Published online Jun 28, 2009. doi: 10.3748/wjg.15.2995
Revised: May 11, 2009
Accepted: May 18, 2009
Published online: June 28, 2009
AIM: To study the methods of preparing the magnetic nano-microspheres of Fe2O3 and As2O3/Fe2O3 complexes and their therapeutic effects with magnetic fluid hyperthermia (MFH).
METHODS: Nanospheres were prepared by chemical co-precipitation and their shape and diameter were observed. Hemolysis, micronucleus, cell viability, and LD50 along with other in vivo tests were performed to evaluate the Fe2O3 microsphere biocompatibility. The inhibition ratio of tumors after Fe2O3 and As2O3/Fe2O3 injections combined with induced hyperthermia in xenograft human hepatocarcinoma was calculated.
RESULTS: Fe2O3 and As2O3/Fe2O3 particles were round with an average diameter of 20 nm and 100 nm as observed under transmission electron microscope. Upon exposure to an alternating magnetic field (AMF), the temperature of the suspension of magnetic particles increased to 41-51°C, depending on different particle concentrations, and remained stable thereafter. Nanosized Fe2O3 microspheres are a new kind of biomaterial without cytotoxic effects. The LD50 of both Fe2O3 and As2O3/Fe2O3 in mice was higher than 5 g/kg. One to four weeks after Fe2O3 and As2O3/Fe2O3 complex injections into healthy pig livers, no significant differences were found in serum AST, ALT, BUN and Cr levels among the pigs of all groups (P > 0.05), and no obvious pathological alterations were observed. After exposure to alternating magnetic fields, the inhibition ratio of the tumors was significantly different from controls in the Fe2O3 and As2O3/Fe2O3 groups (68.74% and 82.79%, respectively; P < 0.01). Tumors of mice in treatment groups showed obvious necrosis, while normal tissues adjoining the tumor and internal organs did not.
CONCLUSION: Fe2O3 and As2O3/Fe2O3 complexes exerted radiofrequency-induced hyperthermia and drug toxicity on tumors without any liver or kidney damage. Therefore, nanospheres are ideal carriers for tumor-targeted therapy.
- Citation: Wang ZY, Song J, Zhang DS. Nanosized As2O3/Fe2O3 complexes combined with magnetic fluid hyperthermia selectively target liver cancer cells. World J Gastroenterol 2009; 15(24): 2995-3002
- URL: https://www.wjgnet.com/1007-9327/full/v15/i24/2995.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2995
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors in China, and the incidence has increased in recent years. Current therapeutic options remain unsatisfactory for most patients. Surgical resection has been recognized as the most effective method for the treatment of hepatocarcinoma, but it is only indicated for a small number of hepatocarcinoma patients[12]. Therefore, it is crucial to identify a new method to treat hepatocarcinoma.
In recent years, radiofrequency-induced hyperthermia has increasingly attracted attention for the generation of heat in a desired zone, even in tumors deeply located inside a patient’s body. During exposure to alternating magnetic field (AMF), magnetic particles can absorb energy and transform it into heat at temperatures of 42-45°C, at which tumor cells are very sensitive[3]. In addition, the use of magnetic nanospheres, which can carry the magnetic particles into the tumor cells very easily, can greatly enhance the effects of the thermotherapy. In our research, we attempted to prepare a kind of new magnetic material that contained As2O3. We transformed the energy of the radio waves into heat to kill tumor cells and explored the therapeutic effects of nanospheres for the treatment of hepatocarcinoma.
As2O3 and dimethyl sulfoxide (DMSO) was purchased from Sigma. RPMI-1640 medium was obtained from GIBCOL-BRL. Newborn calf serum was from Si-Ji-Qing Biotechnology Co. (China). HEPES, Trypsin and methyl thiazolyl tetrazolium (MTT) were purchased from AMRESCO. The transmission electron microscope (TEM) used was a H-600 model (Hitachi, Japan) and the scanning electron microscope (SEM) model was JEOL JSM-6360LV (Japan). The energy dispersive spectrometer (EDS) was purchased from Thermo NORAN Vantage (USA).
L929 cells (human fibroblast cell line) and SMMC-7721 cells (human liver cancer cell line) were purchased from the Institute of Biochemistry and Cell Biology, Shanghai Institute of Biological Sciences, Chinese Academy of Sciences.
BALB/C nude mice (male, 10-wk-old) were purchased from the Lakes Animal Experimental Center of the Institute of Biochemistry and Cell Biology, Shanghai Institute of Biological Sciences, Chinese Academy of Sciences.
Preparation and characteristics of Fe2O3 and As2O3/Fe2O3 nanoparticles: Fe2O3 magnetic nanoparticles were prepared according to the method previously described[4]. Fe2O3 magnetic nanoparticles were added into a solution of As2O3 (0.01 mg/mL, pH = 5, adjusted by acetic acid) under a condition of supersonic dispersion. After 30 min at 80°C, the products were centrifuged at 2000 r/min for 10 min, rinsed twice by absolute alcohol, and then dried in a vacuum. The diameter and composition of Fe2O3 and As2O3/Fe2O3 were examined under TEM and EDS.
A heating test was performed to detect the thermodynamic characteristics of the magnetic particles. Various doses of Fe2O3 and As2O3/Fe2O3 particles were decentralized in 0.9% NaCl. The concentrations of Fe2O3 were 2, 4, 6 and 8 g/L. Two milliliters of nanoparticle fluid was then added to a flat-bottomed cuvette to reach a level of 5 mm from the bottom of the cuvette and in the center of the hyperthermia-coil of a high frequency electromagnetic field. The output electric current was 300 A, and the fluid was heated for 1 h with temperature measurement at 5 min intervals.
Biocompatibility study of Fe2O3 nanoparticles: MTT assay, hemolytic test, and micronucleus assay were performed to test the in vitro cytotoxicity of Fe2O3 nanoparticles. To perform the MTT assay, L929 cells were cultured in RPMI-1640 media supplemented with 10% heat-inactivated calf serum, penicillin (100 U/mL) and streptomycin (100 mg/mL) and grown in the presence of 5% CO2 at 37°C. Cells were seeded in a 96-well plate and treated with 200 &mgr;L Fe2O3 nanoparticle fluid at various concentrations (100%, 75%, 50% and 25%) for 48 h and with 5 &mgr;mol/L of As2O3 as a positive control. Subsequently, 20 &mgr;L (5 g/L) MTT was added to the cells in each well and incubated for 4 h at 37°C. Culture media was discarded and 150 &mgr;L of DMSO was added and subjected to vibration for 10 min. The absorbance (A) value was measured at a wavelength of 493 nm. The cell relative growth rate (RGR) was calculated as follows: (A of experimental group/A of control group) × 100%.
For the hemolytic test, 50 mL of Fe2O3 and As2O3/Fe2O3 was centrifuged at 2000 r/min for 10 min 3 times, then suspended and incubated at 37°C, and after 30 min a liquid-extract was obtained. Ten milliliters of 0.9% NaCl and 10 mL of double distilled water were used as negative (0% hemolysis) and positive (100% hemolysis) controls, respectively. Each group contained three tubes. Diluted anticoagulated rabbit blood (0.2 mL) was added to each tube, which had been pre-heated at 37°C for 30 min. Contents of all the tubes were incubated in a water bath at 37°C for 60 min. All tubes were centrifuged at 2500 r/min for 5 min and the supernatant was taken to estimate free hemoglobin. Absorbance was measured and recorded at 540 nm. In general, the optical density was 0.8 ± 0.3 in positive control groups and was no more than 0.03 in negative control groups. The hemolysis rate (HR) was calculated as follows: HR (%) = (A of experimental group - A of negative control group)/(A of positive control group - A of negative control group) × 100%.
For the micronucleus assay, 60 mice were randomly divided into six groups, with five females and five males in each group. Animals were injected intraperitoneally with 100 g/L of Fe2O3 or As2O3/Fe2O3 (40 mg/kg) twice at a 24 h interval. The negative group (with 0.9% NaCl) and positive group (with CT × 40 mg/kg) were set as control groups. Six hours after the second injection, all the mice were killed. The thighbone marrows were extracted for smears, methanol-fixed for 5 min, then dyed with Giemsa for 15 min. For each smear, 1000 polychromatic erythrocytes (PEC) were counted, and the number of PEC containing micronucleus was calculated (MN). Poisson distribution verified the statistical difference of each group.
We also studied the in vivo histotoxicity of nanoparticles. The Kun Ming mice were divided into 15 groups randomly with five females and five males in each group. Various amounts of 100 g/L Fe2O3 and As2O3/Fe2O3 nanoparticles were intraperitoneally injected into each mouse of seven groups at 1.25, 1.75, 2.5, 3.5, 5, 7.07 and 10 g/kg according to their weight. The negative control group was injected with the same volume of 0.9% NaCl, and the mice were observed in the following 15 d. The median lethal dose (LD50) was evaluated by the Karber method.
Sixteen healthy pigs were divided randomly into four groups (control group, Fe2O3 low dose, Fe2O3 high dose, and the As2O3/Fe2O3 group). Fe2O3 or As2O3/Fe2O3 (10 g/L) was injected into the liver of pigs in the experimental groups. Four pigs from each group were killed from 1 to 4 wk after injection. Serum AST, ALT, BUN and Cr were measured. Livers were harvested and dissected into 1 mm3 specimens. Subsequently, the samples were fixed in 4% glutaraldehyde and were prepared into ultrathin sections (60 nm) to be examined under TEM and EDS.
Inhibition of SMMC-7721 cell proliferation was measured by MTT assay according to the method described above. Xenograft tumors were induced in the subcutaneous tissue around the right shoulder of nude mice with SMMC-7721 cells. Once the tumor diameter increased to 0.2-0.4 cm, mice were divided into 6 groups: (1) the control (sterile 0.9% NaCl); (2) As2O3 (5 &mgr;mol/L As2O3); (3) Fe2O3 (5 g/L Fe2O3); (4) As2O3/Fe2O3 (5 g/L Fe2O3); (5) Fe2O3with hyperthermia; and (6) As2O3/Fe2O3 with hyperthermia. Each group contained eight mice. They were injected into the tumors at 1/2 of the volume of the tumor. The tumors of the mice in groups 5 and 6 were exposed to a high-frequency alternating magnetic field and irradiated for 30 min. The treatment was given three times at 24 h intervals. After 45 d, all the mice were killed. The weight and volume inhibitory rates of the tumor were calculated as follows: IW = (1 - the weight of tumor of experimental group/the weight of tumor of control group) ×100%; Iv = (1 - the volume of tumor of experimental group/the volume of tumor of control group).
Values were expressed as mean ± SD. The data were analyzed by SPSS 11.5 and SAS 10.0 software packages. Differences in the results were considered statistically significant when P < 0.05.
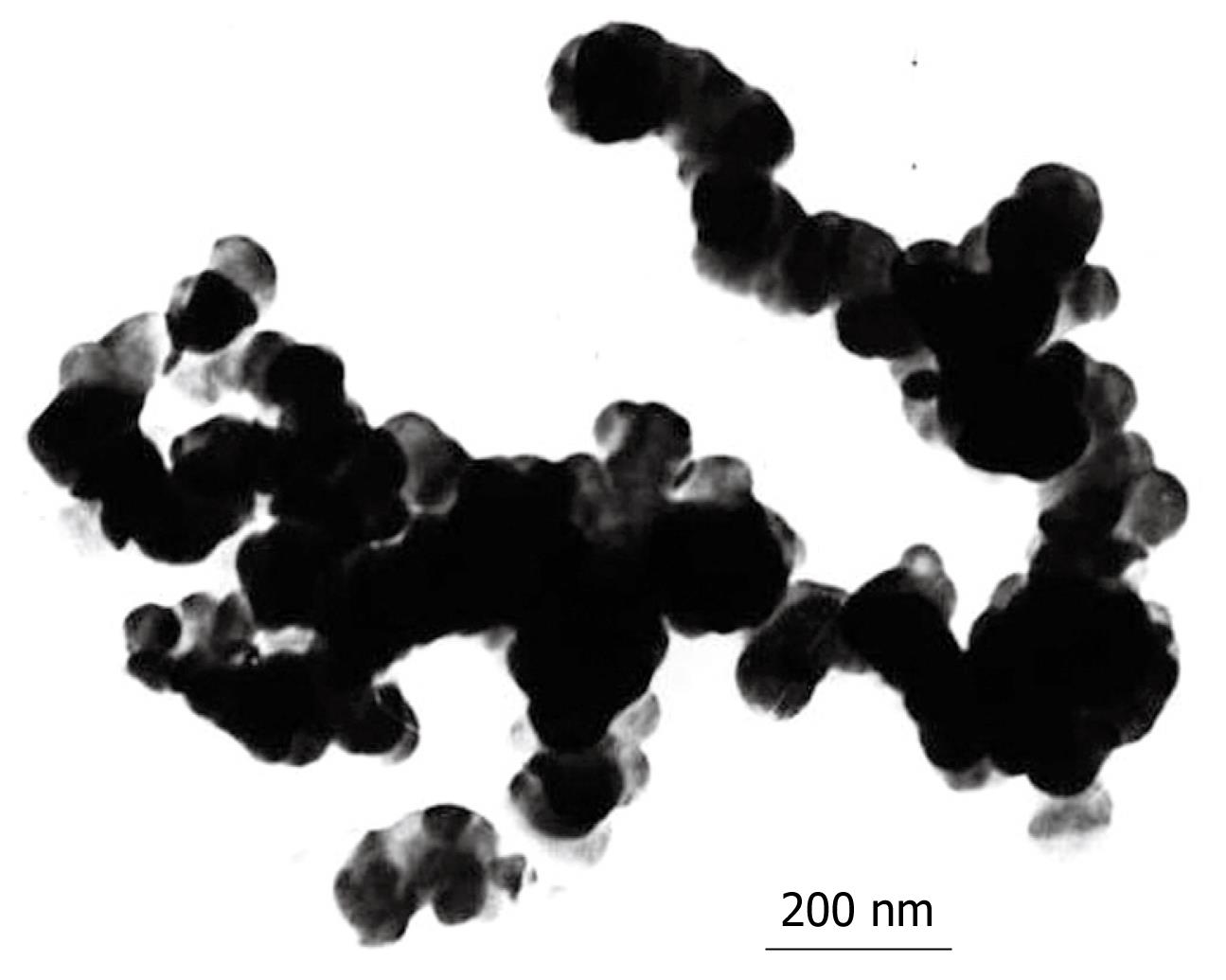
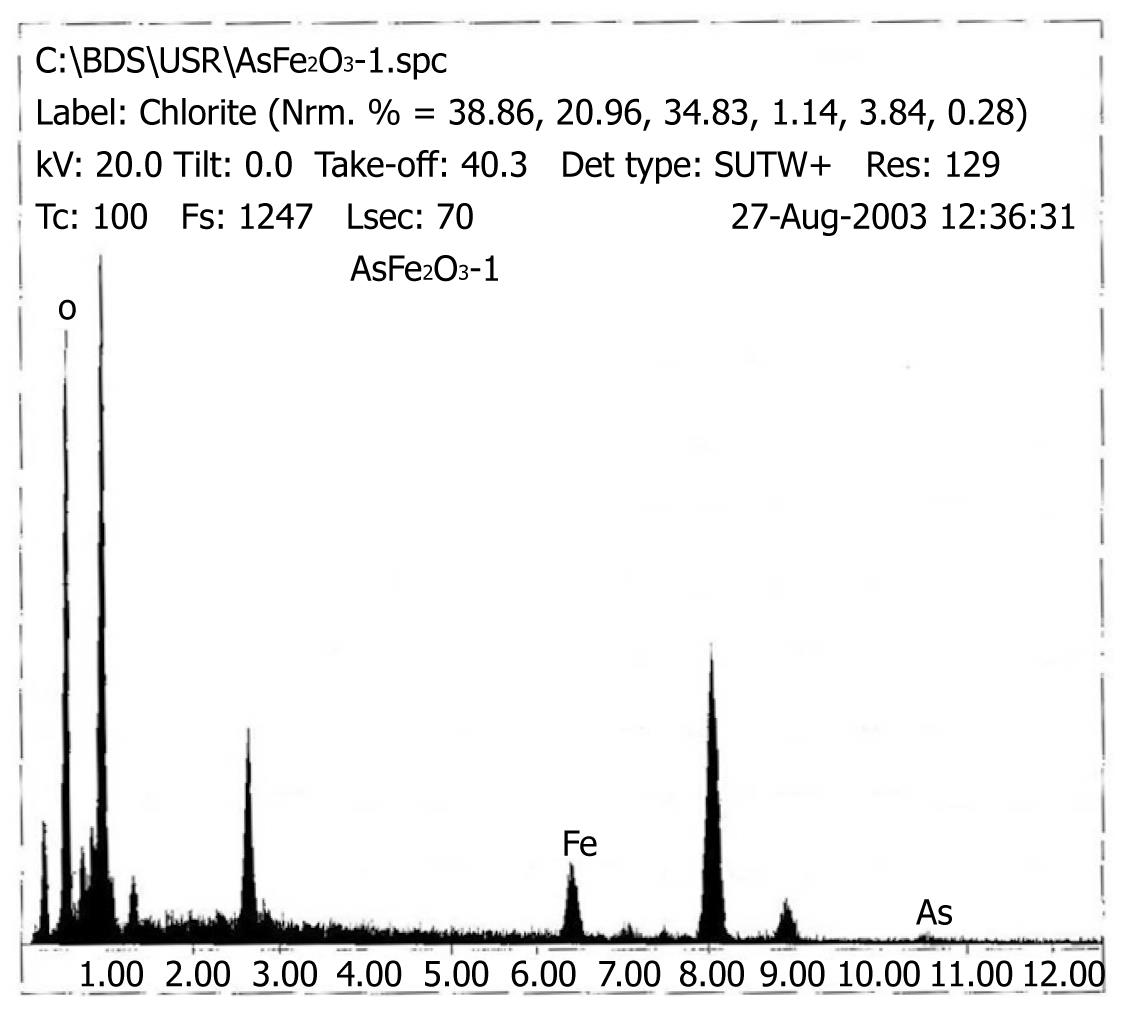
Under TEM, the nanospheres appeared to be roughly spherical, brown particles that could be suspended stably in water with good dispersibility. The diameter of Fe2O3 particles was about 20 nm, and the diameter of As2O3/Fe2O3 particles was about 100 nm as shown in Figure 1. The EDS result verified that the nanoparticles contained magnetic particles and As2O3 (Figure 2).
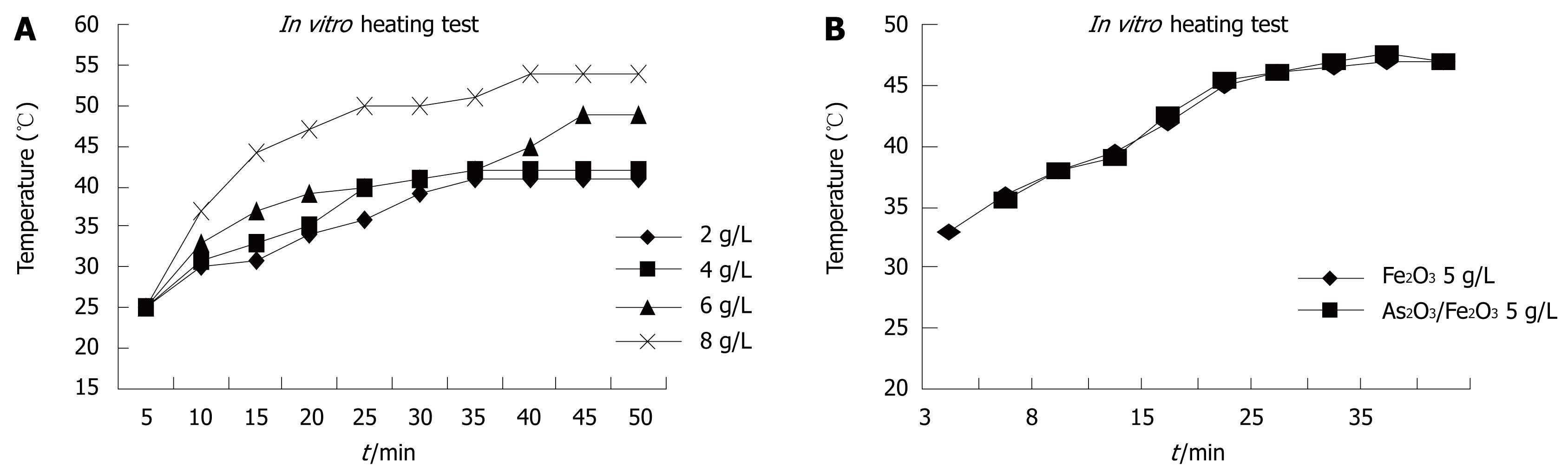
Figure 3A shows the thermodynamic tests of various doses of magnetic nanoparticles. Fe2O3 particles were decentralized in 0.9% NaCl and exposed to a high-frequency alternating electromagnetic field (output current equal to 300 A) for 60 min. The temperature rose rapidly within 5 min and slowly continued to increase from 5-40 min, and remained stable after 40 min. The temperature of the magnetic fluid (MF) rose from 41°C to 51°C, depending on the different concentrations. The results showed that Fe2O3 nanoparticles had good power absorption capabilities in the high-frequency alternating electromagnetic field, and had strong magnetic responsiveness. We selected a suitable temperature range (42-46°C) for tumor hyperthermia[5] by adjusting the concentration of Fe2O3. We chose a concentration of 5 g/L for Fe2O3, and at this concentration, the temperature rose to 46°C in MFH (Figure 3B).
MTT assay: The RGR of L929 cells treated with 25%, 50%, and 100% of liquid-extract of Fe2O3 were 102.63%, 96.39%, and 95.35%; for As2O3/Fe2O3 were 93.64%, 38.29%, and 27.26%, respectively. The value of the As2O3 group (5 &mgr;mol/L) was 30.12% (Table 1). The results corresponded to the cellular morphological changes observed under an inverted microscope.
| Group | Absorbance value | RGR (%) | Cytotoxicity gradations |
| Negative control | 0.4671 ± 0.0103 | 100.00 | 0 |
| 25% Fe2O3 | 0.4793 ± 0.0210 | 102.63 | 0 |
| 50% Fe2O3 | 0.4501 ± 0.0101 | 96.39 | 0 |
| 100% Fe2O3 | 0.4453 ± 0.0108 | 95.35 | 0 |
| 25% As2O3/Fe2O3 | 0.4373 ± 0.0210 | 93.64 | 0 |
| 50% As2O3/Fe2O3 | 0.1788 ± 0.0247 | 38.29 | 3 |
| 100% As2O3/Fe2O3 | 0.1273 ± 0.0073 | 27.26 | 3 |
| As2O3 (5 &mgr;mol/L) | 0.1322 ± 0.0090 | 30.12 | 3 |
| Positive control | 0.0733 ± 0.0050 | 15.70 | 4 |
Hemolytic test: The Absorbance of each group was observed at 545 nm. As shown in Table 2, the HR of Fe2O3 and As2O3/Fe2O3 nanoparticles was 0% and 0.77%, which is far less than the standard 5% that indicates a hemolytic reaction.
| Group | Absorbance (A) | Average A | Hemolysis rate (HR, %) | ||
| 1 | 2 | 3 | |||
| Negative control | 0.234 | 0.234 | 0.236 | 0.235 | |
| Fe2O3 extract | 0.232 | 0.235 | 0.233 | 0.233 | 0.00 |
| As2O3/Fe2O3 extract | 0.246 | 0.246 | 0.246 | 0.246 | 0.77 |
| Positive control | 1.688 | 1.776 | 1.521 | 1.662 | |
Micronucleus assay: The MN formation rates of Fe2O3, As2O3/Fe2O3, the negative control and the positive control groups were 0.28%, 0.26%, 0.24%, and 24.1%, respectively (Table 3).
Median lethal dose (LD50) determination: The mice receiving various doses were observed over the subsequent 15 d and the experimental animals died in succession. The LD50 was evaluated by the Karber method. The LD50 of mice receiving Fe2O3 and As2O3/Fe2O3 were 5.75 g/kg and 5.56 g/kg, respectively (Tables 4 and 5).
| Groups | Dose (g/kg) | Log | N | Deaths (N) | Mortality % (p) | Survival % (q) | p×q |
| 1 | 10.00 | 1.000 | 10 | 10 | 100 | 0 | 0.00 |
| 2 | 7.07 | 0.849 | 10 | 4 | 40 | 60 | 0.24 |
| 3 | 5.00 | 0.699 | 10 | 4 | 40 | 60 | 0.24 |
| 4 | 3.50 | 0.544 | 10 | 2 | 20 | 80 | 0.16 |
| 5 | 2.50 | 0.398 | 10 | 0 | 0 | 100 | 0.00 |
| 6 | 1.75 | 0.243 | 10 | 1 | 10 | 90 | 0.09 |
| 7 | 1.25 | 0.097 | 10 | 0 | 0 | 100 | 0.00 |
| i = 0.15 | ∑p = 2.1 | ||||||
| Groups | Dose (g/kg) | Log | N | Deaths (N) | Mortality % (p) | Survival % (q) | p×q |
| 1 | 10.00 | 1.000 | 10 | 10 | 100 | 0 | 0.00 |
| 2 | 7.07 | 0.849 | 10 | 5 | 50 | 50 | 0.25 |
| 3 | 5.00 | 0.699 | 10 | 3 | 30 | 70 | 0.21 |
| 4 | 3.50 | 0.544 | 10 | 3 | 30 | 70 | 0.21 |
| 5 | 2.50 | 0.398 | 10 | 1 | 10 | 90 | 0.09 |
| 6 | 1.75 | 0.243 | 10 | 0 | 0 | 100 | 0.00 |
| i = 0.15 | ∑p = 2.2 | ||||||
Biocompatibility study in pigs: One to four weeks after injection of Fe2O3 and As2O3/Fe2O3 into healthy pig livers, no significant differences were found in serum AST, ALT, BUN and Cr levels among pigs of all groups (P > 0.05), and no obvious pathological alterations were observed (Table 6). EDS examination revealed that in the As2O3/Fe2O3 complex group, numerous black nanosized As2O3/Fe2O3 complexes had accumulated in the liver tissue of pigs.
| Groups | TB | ALT | AST | Bun | Cr |
| Control | 4.8 ± 1.67 | 41.5 ± 10.08 | 92.25 ± 46.81 | 3.3 ± 0.36 | 86.5 ± 6.19 |
| Fe2O3 low dose | 8.025 ± 2.70 | 44.25 ± 16.35 | 87 ± 29.01 | 3 ± 0.99 | 83.25 ± 4.65 |
| Fe2O3 high dose | 6.45 ± 2.97 | 34.5 ± 9.61 | 71.25 ± 35.64 | 2.475 ± 0.46 | 77.5 ± 15.44 |
| As2O3/Fe2O3 | 7.93 ± 2.66 | 47.25 ± 14.19 | 52.75 ± 5.5 | 2.5 ± 0.25 | 92.25 ± 19.69 |
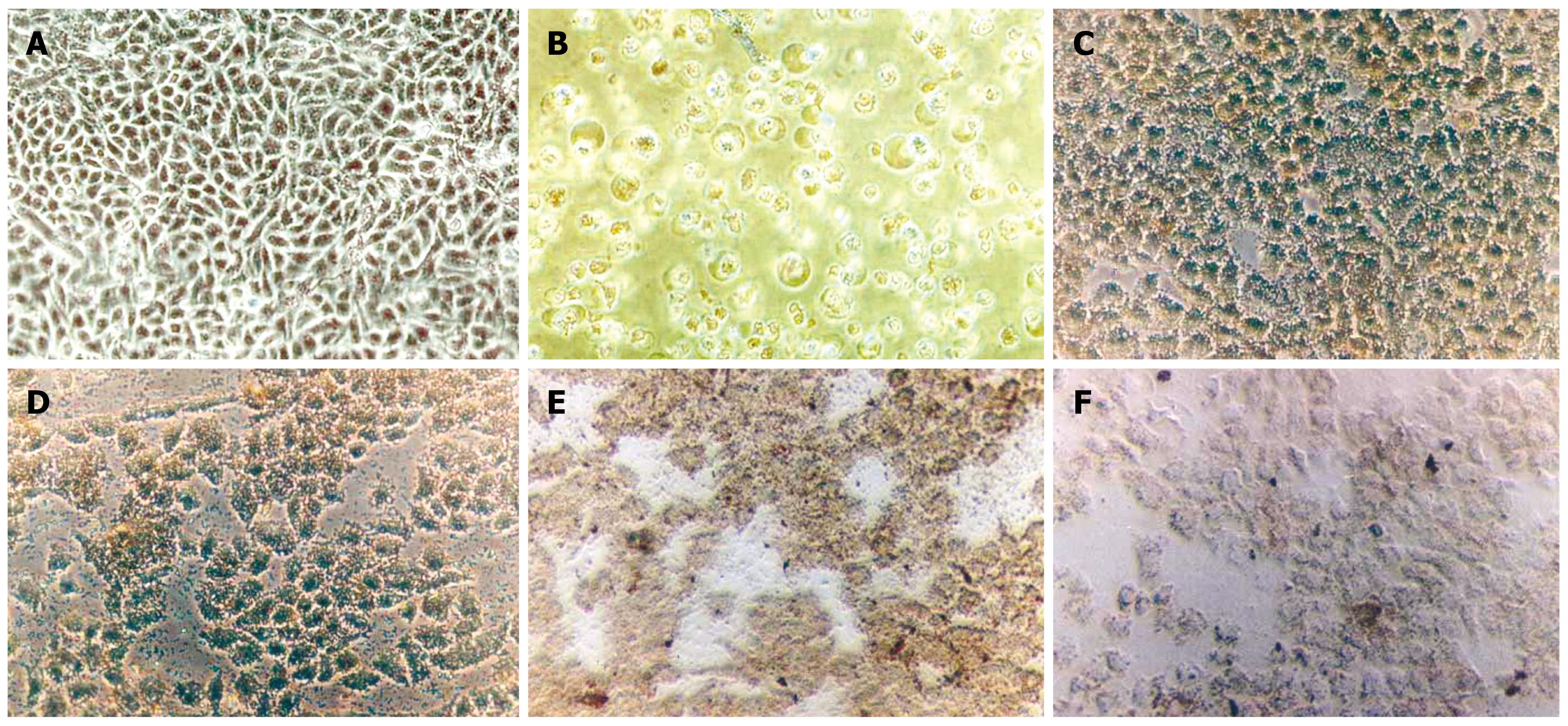
Morphological changes of apoptotic SMMC-7721 cells: The morphological changes of SMMC-7721 cells after treatment were observed under inverted microscopy. As shown in Figure 4, cells in the control and the Fe2O3 groups exhibited a normal shape, clear edge, and no cell fragmentation (Figure 4A). In the groups of As2O3, the As2O3/Fe2O3, the Fe2O3 and As2O3/Fe2O3 combined with MFH, the SMMC-7721 cells became small and global. Some shrunk and even a portion of the cells were suspended, which revealed the typical changes associated with apoptosis (Figure 4B-D). The results showed that both As2O3 (5 &mgr;mol/L) and MFH (at 46°C) could damage liver cells by inducing apoptosis.
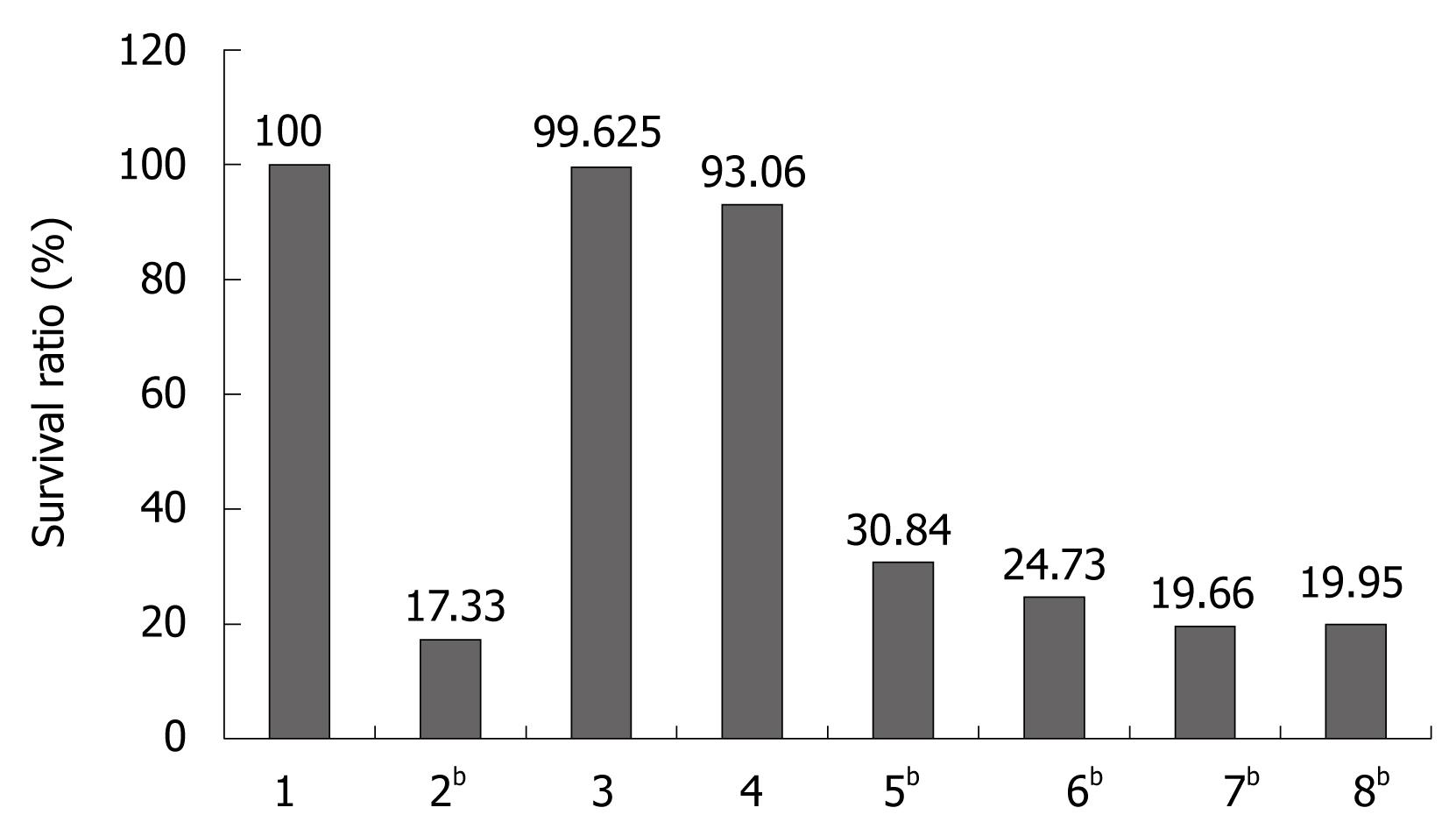
Inhibition of SMMC-7721 cell proliferation after treatment with Fe2O3 and As2O3/Fe2O3 combined with MFH: The results of MTT assay are shown in Figure 5. The cell survival rates of cells treated with Fe2O3 and As2O3/Fe2O3 combined with MFH were 19.66% and 19.95%, respectively, which was statistically different from the negative group (P < 0.01). So, the therapeutic effect of nanosized As2O3/Fe2O3 complexes in combination with MFH on SMMC-7712 cells is much better than that of As2O3 or Fe2O3 nanoparticles alone.
In vivoinhibitory effect of Fe2O3 and As2O3/Fe2O3 combined with MFH on xenograft liver cancer in nude mice: Animal experiments showed that tumors in the experimental groups became smaller (Figure 6). The mass and volume inhibitory ratio of the As2O3/Fe2O3 combined with MFH were IM = 82.79% and IV = 83.56%, respectively, which was much higher than that of the other groups (Table 7). Compared with control and experimental groups, each group was markedly different from the controls (P < 0.01). Histological examination in the As2O3/Fe2O3 group revealed that there was an accumulation of black nanosized As2O3/Fe2O3 particles at the stroma in the margin of the tumors. Many of the tumor cells disappeared at the site adjacent to this accumulation, and a necrotic zone was found surrounding the material (Figure 6).
| Groups | Tumor mass (g, mean ± SD) | Mass inhibitory rate (%) | Tumor volume (cm3, mean ± SD) | Volume inhibitory rate (%) |
| Control | 0.6145 ± 0.2296 | 0.00 | 0.7195 ± 0.3231 | 0.00 |
| As2O3 | 0.5885 ± 0.1628 | 5.86 | 0.6015 ± 0.2282 | 16.40 |
| Fe2O3 | 0.5365 ± 0.2792 | 12.69 | 0.5997 ± 0.2518 | 16.65 |
| As2O3/Fe2O3 | 0.4548 ± 0.2591 | 26.04 | 0.6252 ± 0.4034 | 21.45 |
| Fe2O3 with MFH | 0.1921 ± 0.0395 | 68.74b | 0.2373 ± 0.0874 | 67.02b |
| As2O3/Fe2O3 with MFH | 0.1057 ± 0.0510 | 82.79bc | 0.1183 ± 0.0726 | 83.56bc |
As2O3, a traditional Chinese medicine, plays an important role in the treatment and research for human cancers such as acute promyelocytic leukemia (APL), myeloid leukemia, gastric cancer, breast cancer, neuroblastoma and esophageal carcinoma, as well as head and neck cancers[6–8], however, there are many limitations to its use due to its form. Patients treated with As2O3 suffered from acute and chronic side effects such as gastrointestinal reactions, which are often severe or fatal[9–11]. Moreover, it has been generally considered to be an extremely effective environmental cocarcinogen for some human malignancies, especially for skin and lung cancer[12]. Therefore, enhancing the curative effect and reducing the toxicity of As2O3 by changing its form is of great importance.
Hyperthermia for tumor therapy has been a long-standing modality. At present, thermotherapy is commonly used clinically with such applications as radiofrequency, microwave and lasers, all of which have many limitations for tumor hyperthermia. In 1997, Jordan[13] discovered that a nanoscaled magnetic fluid could be absorbed with much higher power in an alternating magnetic field, and used to treat disease or tumors. This treatment is named “Magnetic Fluid Hyperthermia (MFH)”. MFH has a high ability to target and localize thermogenic actions. Therefore, tissue without magnetic particles would not be damaged.
Nanoparticles combined with MFH may be a potential method to treat tumors. In our study, As2O3/Fe2O3 complexes were prepared as a new magnetic material. Observed under TEM, they are round or elliptical, disperse well and are about 100 nm in diameter. In addition, they have good power absorption capabilities in a high-frequency alternating electromagnetic field. The temperature can rapidly reach 46°C within 5 min, which can kill tumor cells while having little effect on normal cells.
However, the biomaterials would be in direct contact with tissues and cells when introduced into the body, so their biocompatibility had to be evaluated before they could be applied in a clinic setting. Many studies have shown that some materials show signs of toxicity when their diameters are reduced to nanoscale[14]. Therefore, the potential hazards and bio-safety of Fe2O3 microspheres should be particularly observed when they are applied to tissues. Biomaterials must not only have long-term stability in biotic conditions, but also have no harmful effects on tissues, blood or the immune system. In our work, referring to ISO10993-1992 and other international standards[15–17], we evaluated the nanoparticles using an in vitro cytotoxicity test, hemolytic test, a micronucleus experiment, by calculating the LD50, and an in vivo study. MTT results showed that Fe2O3 nanoparticles had no significant effect on cellular proliferation when treated with various doses of extracted liquids of Fe2O3 nanoparticles. The cytotoxicities were 0 grade (RGR > 75%) indicating that there was no evidence of cytotoxicity. The results of the hemolytic test demonstrated that the hemolytic rate of the liquid-extracts of Fe2O3 and As2O3/Fe2O3 were 0.% and 0.77%, far less than 5%. This finding indicated that Fe2O3 had no hemolytic reaction when in direct contact with blood and was consistent with the requirement of hemolytic tests for biomaterials. Genotoxicity and carcinogenicity tests answer the most complicated questions about biomaterials. The micronucleus assay is a rapid detection method to evaluate whether a biomaterial would damage chromosomes or interfere with cellular mitosis. This method can rapidly monitor acute and/or chronic genotoxicity, and does not require cultured cells[18]. In our study, we compared Fe2O3 groups with the negative control group and found no significant difference (P > 0.05) in the micronucleus formation rate. However, when we compared these groups with the positive control group, the result was significantly different (P < 0.05).
Therefore, Fe2O3 nanoparticles were not carcinogenic or mutagenic. However, the results of the acute toxicity test revealed that Fe2O3 nanoparticles intraperitoneally injected into the mice had low toxicity. The LD50 was equal to 5 g/kg, which is in the “no toxicity” category according to the standard of acute toxicity gradation of WHO. The LD50 of Fe2O3 for the mice was 5.75 g/kg with a 95% confidence interval of 4.8-6.9 g/kg. So, Fe2O3 also belonged to the “no toxicity” category and had a wide safety value margin. When we injected Fe2O3 into livers of healthy pigs, no significant differences in serum AST, ALT, BUN and Cr levels were found among pigs of all groups (P > 0.05), and no obvious pathological alterations were observed. From the results of our experiment, we believe that Fe2O3 demonstrated no toxic effects, is a highly biocompatible material and may be suitable for further applications in tumor hyperthermia.
We studied the therapeutic effect of Fe2O3 and As2O3/Fe2O3 combined with MFH on liver cancer in vitro and in vivo. We injected Fe2O3 and As2O3/Fe2O3 into the tumor tissues instead of the normal tissue boundary of the tumor. Thus, the nanoparticles were delivered into the desired zone. This method allows thermogenic action to be administered locally, even in tumors located deep inside bodies, while minimizing heating of normal tissue around the tumor[1920]. Compared with As2O3/Fe2O3 groups, As2O3/Fe2O3 combined with MFH had a better inhibitory effect on xenograft liver tumors, which indicates that MFH had a significant therapeutic effect. Much to our surprise, As2O3/Fe2O3 combined with MFH was the best therapeutic agent among all the groups tested. This result revealed that As2O3/Fe2O3 combined with MFH had two functions: chemotherapy of As2O3 and thermotherapy of magnetic Fe2O3 nanoparticles.
In conclusion, As2O3/Fe2O3 combined with MFH is a new biomaterial with low toxicity. However, we must acknowledge that our studies have limitations, and more researches should be carried out in the future. Although there is still a long way to go before the technology can be applied to clinical treatment, this method may develop into a new approach for the treatment of liver cancer and other solid tumors.
Hepatocellular carcinoma is one of the most common malignant tumors in China, and has a low recovery rate, so it is necessary to search for a new method to treat liver tumors.
Nanoparticles combined with magnetic fluid hyperthermia (MFH) have become a potential method to treat tumors. In this study, As2O3/Fe2O3 complexes were found to have two functions, chemotherapy of As2O3, and thermotherapy of magnetic Fe2O3 nanoparticles.
As2O3/Fe2O3 combined with MFH is a new biomaterial with good therapeutic effects on liver cancer. This preparation may be developed into a new agent for the treatment of liver cancer and other solid tumors.
MFH is a method that can target and localize thermogenic actions. Therefore, the tissue surrounding the tumor without magnetic particles would not be damaged.
The manuscript describes the therapeutic potential of nanosized As2O3/Fe2O3 complexes in combination with MFH on liver cancer cells. The authors analyzed the toxicity and therapeutic potentials of various concentrations of Fe2O3 and As2O3/Fe2O3. Interestingly, they found significant antitumor effects of those compounds against xenograft tumors of liver cancer cells when combined with magnetic fluid hyperthermia. The data are important and promising.
| 1. | Zhang YY, Xia HH. Novel therapeutic approaches for hepatocellulcar carcinoma: fact and fiction. World J Gastroenterol. 2008;14:1641-1642. |
| 2. | Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445-454. |
| 3. | Gneveckow U, Jordan A, Scholz R, Bruss V, Waldofner N, Ricke J, Feussner A, Hildebrandt B, Rau B, Wust P. Description and characterization of the novel hyperthermia- and thermoablation-system MFH 300F for clinical magnetic fluid hyperthermia. Med Phys. 2004;31:1444-1451. |
| 4. | Yan S, Zhang D, Gu N, Zheng J, Ding A, Wang Z, Xing B, Ma M, Zhang Y. Therapeutic effect of Fe2O3 nanoparticles combined with magnetic fluid hyperthermia on cultured liver cancer cells and xenograft liver cancers. J Nanosci Nanotechnol. 2005;5:1185-1192. |
| 5. | Wust P, Gneveckow U, Johannsen M, Bohmer D, Henkel T, Kahmann F, Sehouli J, Felix R, Ricke J, Jordan A. Magnetic nanoparticles for interstitial thermotherapy--feasibility, tolerance and achieved temperatures. Int J Hyperthermia. 2006;22:673-685. |
| 6. | Luo L, Qiao H, Meng F, Dong X, Zhou B, Jiang H, Kanwar JR, Krissansen GW, Sun X. Arsenic trioxide synergizes with B7H3-mediated immunotherapy to eradicate hepatocellular carcinomas. Int J Cancer. 2006;118:1823-1830. |
| 7. | Jiang XH, Wong BC, Yuen ST, Jiang SH, Cho CH, Lai KC, Lin MC, Kung HF, Lam SK. Arsenic trioxide induces apoptosis in human gastric cancer cells through up-regulation of p53 and activation of caspase-3. Int J Cancer. 2001;91:173-179. |
| 8. | Chow SK, Chan JY, Fung KP. Inhibition of cell proliferation and the action mechanisms of arsenic trioxide (As2O3) on human breast cancer cells. J Cell Biochem. 2004;93:173-187. |
| 9. | Miller WH Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893-3903. |
| 10. | Li LH, Li HM. [Progression of foundational and clinical studies on use of arsenic trioxide in treatment of lymphoma--review]. Zhongguo Shiyan Xueyexue Zazhi. 2007;15:1335-1339. |
| 11. | Zhao X, Feng T, Chen H, Shan H, Zhang Y, Lu Y, Yang B. Arsenic trioxide-induced apoptosis in H9c2 cardiomyocytes: implications in cardiotoxicity. Basic Clin Pharmacol Toxicol. 2008;102:419-425. |
| 12. | Dong JT, Luo XM. Effects of arsenic on DNA damage and repair in human fetal lung fibroblasts. Mutat Res. 1994;315:11-15. |
| 13. | Jordan A, Scholz R, Wust P, Fahling H, Krause J, Wlodarczyk W, Sander B, Vogl T, Felix R. Effects of magnetic fluid hyperthermia (MFH) on C3H mammary carcinoma in vivo. Int J Hyperthermia. 1997;13:587-605. |
| 14. | Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77:126-134. |
| 15. | Harmand MF. In vitro study of biodegradation of a Co-Cr alloy using a human cell culture model. J Biomater Sci Polym Ed. 1995;6:809-814. |
| 16. | Richardson RR Jr, Miller JA, Reichert WM. Polyimides as biomaterials: preliminary biocompatibility testing. Biomaterials. 1993;14:627-635. |
| 17. | Yang X, Xi T. [Progress in the studies on the evaluation of biocompatibility of biomaterials]. Shengwu Yixue Gongchengxue Zazhi. 2001;18:123-128. |
| 18. | Fuic A, Mijic A. [In vitro and in vivo micronucleus tests in genotoxicity research]. Arh Hig Rada Toksikol. 1999;50:299-306. |
| 19. | Moroz P, Jones SK, Gray BN. The effect of tumour size on ferromagnetic embolization hyperthermia in a rabbit liver tumour model. Int J Hyperthermia. 2002;18:129-140. |