Published online Jun 21, 2009. doi: 10.3748/wjg.15.2904
Revised: May 13, 2009
Accepted: May 20, 2009
Published online: June 21, 2009
AIM: To share our surgical experience and the outcome of limited pancreatic head resection for the management of branch duct intraductal papillary mucinous neoplasm (IPMN).
METHODS: Between May 2005 and February 2008, nine limited pancreatic head resections (LPHR) were performed for IPMN of the pancreatic head. We reviewed the nine patients, retrospectively.
RESULTS: Tumor was located in the uncinate process of the pancreas in all nine patients. Three patients had stents inserted in the main pancreatic duct due to injury. The mean size of tumor was 28.4 mm. Postoperative complications were found in five patients: 3 pancreatic leakages, a pancreatitis, and a duodenal stricture. Pancreatic leakages were improved by external drainage. No perioperative mortality was observed and all patients are recorded alive during the mean follow-up period of 17.2 mo.
CONCLUSION: In selected patients after careful evaluation, LPHR can be used for the treatment of branch duct type IPMN. In order to avoid pancreatic ductal injury, pre- and intra-operative definite localization and careful operative techniques are required.
- Citation: Paik KY, Choi SH. Experience of limited pancreatic head resection for management of branch duct intraductal papillary mucinous neoplasm in a single center. World J Gastroenterol 2009; 15(23): 2904-2907
- URL: https://www.wjgnet.com/1007-9327/full/v15/i23/2904.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2904
Branch duct type intraductal papillary mucinous neoplasm (IPMN) has a low malignant potential and is more frequently located in the head of the pancreas[1–4]. When this lesion is located in the pancreatic head, the conventional treatment for IPMN has been pancreaticoduodenectomy (PD). Also, partial pancreatic resection is feasible as a treatment for small branch duct type IPMN, which shows less aggressive behavior.
In recent years, some surgeons have advocated limited pancreatectomy for management of benign IPMN and regarding this the following procedures have been reported in several papers: inferior head resection of the pancreas[5], partial pancreatic head resection[6], uncinate process resection[78], single branch resection of the pancreas[9], and ductal branch oriented minimal pancreatectomy[10]. It had been expected that there would be advantages of minimal pancreatic parenchymal loss and prevention of functional insufficiency by performing limited pancreatectomy. However, recommendations and reports of postoperative complications and clinical outcomes following these procedures have been limited. Therefore we report a single center surgical experience and short-term outcome of limited pancreatic head resection (LPHR) for the management of branch duct type IPMN.
From May 2005 to February 2008, a retrospective review was undertaken of 12 patients who underwent partial pancreatectomy for IPMN at our institution, among whom, nine patients underwent LPHR. In-hospital clinical course and method of operation, postoperative complications and follow-up data were analyzed.
Assessment of the tumor location was carried out before surgery using computed tomography (CT) in all nine cases, upon suspicion of IPMN.
Additional evaluations were routinely performed with endoscopic retrograde cholagiopancreatography (ERCP) or magnetic resonance cholagiopancreatography (MRCP) for evaluating the pancreatic duct, which were confirmed during surgery through direct visualization of lesion by dissection of pancreas head and intraoperative ultrasonography (IOUSG). All pancreatectomies were performed by a single surgeon. Pancreatic parenchymal control was performed using bipolar coagulator for fine dissection and easy bleeding control. All nine resected tumors were examined by a single pathologist with regard to resection margin and tumor characteristics during operation. The definition of postoperative pancreatic leakage was in accordance with the International Study Group on Pancreatic Fistula (ISGPF) definition[11].
Prior to 2005, PD was the standard treatment for IPMN of the pancreas head. Sugiyama et al[12] reported that size > 30 mm and presence of mural nodules were the strongest predictors of malignancy in branch duct IPMN. But our previous study revealed that many of < 30 mm resected branch duct IPMN were malignant[13]. For these reason, under 30 mm-sized branch duct IPMN were resected at our center, and < 20 mm lesions were observed with close follow-up. Since 2005, LPHR procedure was tried for those lesions > 20 mm, mainly located in the uncinate process in which the main pancreatic duct was intact on imaging study, and branched IPMN was suspected on pancreatogram.
Pancreatic head was exposed by omentectomy and superior mesenteric vein (SMV) branch ligation such as in PD. After the exposure, exploration of the lesion and main pancreatic duct was carried out using IOUSG. Preoperative or intraoperative pancreatic stents were not used before resection. Bipolar coagulator was used for fine dissection of pancreas head.
The mean age of the patients was 63 years (range: 42-72 years), with six male patients. Six patients presented clinical symptoms and incidental lesions were found in three patients.
All nine patients underwent multidetector abdominal CT, four of nine patients underwent MRCP, and another five patients underwent ERCP. All nine tumors were located in the uncinate process, and two patients had another lesion in the tail portion of the pancreas. Five patients were confirmed IPMN by imaging studies, another four patients were suspected IPMN or other cystic neoplasm including mucinous cystic neoplasm (MCN). Clinical profiles are summarized in Table 1.
| Case No. | F/U (mo) | Complication | Pathology | Size (mm) | Preop Dx | Pancreatogram | Age | Gender |
| 1 | 24 | P-leakage | Adenoma | 30 | MCN | ERCP | 69 | F |
| 2 | 20 | Pancreatitis | Adenoma | 25 | Cystic.n | ERCP | 67 | M |
| 3 | 22 | P-leakage | Adenoma | 20 | IPMN | MRCP | 71 | M |
| 4 | 22 | D-stricture | Adenoma | 22 | Cystic.n | ERCP | 72 | M |
| 5 | 19 | - | Adenoma | 25 | MCN | ERCP | 50 | F |
| 6 | 14 | - | Adenoma | 42 | IPMN | ERCP | 69 | M |
| 7 | 12 | - | Adenoma | 27 | IPMN | MRCP | 60 | M |
| 8 | 12 | P-leakage | Adenoma | 30 | IPMN | MRCP | 42 | M |
| 9 | 10 | - | Adenoma | 35 | IPMN | MRCP | 67 | F |
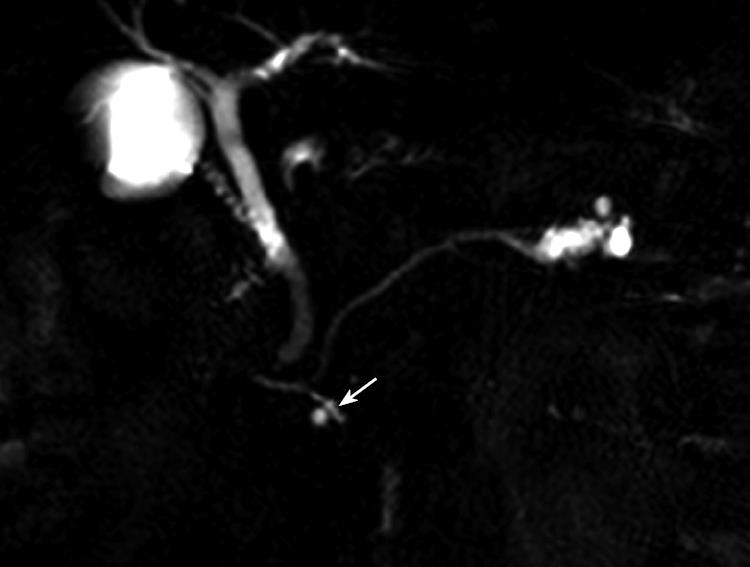
Three quarters of the cases of MRCP showed connection between the main pancreatic duct and the cystic lesion in the uncinate process (Figure 1). These findings aroused suspicion of branched type of IPMN, and LPHR was planned.
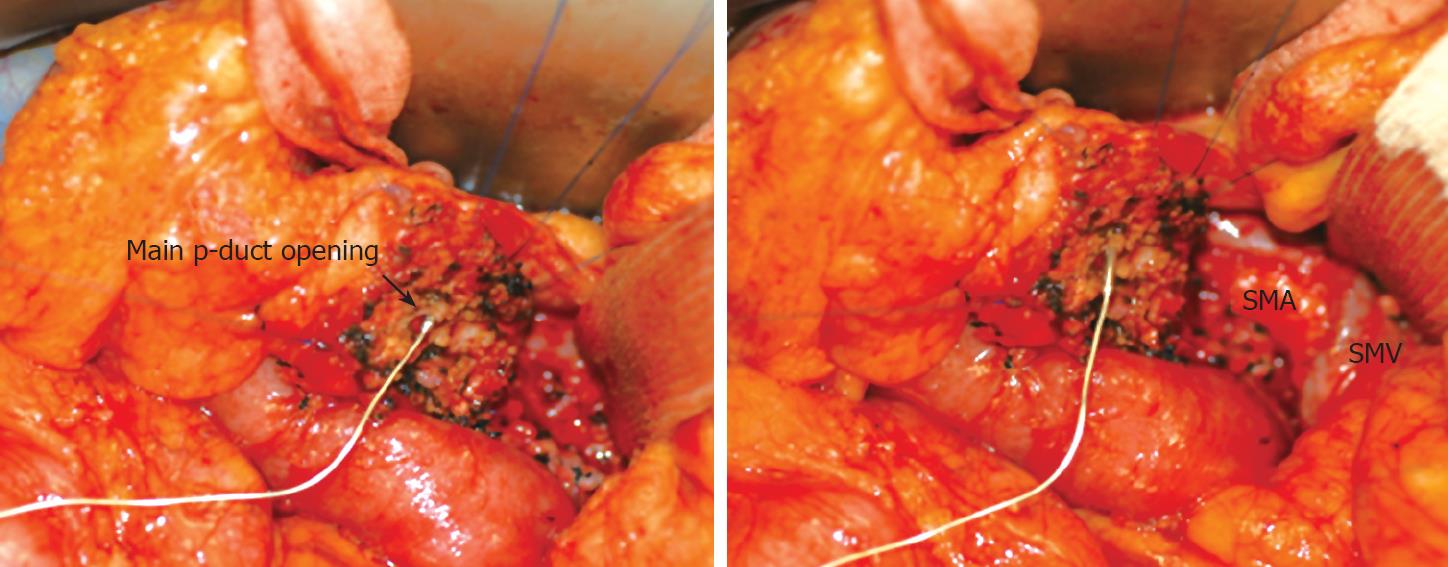
Uncinate process resection or ductal branch-oriented minimal pancreatectomy[710] were performed in six patients. Single-branch resection[9] was performed in three patients. Two of nine patients underwent additional distal pancreatectomy for pancreatic tail IPMN. The Wirsung duct was damaged in three of the six patients who underwent uncinate process resection or ductal branch-oriented minimal pancreatectomy in which internal silastic stents were inserted and primary repair was carried out (Figure 2). After pancreatectomy, we confirmed the pathologic diagnosis with resection margin.
The mean tumor size was 28.4 mm (range 20-42 mm). All nine tumors were confirmed as intraductal papillary mucinous adenoma and resection margins were free of tumor.
The mean number of hospital days was 21.1 d (range: 8-48 d). There was no mortality and five morbidities. Pancreatic leakage occurred in three patients, two of which involved injured pancreatic duct during operation and an inserted silastic internal pancreatic stent. The third ductal injury was not detected during operation. Pancreatic leakage was detected on postoperative days 4 and 5 respectively. One patient was discharged on postoperative day 32 with a Jackson-Pratt (JP) drain which was removed on day 62 after drain amylase was normalized. Another patient with re-exploration at diagnosis of leakage showed severe inflammation, because of which an external drain (sump drain) was added. Both patients were discharged at postoperative day 43 after removal of the drain. In the case of non-injury of the pancreatic duct, drain amylase increased after operation but normalized at postoperative day 15. One pancreatitis and one duodenal stricture were observed. Duodenal stricture was improved after gastrojejunostomy. The mean follow-up time was 17.2 mo during which there were no recurrences or metastases.
Despite the small number of cases included in this study, to our knowledge this study and evaluation of the nine cases of LPHR for branched IPMN is so far the largest amongst related studies. It can be said that partial pancreatic head resection can be a better treatment option than conventional PD for branch duct IPMN on pancreas head under free resection margin. PD may present as surgical overkill for benign and low-grade malignant tumors such as branch duct type IPMN of the pancreatic head. Such procedures result in a significant loss of normal pancreatic parenchyma with subsequent impairment of exocrine and endocrine pancreatic functions[14–16]. Resection of the distal bile duct in patients undergoing PD requires a bilio-enteric anastomosis, which increases the risk of ascending cholangitis and subsequent intrahepatic abscesses[17]. Following PD, the incidence of diabetes mellitus varies between 15% and 40%[1819]. It can be noted in reports of recent papers that there is unchanged endocrine and exocrine pancreatic function following segmental pancreatic resection[20–22].
Ventral pancreatectomy was performed for management of cystic tumor as limited pancreatic head resection in 1993[23]. This procedure resected too great a width of normal pancreas for a small lesion and reconstructed pancreatic duct and bile duct by entric-anastomosis. Thereafter, pancreatic duct preserving procedures were reported, such as inferior pancreatic head resection, and uncinate process resection[578]. More minimal pancreatic head resections were performed such as single branch resection of the pancreas, and ductal branch-oriented minimal pancreatectomy[910].
Pancreatic leakage is one of the most frustrating complications after limited pancreatic resection. Although this study showed no mortality, three cases of pancreatic leakages occurred. Three pancreatic injuries were found during pancreatectomy. Firstly, the main pancreatic duct was injured after pancreatic dissection from the superior mesenteric vein (SMV) where mucin leaked from a small ductal opening in which a stent was inserted. Secondly, due to ductal tearing, the main pancreatic duct opening was widened during which a stent was inserted through the opening site. Lastly, minutely injured duct was repaired without stent insertion, without leakage after operation. Sata et al[9] experienced pancreatic leakages which were managed by the insertion of an endoscopic naso-pancreatic drainage tube. In segmental pancreas resection, pancreatic leakage rates reach up to 40%[20–22].
Generally, benign branch duct type IPMN in the pancreas head, especially the uncinate process, does not involve the main pancreatic duct. However IPMN too close to the main duct, or a large mass, need to be carefully evaluated. For safe dissection during operation without injuring the main pancreatic duct, pre- or intra-operative pancreatic duct evaluation is crucial. IOUSG or MRCP were insufficient for detecting the pancreatic duct or distance of duct to mass during operation. Although all nine patients had IOUSG performed, three pancreatic injuries occurred. Takada et al[7] applied preoperative pancreatic duct stents guided by ERCP, during which the patients did not experience pancreatic leakage. They mentioned that the purpose of the stent was intraoperative identification of pancreatic duct with protection against iatrogenic injury, and postoperative drainage for minimizing pancreatic fistula. Yamaguchi et al[10] proposed the placement of a main duct tube for preventing transient stenosis of pancreatic duct. However in this study, preoperative stents were not applied. We speculated that the pancreatic drainage tube produced a pancreatitis.
LPHR does not necessitate anastomosis between the pancreatic duct, bile duct and the bowel. A disadvantage of LPHR was the higher rate of leakage (33.3%). In order to avoid pancreatic ductal injury, preoperative or intraoperative definite localization and careful surgical techniques were important. If the pancreatic duct was injured during operation, internal drainage procedure was necessary. If the disadvantage of ductal injury can be overcome, LPHR can be a useful procedure for the treatment of branch duct type IPMN in selected patients.
Branch duct type intraductal papillary mucinous neoplasm (IPMN) has a low malignant potential. Limited pancreatectomy is advocated for those lesions, thereby reducing the risk of functional insufficiency and morbidity in extensive resection.
Recommendations and reports of clinical outcomes following limited pancreatic head resection procedures were limited. Several case reports have been published. Despite the small number of cases included in this study, evaluation of the nine cases of limited pancreatic head resections (LPHR) for branched IPMN is so far the largest amongst related studies.
Disadvantage of LPHR was the higher rate of leakage. If the disadvantage of ductal injury can be overcome, LPHR can be a useful procedure for the treatment of branch duct type IPMN in selected patients.
This interesting paper explores the possibility of limited resections for branch duct-type IPMN. But, three of 9 patients also developed a pancreatic fistula; a rate that is also higher than after pancreaticoduodenectomy.
| 1. | Terris B, Ponsot P, Paye F, Hammel P, Sauvanet A, Molas G, Bernades P, Belghiti J, Ruszniewski P, Flejou JF. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372-1377. |
| 2. | Sugiyama M, Atomi Y. Intraductal papillary mucinous tumors of the pancreas: imaging studies and treatment strategies. Ann Surg. 1998;228:685-691. |
| 3. | Tanaka M. Intraductal papillary mucinous neoplasm of the pancreas: diagnosis and treatment. Pancreas. 2004;28:282-288. |
| 4. | Kobari M, Egawa S, Shibuya K, Shimamura H, Sunamura M, Takeda K, Matsuno S, Furukawa T. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131-1136. |
| 5. | Nakagohri T, Kenmochi T, Kainuma O, Tokoro Y, Kobayashi S, Asano T. Inferior head resection of the pancreas for intraductal papillary mucinous tumors. Am J Surg. 2000;179:482-484. |
| 6. | Nakagohri T, Konishi M, Inoue K, Izuishi K, Kinoshita T. Partial pancreatic head resection for intraductal papillary mucinous carcinoma originating in a branch of the duct of santorini. Eur Surg Res. 2002;34:437-440. |
| 7. | Takada T, Amano H, Ammori BJ. A novel technique for multiple pancreatectomies: removal of unicinate process of the pancreas combined with medial pancreatectomy. J Hepatobiliary Pancreat Surg. 2000;7:49-52. |
| 8. | Sharma MS, Brams DM, Birkett DH, Munson JL. Uncinatectomy: a novel surgical option for the management of intraductal papillary mucinous tumors of the pancreas. Dig Surg. 2006;23:121-124. |
| 9. | Sata N, Koizumi M, Tsukahara M, Yoshizawa K, Kurihara K, Nagai H. Single-branch resection of the pancreas. J Hepatobiliary Pancreat Surg. 2005;12:71-75. |
| 10. | Yamaguchi K, Shimizu S, Yokohata K, Noshiro H, Chijiiwa K, Tanaka M. Ductal branch-oriented minimal pancreatectomy: two cases of successful treatment. J Hepatobiliary Pancreat Surg. 1999;6:69-73. |
| 11. | Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, Neoptolemos J, Sarr M, Traverso W, Buchler M. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8-13. |
| 12. | Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90:1244-1249. |
| 13. | Chung JC, Jo SH, Choi SH, Choi DW, Kim YI. Surgical management for intraductal papillary mucinous tumor of pancreas confined to branch duct. J Korean Surg Soc. 2006;70:288-293. |
| 14. | Sakorafas GH, Farnell MB, Nagorney DM, Sarr MG, Rowland CM. Pancreatoduodenectomy for chronic pancreatitis: long-term results in 105 patients. Arch Surg. 2000;135:517-523; discussion 523-524. |
| 15. | Tran K, Van Eijck C, Di Carlo V, Hop WC, Zerbi A, Balzano G, Jeekel H. Occlusion of the pancreatic duct versus pancreaticojejunostomy: a prospective randomized trial. Ann Surg. 2002;236:422-428; discussion 428. |
| 16. | Rault A, SaCunha A, Klopfenstein D, Larroude D, Epoy FN, Collet D, Masson B. Pancreaticojejunal anastomosis is preferable to pancreaticogastrostomy after pancreaticoduodenectomy for longterm outcomes of pancreatic exocrine function. J Am Coll Surg. 2005;201:239-244. |
| 17. | Yamaguchi K, Tanaka M, Chijiiwa K, Nagakawa T, Imamura M, Takada T. Early and late complications of pylorus-preserving pancreatoduodenectomy in Japan 1998. J Hepatobiliary Pancreat Surg. 1999;6:303-311. |
| 18. | Beger HG, Buchler M, Bittner RR, Oettinger W, Roscher R. Duodenum-preserving resection of the head of the pancreas in severe chronic pancreatitis. Early and late results. Ann Surg. 1989;209:273-278. |
| 19. | Martin RF, Rossi RL, Leslie KA. Long-term results of pylorus-preserving pancreatoduodenectomy for chronic pancreatitis. Arch Surg. 1996;131:247-252. |
| 20. | Rotman N, Sastre B, Fagniez PL. Medial pancreatectomy for tumors of the neck of the pancreas. Surgery. 1993;113:532-535. |
| 21. | Sperti C, Pasquali C, Ferronato A, Pedrazzoli S. Median pancreatectomy for tumors of the neck and body of the pancreas. J Am Coll Surg. 2000;190:711-716. |
| 22. | Warshaw AL, Rattner DW, Fernandez-del Castillo C, Z'graggen K. Middle segment pancreatectomy: a novel technique for conserving pancreatic tissue. Arch Surg. 1998;133:327-331. |
| 23. | Takada T. Ventral pancreatectomy: resection of the ventral segment of the pancreas. J Hepatobiliary Pancreat Surg. 1993;1:36-40. |