C, Bernstein H, Garewal H, Dinning P, Jabi R, Sampliner RE, McCuskey MK, Panda M, Roe DJ, L'Heureux L. A bile acid-induced apoptosis assay for colon cancer risk and associated quality control studies. Cancer Res. 1999;59:2353-2357. |
| 139. | Bernstein H, Holubec H, Warneke JA, Garewal H, Earnest DL, Payne CM, Roe DJ, Cui H, Jacobson EL, Bernstein C. Patchy field defects of apoptosis resistance and dedifferentiation in flat mucosa of colon resections from colon cancer patients. Ann Surg Oncol. 2002;9:505-517. |
| 140. | Hague A, Elder DJ, Hicks DJ, Paraskeva C. Apoptosis in colorectal tumour cells: induction by the short chain fatty acids butyrate, propionate and acetate and by the bile salt deoxycholate. Int J Cancer. 1995;60:400-406. |
| 141. | Marchetti MC, Migliorati G, Moraca R, Riccardi C, Nicoletti I, Fabiani R, Mastrandrea V, Morozzi G. Possible mechanisms involved in apoptosis of colon tumor cell lines induced by deoxycholic acid, short-chain fatty acids, and their mixtures. Nutr Cancer. 1997;28:74-80. |
| 142. | Milovic V, Teller IC, Faust D, Caspary WF, Stein J. Effects of deoxycholate on human colon cancer cells: apoptosis or proliferation. Eur J Clin Invest. 2002;32:29-34. |
| 143. | Martinez JD, Stratagoules ED, LaRue JM, Powell AA, Gause PR, Craven MT, Payne CM, Powell MB, Gerner EW, Earnest DL. Different bile acids exhibit distinct biological effects: the tumor promoter deoxycholic acid induces apoptosis and the chemopreventive agent ursodeoxycholic acid inhibits cell proliferation. Nutr Cancer. 1998;31:111-118. |
| 144. | Powell AA, LaRue JM, Batta AK, Martinez JD. Bile acid hydrophobicity is correlated with induction of apoptosis and/or growth arrest in HCT116 cells. Biochem J. 2001;356:481-486. |
| 145. | Yui S, Saeki T, Kanamoto R, Iwami K. Characteristics of apoptosis in HCT116 colon cancer cells induced by deoxycholic acid. J Biochem. 2005;138:151-157. |
| 146. | Yui S, Kanamoto R, Saeki T. Deoxycholic acid can induce apoptosis in the human colon cancer cell line HCT116 in the absence of Bax. Nutr Cancer. 2008;60:91-96. |
| 147. | Crowley CL, Payne CM, Bernstein H, Bernstein C, Roe D. The NAD+ precursors, nicotinic acid and nicotinamide protect cells against apoptosis induced by a multiple stress inducer, deoxycholate. Cell Death Differ. 2000;7:314-326. |
| 148. | Im E, Martinez JD. Ursodeoxycholic acid (UDCA) can inhibit deoxycholic acid (DCA)-induced apoptosis via modulation of EGFR/Raf-1/ERK signaling in human colon cancer cells. J Nutr. 2004;134:483-486. |
| 149. | Zhou J, Liu M, Zhai Y, Xie W. The antiapoptotic role of pregnane X receptor in human colon cancer cells. Mol Endocrinol. 2008;22:868-880. |
| 150. | Washo-Stultz D, Crowley C, Payne CM, Bernstein C, Marek S, Gerner EW, Bernstein H. Increased susceptibility of cells to inducible apoptosis during growth from early to late log phase: an important caveat for in vitro apoptosis research. Toxicol Lett. 2000;116:199-207. |
| 151. | Katona BW, Anant S, Covey DF, Stenson WF. Characterization of enantiomeric bile acid-induced apoptosis in colon cancer cell lines. J Biol Chem. 2009;284:3354-3364. |
| 152. | Haza AI, Glinghammar B, Grandien A, Rafter J. Effect of colonic luminal components on induction of apoptosis in human colonic cell lines. Nutr Cancer. 2000;36:79-89. |
| 153. | Schlottman K, Wachs FP, Krieg RC, Kullmann F, Schölmerich J, Rogler G. Characterization of bile salt-induced apoptosis in colon cancer cell lines. Cancer Res. 2000;60:4270-4276. |
| 154. | Wachs FP, Krieg RC, Rodrigues CM, Messmann H, Kullmann F, Knüchel-Clarke R, Schölmerich J, Rogler G, Schlottmann K. Bile salt-induced apoptosis in human colon cancer cell lines involves the mitochondrial transmembrane potential but not the CD95 (Fas/Apo-1) receptor. Int J Colorectal Dis. 2005;20:103-113. |
| 155. | Payne CM, Waltmire CN, Crowley C, Crowley-Weber CL, Dvorakova K, Bernstein H, Bernstein C, Holubec H, Garewal H. Caspase-6 mediated cleavage of guanylate cyclase alpha 1 during deoxycholate-induced apoptosis: protective role of the nitric oxide signaling module. Cell Biol Toxicol. 2003;19:373-392. |
| 156. | Holubec H, Payne CM, Bernstein H, Dvorakova K, Bernstein C, Waltmire CN, Warneke JA, Garewal H. Assessment of apoptosis by immunohistochemical markers compared to cellular morphology in ex vivo-stressed colonic mucosa. J Histochem Cytochem. 2005;53:229-235. |
| 157. | Badvie S, Hanna-Morris A, Andreyev HJ, Cohen P, Saini S, Allen-Mersh TG. A "field change" of inhibited apoptosis occurs in colorectal mucosa adjacent to colorectal adenocarcinoma. J Clin Pathol. 2006;59:942-946. |
| 158. | Fang C, Dean J, Smith JW. A novel variant of ileal bile acid binding protein is up-regulated through nuclear factor-kappaB activation in colorectal adenocarcinoma. Cancer Res. 2007;67:9039-9046. |
| 159. | Crowley-Weber CL, Payne CM, Gleason-Guzman M, Watts GS, Futscher B, Waltmire CN, Crowley C, Dvorakova K, Bernstein C, Craven M. Development and molecular characterization of HCT-116 cell lines resistant to the tumor promoter and multiple stress-inducer, deoxycholate. Carcinogenesis. 2002;23:2063-2080. |
| 160. | Bree RT, Neary C, Samali A, Lowndes NF. The switch from survival responses to apoptosis after chromosomal breaks. DNA Repair (Amst). 2004;3:989-995. |
| 161. | Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440-450. |
| 162. | Plesca D, Mazumder S, Almasan A. DNA damage response and apoptosis. Methods Enzymol. 2008;446:107-122. |
| 163. | Matés JM, Segura JA, Alonso FJ, Márquez J. Intracellular redox status and oxidative stress: implications for cell proliferation, apoptosis, and carcinogenesis. Arch Toxicol. 2008;82:273-299. |
| 164. | Payne CM, Crowley-Skillicorn C, Holubec H, Dvorak K, Bernstein C, Moyer MP, Garewal H, Bernstein H. Deoxycholate, an endogenous cytotoxin/genotoxin, induces the autophagic stress-survival pathway: Implications for colon carcinogenesis. J Toxicol. 2009;82:Epub ahead of print. |
| 165. | Schreck R, Albermann K, Baeuerle PA. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun. 1992;17:221-237. |
| 166. | Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777-790. |
| 167. | Wang Q, Gao F, May WS, Zhang Y, Flagg T, Deng X. Bcl2 negatively regulates DNA double-strand-break repair through a nonhomologous end-joining pathway. Mol Cell. 2008;29:488-498. |
| 168. | Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362-1365. |
| 169. | Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365-1368. |
| 170. | Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543-553. |
| 171. | Modica S, Murzilli S, Salvatore L, Schmidt DR, Moschetta A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 2008;68:9589-9594. |
| 172. | Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313-1316. |
| 173. | Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, Evans RM. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci USA. 2001;98:3375-3380. |
| 174. | Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci USA. 2001;98:3369-3374. |