Published online Jun 28, 2009. doi: 10.3748/wjg.15.3009
Revised: January 31, 2009
Accepted: February 7, 2009
Published online: June 28, 2009
AIM: To investigate the effect of heme oxygenase-1 (HO-1) expression on immune liver fibrosis induced by cobalt protoporphyrin (CoPP) in rats.
METHODS: An immune liver fibrosis model of rat was established by administering human serum albumin (HSA). The rats were divided into CoPP, liver fibrosis and normal control groups. Rats in the CoPP group received intraperitoneal CoPP concurrently with HSA. Expression of HO-1 protein was observed by Western blotting and immunohistochemistry. Hematoxylin and eosin (HE) staining was performed to assess fibrosis proliferation and distribution, proliferation extent of fibroblasts, and alterations in hepatocytes and inflammatory cells. Type I and III collagens were detected with Van Gieson’s (VG) staining and Foot’s reticular fiber staining, respectively. In addition, spindle-shaped cells existing at perisinusoidal locations beyond portal and septa areas were investigated with HE staining.
RESULTS: Western blotting and immunohistochemistry showed that the expression of HO-1 protein was higher in the CoPP group than in the liver fibrosis group (P < 0.05). Compared with the liver fibrosis group, the serological index of hepatic fibrosis in the CoPP group decreased significantly (P < 0.05). HE, VG and Foot’s staining revealed that administration of CoPP reduced the extent of hepatic fibrosis. The levels of serological indicators and the number of spindle-shaped cells at perisinuous locations beyond the portal and septa areas were reduced in the CoPP group. Only a few inflammatory cells were seen around the portal areas and central veins in the CoPP group.
CONCLUSION: Increased endogenous HO-1 may suppress liver fibrosis by protecting liver cells, inhibiting inflammatory cell infiltration and hepatic stellate cell transformation.
- Citation: Wang F, Duan ZJ, Sun YJ. Influence of heme oxygenase-1 expression on immune liver fibrosis induced by cobalt protoporphyrin in rats. World J Gastroenterol 2009; 15(24): 3009-3014
- URL: https://www.wjgnet.com/1007-9327/full/v15/i24/3009.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3009
Heme oxygenase-1 (HO-1) and heme degradation products of biliverdin, bilirubin, CO and free iron play an important role in many physiological and pathological processes, such as liver ischemia-reperfusion injury, liver transplantation, and acute liver injury[1–5]. In the present study, we investigated the effects of HO-1 expression on immune liver fibrosis induced by cobalt protoporphyrin (CoPP) in rats.
Healthy male Sprague-Dawley rats, weighing 220-270 g, were obtained from the Laboratory Animal Center of Dalian Medical University.
CoPP injections were prepared by dissolving the compound in 0.2 mmol/L NaOH, adjusting its pH value to 7.4 with 1 mmol/L HCl, and diluting it with 0.9% NaCl to a final concentration of l mg/mL, as previously described[6]. Twenty percent human serum albumin (HSA) injection (Instituto Grifols, S.A. Barcelona, Spain), Freunds’ incomplete adjuvant (Santa Cruz Biotechnology, Santa Cruz, CA, USA), protoporphyrin IX cobalt chloride (Sigma, St Louis, MO, USA), rabbit anti-HO-1 (Boster Biological Technology, Wuhan, China), and anti-mouse IgG, anti-rabbit IgG (Zhong Shan Golden Bridge Biotechnology, Beijing, China) were used in the study.
Animals were divided randomly into a liver fibrosis group (n = 20), a CoPP group (n = 20), and a normal control group (n = 12). An immune liver fibrosis rat model was established as previously described[7]. Rats were allergized with HSA, and then injured by injecting albumin into the tail vein (2.5 mg per rat each time and increased gradually to 4.5 mg, twice weekly for 6 wk). Rats in the CoPP group received intraperitoneal CoPP (5 mg/kg) concurrently with HSA administration.
Rats were attacked by HSA for 6 wk, fasted for 12 h, and then weighed. Three percent sodium pentobarbital injection (2 mL/kg) was used as an anesthetic agent. Four milliliters of blood was collected from the eyeball of rats and stored at -20°C. Livers were removed from the rats, fixed in a 10% neutral formalin solution, embedded in paraffin, and preserved at -80°C.
One milliliter of lysate containing 20 mmol/L Tris (pH 7.5), 150 mmol/L NaCl, 1%Triton X-100, 1 mmol/L phenylmethanesulfonylfluoride, was added to 100 mg liver tissue. The mixture was homogenized by centrifugation at 12 000 r/min for 3-5 min at 4°C, and the supernatant was separated. After SDS-PAGE, the sample was transferred to a polyvinylidine fluoride membrane and stained with 3,3' diaminobenzidine. The sample was incubated with primary antibody (rabbit-anti-mouse HO-1 monoclonal antibody, 1:100) and secondary antibody (peroxidase-labeled sheep-anti-rabbit antibody, 1:100), with β-actin as an internal reference.
Paraffin-embedded liver tissue was cut into sections, which were routinely stained with HE. Cells in good condition were stained using an immunohistochemical method (streptavidin-peroxidase method), after dewaxing, hydration, inactivation, incubation with primary antibody (rabbit-anti-mouse HO-1 monoclonal antibody; 1:150) and secondary antibody (biotin-labeled sheep-anti-rabbit antibody; 1:100), and mounting. The primary antibody was administered with PBS to serve as a negative control. Yellow material in the cytolymph was considered to be a positive cell. Five high-power microscopic fields were randomly chosen per slice. The percentage of positive cells increased as the intensity of staining increased in every field. Ultimately, the average of five fields was used to compare differences between groups.
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured with an automatic biochemical analyzer.
The levels of serum hyaluronic acid (HA), laminin (LN), type III procollagen (PC III) and type IV collagen (IV-C) were measured using a gamma radioimmunoassay counter.
Proliferation and distribution of liver fibrous tissue, proliferation of fibroblasts and changes in liver cells, portal areas and central veins were observed by HE staining[8]. Spindle-shaped cells existing at the perisinuous location beyond the portal and septal areas were counted. The proliferation degree of type I and III collagen was observed with Van Gieson’s (VG) and Foot’s staining, respectively[8].
Data analysis was performed using SPSS 10.0 software (Chicago, IL, USA). Analysis of variance (ANOVA) or Wilcoxon statistical methods were used to determine statistical significance. The results were expressed as mean ± SD. P < 0.05 was considered statistically significant.
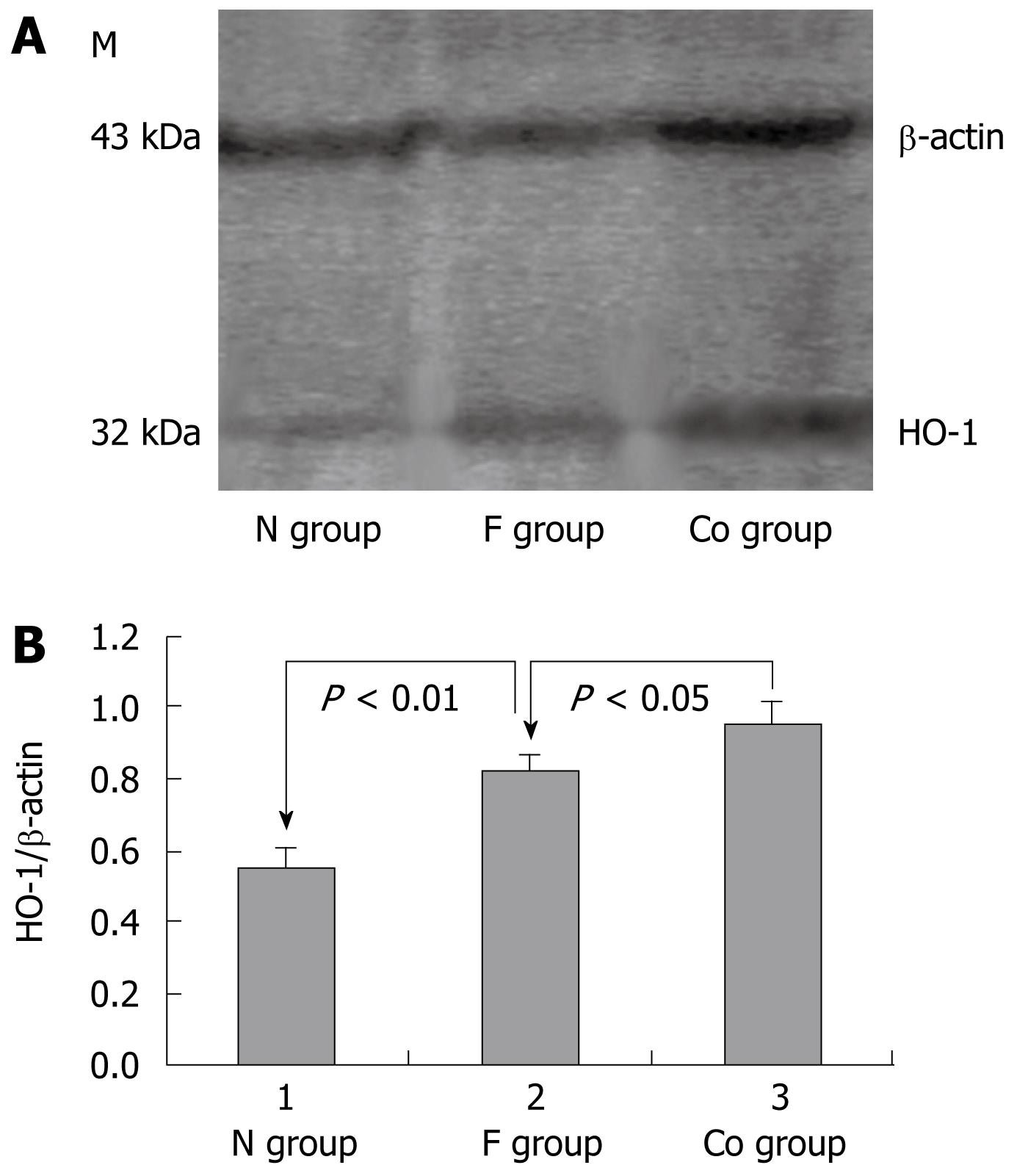
The HO-1 protein expression level was significantly higher in the liver fibrosis group than in the normal control group (P < 0.01) and higher in the CoPP group than in the liver fibrosis group (P < 0.05, Figure 1).
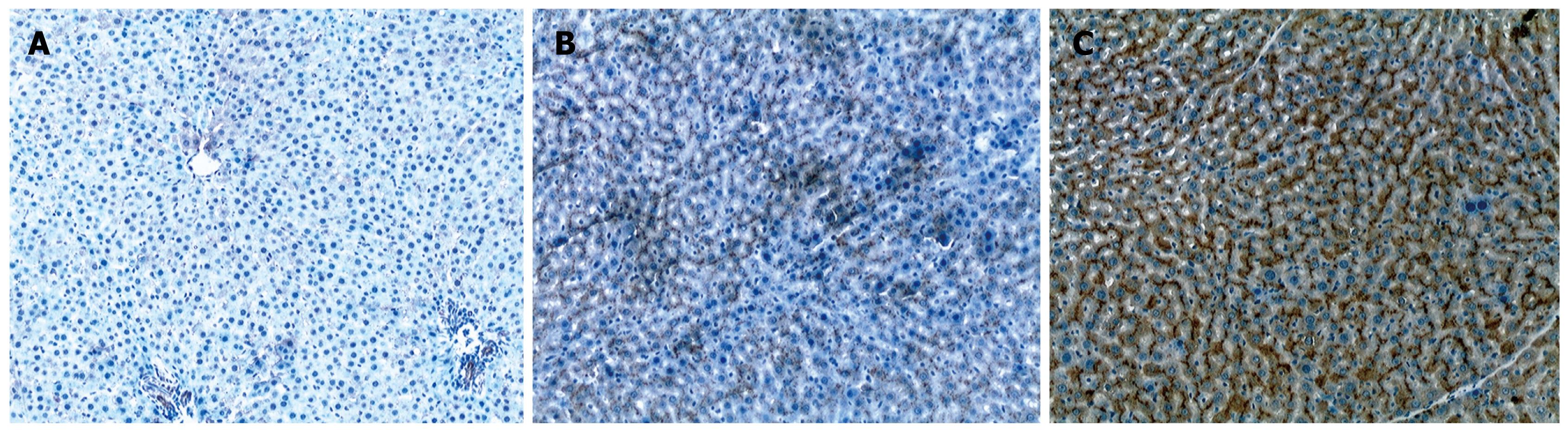
Liver cells were not or only slightly stained in the normal control group (Figure 2A). Liver cells and mesenchymal cells were diffusely and unevenly stained in the liver fibrosis group (Figure 2B) and diffusely but strongly stained in the CoPP group (Figure 2C). Compared with the liver fibrosis group, the staining intensity and scope increased significantly in the CoPP group. The score for HO-1 protein expression was 0.80 ± 0.79 in the normal control group, 4.00 ± 1.31 in the liver fibrosis group, and 5.52 ± 1.15 in the CoPP group. The score for HO-1 protein expression was significantly higher in the CoPP group than in the liver fibrosis group (P < 0.05).
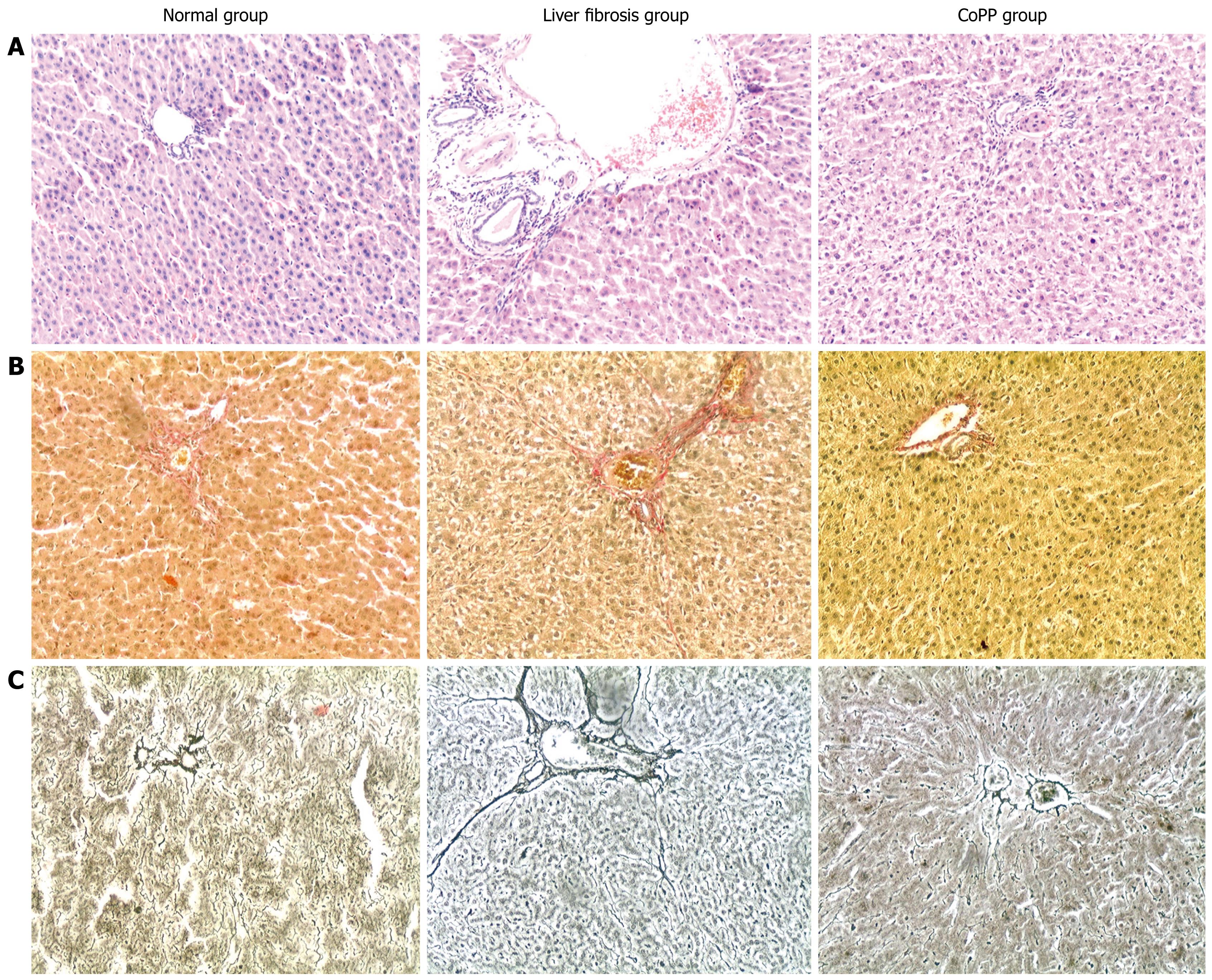
The level of serological indicators was significantly higher in the liver fibrosis group than in the normal control group (P < 0.01), and significantly lower in the liver fibrosis group than in the CoPP group (P < 0.05, Table 1). The structure of hepatic lobules seemed integral without fibrous hyperplasia, and some collagen fibers were observed in the portal areas of the normal control group (Figure 3). Significant fibrous hyperplasia and fibrosis extension in the portal areas with fibroblast proliferation, widened lobular septa with more fiber deposition, and formation of fibrous septa were found in the liver fibrosis group (Figure 3). Compared with the liver fibrosis group, fibrous hyperplasia was significantly reduced and fine fibers were seen occasionally and distributed mainly in the portal areas of the CoPP group. The number of fibroblasts was also decreased in the CoPP group (Figure 3). Compared with the normal control group, there was a significant increase in fibrosis and hyperplasia of fibroblasts, and type I and III collagens in the liver fibrosis group (P < 0.01). The extent of fibrosis was lower in the CoPP group than in the liver fibrosis group (Table 2).
| Group | n | - | + | ++ | +++ |
| HE staining | |||||
| N | 11 | 11 | 0 | 0 | 0 |
| F | 15 | 0 | 8 | 7 | 0 |
| Co | 17 | 0 | 15 | 2 | 0 |
| Foot staining | |||||
| N | 11 | 11 | 0 | 0 | 0 |
| F | 15 | 0 | 4 | 8 | 3 |
| Co | 17 | 0 | 10 | 7 | 0 |
| VG staining | |||||
| N | 11 | 11 | 0 | 0 | 0 |
| F | 15 | 0 | 7 | 6 | 2 |
| Co | 17 | 0 | 14 | 3 | 0 |
Injection of HSA increased serum ALT and AST levels (P < 0.01). The serum ALT and AST levels were lower in the CoPP group than in the liver fibrosis group (P < 0.05, Table 3). Rat liver cells were relatively uniform, yet most were swollen with a small amount of fatty degeneration and few nuclei were strongly stained and dissolved in the liver fibrosis group. In the CoPP group, most liver cells were normal. Only a small number were swollen and no degeneration of cells was observed, suggesting that HO-1 expression can protect impaired hepatocytes at a certain extent. The number of spindle-shaped cells was 37.2 ± 4.7 in the normal control group, 55.2 ± 3.5 in the liver fibrosis group, and 50.6 ± 3.6 in the CoPP group (P < 0.01). Meanwhile, the number of spindle-shaped cells, which were confirmed by electron microscopy to be activated hepatic stellate cells (HSCs), was reduced in the CoPP group. In the liver fibrosis group, a large number of inflammatory cells infiltrated the portal areas. Most of them were lymphocytes, and a small number were neutrophils. A small number of inflammatory cells were observed in the CoPP group.
Many chronic liver diseases progress to liver fibrosis[9]. The core aspect of the occurrence and development of hepatic fibrosis is to activate HSCs and transform them into myofibroblast-like cells[1011]. Various factors result in hepatic fibrosis. Interactions between cells, cells and matrix, or transmitters and matrix, constitute a complex network that underlies the occurrence and development of liver fibrosis[12]. If the cause could be suppressed and the network systems could be impeded, hepatic fibrosis or cirrhosis could be effectively prevented.
Heme is catalyzed into CO, biliverdin and free iron by HO-1, a rate-limiting enzyme in heme metabolism. HO-1 expression can be induced by various stress factors, such as oxidative stress, inflammatory factors and heavy metals. In addition, some protoporphyrins, especially CoPP, can increase HO-1 expression in vivo. HO-1, as a protective protein in vivo, plays a vital role in many aspects, such as anti-oxidative stress, anti-inflammation, anti-cell proliferation, and regulation of cytokine expression.
HO-1 is only expressed in Kupffer cells (KCs) of normal liver tissue. In hepatic cirrhosis, however, it is expressed mainly in KCs and fibroblasts[1314]. HO-1 can inhibit HSC proliferation and type I collagen mRNA expression in cultured human liver fibroblasts. When the adeno-associated-virus-mediated HO-1 gene is injected into portal veins of rats with micronodular cirrhosis induced by CCl4, HO-1-transduced HSCs can reduce type I collagen transcription, proliferation ability and macrophage infiltration, thus improving the biochemical function of the liver[1516].
The main cause for chronic liver disease in China is hepatitis B virus (HBV) infections HBV cannot be used to establish animal models of liver fibrosis. Some scholars believe that rat models of immune liver fibrosis induced by HSA have some similarities in morphology and pathogenesis to liver fibrosis caused by HBV[17]. Therefore, it is of great significance to study the effects of HO-1 on immune liver fibrosis.
In the present study, HO-1 protein expression was observed in the CoPP group. Type III collagen was significantly increased early in hepatic fibrosis. Type I collagen fibers are mainly distributed at the fibrous septa, while type III collagen fibers are associated with the reticular fibers[1819]. In addition, serum HA, PC III, LN and IV-C levels are closely correlated with liver fibrosis[2021], indicating that increased HO-1 expression can suppress the occurrence and development of liver fibrosis.
We investigated preliminarily the effect of HO-1 expression on immune liver fibrosis induced by CoPP in rats. When liver cells were destroyed, the membrane permeability was increased and the mitochondria were damaged, and the activity of AST and ALT was elevated. The extent of damage to liver cells is consistent with the level of enzymatic activity[22]. Compared with the liver fibrosis group, serum AST and ALT levels were significantly increased in the CoPP group, indicating that HO-1 protects liver cells. Wen et al[23] have established a liver injury model induced by D-galactosamine and lipopolysaccharide (LPS), and found that pretreatment with hemoglobin increases HO-1, reduces damage to the liver, and lowers serum ALT and AST levels, thus improving liver disease. Nakahira et al[24] have shown that HO-1 activity inhibited by tin-protoporphyrin can lead to severe liver damage and significantly increased ALT levels. Moreover, CO also exerts protective effects on liver cells. Amersi et al[25] have shown that CO can protect liver cells, the AST levels were significantly lower, and the liver structure was normal without swelling or necrosis in the experimental group. Both biliverdin and bilirubin are antioxidants. When biliverdin is transformed into bilirubin, it can strengthen the oxidative capacity, while bilirubin protects the lipid bimolecular layer of the cell membrane[26], suggesting that HO-1 protects liver cells by consuming oxygen molecules and reducing oxygen free radicals, and prevents the peroxidation of free radicals in membrane lipids and maintains membrane integrity and normal physiological functions.
A high HO-1 expression level induced by CoPP plays an important role in reduction of the inflammatory response of liver cells. During inflammatory responses, local blood flow is reduced and leukocytes adhere to activated endothelial cells, thus releasing enzymes and causing cell injury. The key step is adhesion between leukocytes and endothelial cells. Selectin and cell adhesion factors are also involved in the process.
Anti-inflammatory effects of HO/CO are achieved by reducing the expression of adhesion molecules[27]. Macrophages are also activated in inflammatory responses, and produce arachidonic acid substances, tumor necrosis factor-α (TNF-α), and other inflammatory mediators that act on the liver and cause liver injury. LPS can stimulate KCs to generate inflammatory mediators. CO may prevent the expression of inflammatory cytokines [i.e. TNF-α and interleukin (IL)-1β][28], inhibit the secretion of IL-2[29], and reduce T-cell proliferation, thus achieving an anti-inflammatory effect. In addition, CO can increase the expression of anti-inflammatory cytokine IL-10, which can induce HO-1 expression, allowing HO-1 to produce more CO[30]. Thus, a reduction of inflammation induced by HO-1 is achieved by decreasing the activation of KCs, which suppresses the migration and adhesion of leukocytes, and inhibits the release of inflammatory mediators, finally relieves the inflammatory damage to endothelial cells.
A high HO-1 expression level induced by CoPP can inhibit the transformation of HSCs to fibroblasts. In the present study, the number of spindle-shaped cells was decreased in the CoPP group and increased in the liver fibrosis group. Most of these cells were activated HSCs, indicating that HO-1 can inhibit HSC activation. The fibroblasts of α-smooth muscle that express actin are transformed from dormant HSCs to live epithelial or matrix cells. Irrespective of its origin, the activity of fibroblasts is associated with cytokines and chemotactic factors secreted by macrophages[31]. Normal liver cell membranes have a contact-inhibition effect on the proliferation of HSCs and KCs. When liver cells are damaged, destruction of the cell membrane leads to loss of the contact-inhibition effect on HSCs, thus leading to activation of HSCs. The more HSCs are activated, the more cytokines are secreted (including insulin-like growth factor-1). Finally, the feed-forward cycle prevents the reversal of liver fibrosis. In short, HO-1 may inhibit the activation of HSCs.
In conclusion, HO-1 inhibits liver fibrosis and is closely related to other liver diseases. Further study is needed to elucidate its protective effect on immune liver fibrosis.
Heme oxygenase-1 (HO-1) and heme degradation products are involved in acute liver injury. However, their influence on chronic liver injury is still unclear.
There are still many unclear or confusing problems about the development and progression of liver cirrhosis. These problems result in difficulties in the treatment of cirrhosis. It is of great significance to study the effects of HO-1 on immune liver fibrosis. The expression of HO-1 induced by drugs may provide a promising way to treat successfully liver fibrosis.
By establishing a model of immune liver fibrosis in rats, the authors investigated the effect of HO-1 induced by cobalt protoporphyrin (CoPP), which may provide a new treatment modality for liver fibrosis.
The expression of HO-1 induced by drugs in target organs may provide a promising way to treat liver fibrosis.
HO-1 stands for heme oxygenase-1, which is a rate-limiting enzyme. It is also known as heat shock protein 32, and is susceptible to various stress factors such as oxidative stress, inflammatory factors, and heavy metals. In addition, some protoporphyrins, especially CoPP can increase HO-1 expression in vivo.
The study is interesting. The authors showed that increased expression of HO-1 could suppress liver fibrosis, thus providing a new treatment modality for liver fibrosis.
| 1. | Clark JE, Foresti R, Green CJ, Motterlini R. Dynamics of haem oxygenase-1 expression and bilirubin production in cellular protection against oxidative stress. Biochem J. 2000;348 Pt 3:615-619. |
| 2. | Sass G, Seyfried S, Parreira Soares M, Yamashita K, Kaczmarek E, Neuhuber WL, Tiegs G. Cooperative effect of biliverdin and carbon monoxide on survival of mice in immune-mediated liver injury. Hepatology. 2004;40:1128-1135. |
| 3. | Sass G, Soares MC, Yamashita K, Seyfried S, Zimmermann WH, Eschenhagen T, Kaczmarek E, Ritter T, Volk HD, Tiegs G. Heme oxygenase-1 and its reaction product, carbon monoxide, prevent inflammation-related apoptotic liver damage in mice. Hepatology. 2003;38:909-918. |
| 4. | Neto JS, Nakao A, Kimizuka K, Romanosky AJ, Stolz DB, Uchiyama T, Nalesnik MA, Otterbein LE, Murase N. Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. Am J Physiol Renal Physiol. 2004;287:F979-F989. |
| 5. | Camara NO, Soares MP. Heme oxygenase-1 (HO-1), a protective gene that prevents chronic graft dysfunction. Free Radic Biol Med. 2005;38:426-435. |
| 6. | Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104:1631-1639. |
| 7. | Wang BE. [Animals with liver fibrosis induced by albumin immunization]. Zhonghua Yixue Zazhi. 1989;69:503-505, 536. |
| 8. | Duan ZJ, Lu S, Li SR, Wang YD, Huang TW. Protective effect of hepatic stimulating substance (HSS) on immune hepatic fibrosis in rat models. Zhonghua Xiaohua Zazhi. 1997;17:138-140. |
| 9. | Lamireau T, Desmouliere A, Bioulac-Sage P, Rosenbaum J. [Mechanisms of hepatic fibrogenesis]. Arch Pediatr. 2002;9:392-405. |
| 10. | Mann DA, Smart DE. Transcriptional regulation of hepatic stellate cell activation. Gut. 2002;50:891-896. |
| 11. | Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665-672. |
| 12. | Long Y, Tang H. Role of transcription factor in regulation and control of liver cirrhosis. Shijie Huaren Xiaohua Zazhi. 2006;14:969-972. |
| 13. | Li L, Grenard P, Nhieu JT, Julien B, Mallat A, Habib A, Lotersztajn S. Heme oxygenase-1 is an antifibrogenic protein in human hepatic myofibroblasts. Gastroenterology. 2003;125:460-469. |
| 14. | Li L, Julien B, Grenard P, Teixeira-Clerc F, Mallat A, Lotersztajn S. Molecular mechanisms regulating the antifibrogenic protein heme-oxygenase-1 in human hepatic myofibroblasts. J Hepatol. 2004;41:407-413. |
| 15. | Tsui TY, Lau CK, Ma J, Wu X, Wang YQ, Farkas S, Xu R, Schlitt HJ, Fan ST. rAAV-mediated stable expression of heme oxygenase-1 in stellate cells: a new approach to attenuate liver fibrosis in rats. Hepatology. 2005;42:335-342. |
| 16. | Tsui TY, Lau CK, Ma J, Glockzin G, Obed A, Schlitt HJ, Fan ST. Adeno-associated virus-mediated heme oxygenase-1 gene transfer suppresses the progression of micronodular cirrhosis in rats. World J Gastroenterol. 2006;12:2016-2023. |
| 17. | Blackwell JB. Cirrhosis resulting from repeated injections of antigen. J Pathol Bacteriol. 1965;90:245-257. |
| 18. | Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S79-S84. |
| 19. | Kisseleva T, Brenner DA. Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S73-S78. |
| 20. | Lichtinghagen R, Bahr MJ. Noninvasive diagnosis of fibrosis in chronic liver disease. Expert Rev Mol Diagn. 2004;4:715-726. |
| 21. | Xie S, Yao J, Zheng R, Peng X, Gao Z. [Accurate diagnosis of stages of hepatic fibrosis by measuring levels of serum hyaluronic acid, procollagen type III, and collagen type IV]. Zhonghua Ganzangbing Zazhi. 2001;9:334-336. |
| 22. | Holoman J, Glasa J, Galbavy S, Danis D, Molnarova A, Kazar J, Bednarova A, Misianik J. Serum markers of liver fibrogenesis, and liver histology findings in patients with chronic liver diseases. Bratisl Lek Listy. 2002;103:70-75. |
| 23. | Wen T, Wu ZM, Liu Y, Tan YF, Ren F, Wu H. Upregulation of heme oxygenase-1 with hemin prevents D-galactosamine and lipopolysaccharide-induced acute hepatic injury in rats. Toxicology. 2007;237:184-193. |
| 24. | Nakahira K, Takahashi T, Shimizu H, Maeshima K, Uehara K, Fujii H, Nakatsuka H, Yokoyama M, Akagi R, Morita K. Protective role of heme oxygenase-1 induction in carbon tetrachloride-induced hepatotoxicity. Biochem Pharmacol. 2003;66:1091-1105. |
| 25. | Amersi F, Shen XD, Anselmo D, Melinek J, Iyer S, Southard DJ, Katori M, Volk HD, Busuttil RW, Buelow R. Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35:815-823. |
| 26. | Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA. 2002;99:16093-16098. |
| 27. | Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev. 2003;55:551-571. |
| 28. | Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422-428. |
| 29. | Pae HO, Oh GS, Choi BM, Chae SC, Kim YM, Chung KR, Chung HT. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J Immunol. 2004;172:4744-4751. |