Published online Jun 14, 2009. doi: 10.3748/wjg.15.2800
Revised: May 5, 2009
Accepted: May 12, 2009
Published online: June 14, 2009
AIM: To investigate the protein and mRNA expression of semaphorin 5A and its receptor plexin B3 in gastric carcinoma and explore its role in the invasion and metastasis of gastric carcinoma.
METHODS: Expression of semaphorin 5A and its receptor plexin B3 in 48 samples of primary gastric carcinoma, its corresponding non-neoplastic mucosa, and matched regional lymph node metastasis was assayed by reverse transcription-polymerase chain reaction (RT-PCR), real-time RT-PCR and Western blotting.
RESULTS: The protein and mRNA expression of semaphorin 5A and its receptor plexin B3 increased gradually in non-neoplastic mucosa, primary gastric carcinoma and lymph node metastasis (P < 0.05). Moreover, the expression of semaphorin 5A was closely correlated with that of plexin B3.
CONCLUSION: Semaphorin 5A and its receptor plexin B3 play an important role in the invasion and metastasis of gastric carcinoma.
- Citation: Pan GQ, Ren HZ, Zhang SF, Wang XM, Wen JF. Expression of semaphorin 5A and its receptor plexin B3 contributes to invasion and metastasis of gastric carcinoma. World J Gastroenterol 2009; 15(22): 2800-2804
- URL: https://www.wjgnet.com/1007-9327/full/v15/i22/2800.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2800
Gastric cancer is the leading cause of cancer-related death in some Asian countries including China and Japan. Despite the advances in treatment and research efforts over the past few decades, the outcome of gastric cancer remains poor. The overall 5-year survival rate of gastric cancer patients is 5%-15% in the United States and most other Western countries, largely because many gastric cancers are diagnosed at an advanced stage. The aggressive nature of human gastric carcinoma is related to a variety of intracellular events, including activation of various oncogenes, inactivation of tumor suppressor genes. Therefore, great attention has been given to endogenous factors of tumors, which appear to be responsible for tumor cell growth, progression, and invasion. Identification of such endogenous factors not only leads to a better understanding of the carcinogenesis and development of gastric cancer, but also provides new strategies for developing agents that specifically suppress this process.
Semaphorins are the products of a large family of genes currently containing more than 30 members, all of which share a conserved N-terminal domain called the “sema” domain, a segment of approximately 400-500 amino acids[1]. Based on sequence similarity and distinctive structural features, these genes have been grouped into eight subclasses[1–8], of which classes 3-7 are the products of vertebrate semaphorins. Plexins are identified as the best characterized semaphorin receptors, which are segregated into four sub-families containing nine members. It has been shown that some vertebrate semaphorins belonging to classes 4-7 can bind directly to plexins and activate plexin-mediated signal transduction[23]. These semaphorins and plexins have been originally characterized as constituents of the complex regulatory system responsible for the guidance of axons during the development of the central nervous system[45]. However, a growing body of evidence suggests that certain semaphorins, through interacting with its receptors, play a regulatory role in the occurrence and development of tumor[6–9]. Semaphorin 5A is a member of class 5 semaphorins. Plexin B3, belonging to class B plexin subfamily, is a receptor for semaphorin 5A[10]. However, it is unclear whether semaphorin 5A exerts certain biological functions in the progression of human cancers including gastric carcinoma through plexin B3.
In the present study, we investigated the protein and mRNA expression of semaphorin 5A, plexin B3 in primary gastric carcinoma as well as in its corresponding non-neoplastic mucosa and matched regional lymph node metastasis, and preliminarily analyzed their relation with the invasion and metastasis of gastric cancer.
Forty-eight advanced gastric adenocarcinoma (TNM stage III-IV) patients (28 male and 20 female) with lymph node metastasis diagnosed by postoperative pathology were investigated in this study. Their mean age was 58.7 years (range 45-68 years). The patients received neither chemotherapy nor radiation therapy prior to tumor resection and provided their consent for use of tumor tissue. Tissue blocks of non-neoplastic mucosa (> 5 cm away from the edge of tumor), primary tumor and its corresponding metastatic lymph nodes were obtained within 30 min after they were removed from the patients. Each block was cut into two pieces, one for routine pathologic diagnosis and the other for molecular analysis. Samples were frozen in liquid nitrogen immediately and stored at -260°C until use.
Tissues were lysed using Trizol reagent (Invitrogen, Carlsbad, CA), and total RNA was isolated using chloroform and isopropyl alcohol according to the manufacturer’s instructions. After RNA was quantified, 1-5 &mgr;g of RNA was annealed to Oligo (dT) at 65°C for 5 min and cooled at room temperature. Using a proSTAR first strand RT-PCR kit (Stratagene, La Jolla, CA, USA), reverse transcriptase and dNTPs were added to the RNA-Oligo (dT) mixture and the reaction was performed at 42°C for 1 h. Each single-strand cDNA was used for subsequent PCR amplification of semaphorin 5A, plexin B3 and β-actin with the latter used as a quantitative control. PCR was carried out in a reaction volume of 25 &mgr;L under the following conditions: an initial denaturation at 95°C for 5 min, followed by 30 cycles at 94°C for 30 s, at 55°C for 50 s, at 72°C for 40 s, and a final extension at 72°C for 5 min on an authorized thermal cycler. The primer sequences used amplification are 5’-CTCAGTCGCTGTGAGCGTTAT-3’ and 5’-CAGATGTTGGACCGCCAAATA-3’ for semaphorin 5A, 5’-TCTGCTGCTGCGGTTCTG-3’ and 5’-CCTCTCCACCATCTGCTTGTAG-3’ for plexin B3, 5’-CGCACCACTGGCATTGTCAT-3’ and 5’-TTCTCCTTGATGTCACGCAC-3’ for β-actin, respectively. The primer sequences were synthesized by Beijing Genomics Institute (China). The PCR products were resolved in 1.5 % agarose gels and visualized by staining with ethidium bromide. To quantify the PCR products, bands representing the amplified products were analyzed by Quantity One Analysis Software (BIO-RAD Co., USA).
The reaction mixture volume was made up to 50 &mgr;L. Quantitative RT-PCR was performed using SYBR GreenER qPCR SuperMix reagents (Invitrogen) and a Bio-Rad iCycler. Relative transcript quantities were calculated using the ΔΔCt method with β-actin as the endogenous reference gene amplified from the samples. PCR conditions were as follows: an initial melting step at 95°C for 1 min followed by 35 cycles at 95°C for 90 s, at 60°C for 30 s, at 72°C for 30 s, and a final extension at 72°C for 10 min. The primers used for RT-PCR are 5’-GGTACTGTTCTAGCGACGGC-3’ and 5’- ATACTTGGGTTCGGGGTTGT-3’ for semaphorin 5A, 5’-AAAGCCACCGAGGAGCCAGAA-3’ and 5’-ACTTGACGGCGATGGGGATG-3’ for plexin B3, 5’-TGACGTGGACATCCGCAAAG-3’ and 5’-CTGGAAGGTGGACAGCGAGG-3’ for β-actin, respectively.
Frozen specimens were homogenized in a lysis buffer [50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1 mmol/L EDTA, 0.25 % sodium deoxycholate, 1 % Triton X-100, 0.1 % sodium dodecyl sulfate (SDS), 1 mmol/L NaF, 1 mmol/L Na3VO4], and protease inhibitors (10 mg/L aprotinin and 1 mmol/L phenylmethylsulfonyl fluoride) were added to obtain total protein. An equal amount of protein, quantified with a bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL, USA), was subjected to 10% SDS-polyacrylamide gel electrophoresis, and transferred to polyvinylidene difluoride membrane. The membranes were blocked with 5 % nonfat milk in Tris buffered saline with Tween 20 [TBST, 50 mmol/L Tris-HCl (pH 7.6), 150 mmol/L NaCl, 0.1 % Tween 20] for 2 h at room temperature, and subsequently incubated with primary anti-rabbit polyclonal antibody (anti-semaphorin 5A diluted at 1:400 and plexin B3 diluted at 1:500 and β-actin diluted at 1:2000 were purchased from Santa Cruz Biotechnology) in a blocking buffer at 4°C overnight. Following a washing with TBST, the membranes were incubated with horseradish peroxidase-conjugated rabbit anti-mouse secondary antibody (1:1000, Dako, Glostrup, Denmark) for 2 h at room temperature. The membranes were washed with TBST, and protein bands were visualized with enhanced chemiluminescence according to its manufacturer’s instructions (KPL, Gaithersburg, USA). β-actin bands were taken as a loading control. Protein quantity was analyzed using the UTHSCSA Image Tool 3.0. Target protein expression was evaluated using the relative intensity ratio of target protein/loading control.
Results were expressed as mean ± SD. Statistical differences between different groups were assessed by ANOVA using SPSS12.0 statistical software. P < 0.05 was considered statistically significant.
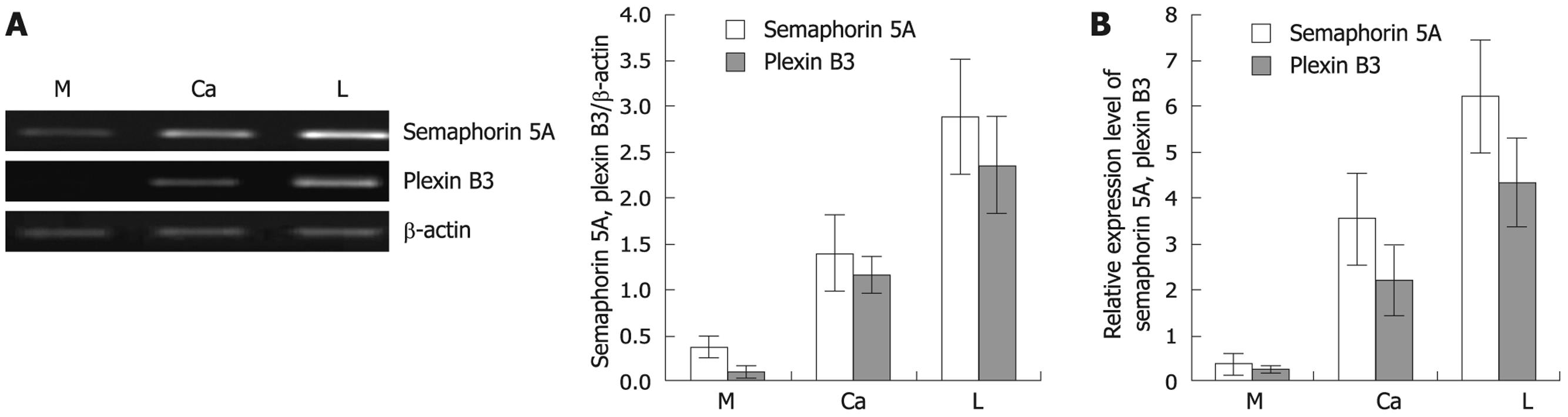
To infer the status of semaphorin 5A and plexin B3 in the invasion and metastasis of gastric carcinogenesis, we evaluated the mRNA expression of semaphorin 5A and plexin B3 using semi-quantitative RT-PCR in 48 samples of primary gastric carcinoma tissue and its corresponding non-neoplastic mucosa as well as matched regional lymph node metastasis. A representative result of RT-PCR for semaphorin 5A and plexin B3 expression is shown in Figure 1A. The expression of semaphorin 5A and plexin B3 mRNA gradually increased in nonneoplastic mucosa, primary gastric carcinoma and lymph node metastasis (P < 0.05). Moreover, the expression of semaphorin 5A was closely correlated with that of plexin B3. To confirm the RT-PCR result of semaphorin 5A and plexin B3, we performed real-time RT-PCR analysis in 20 samples of cDNAs from primary gastric cancer and non-neoplastic mucosa as well as matched regional lymph node metastasis. Similar results were observed (Figure 1B).
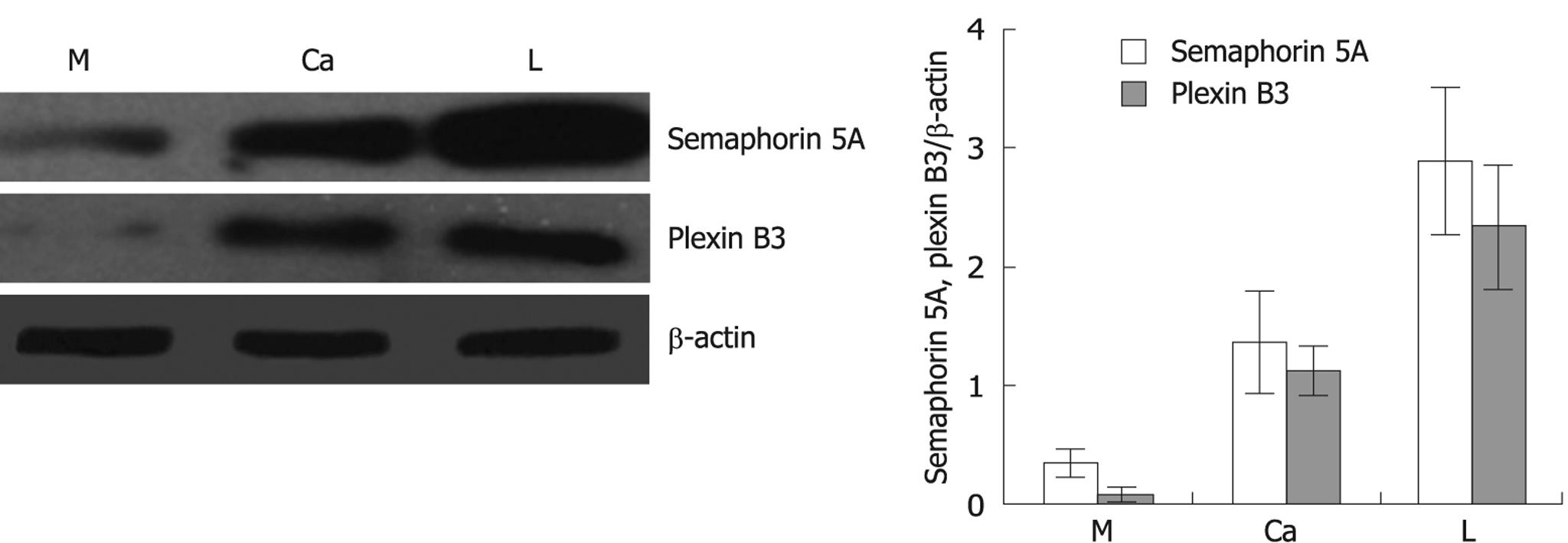
The expression levels of semaphorin 5A, and plexin B3 were also measured by Western blotting in primary gastric carcinoma tissue and its corresponding nonneoplastic mucosa as well as matched regional lymph node metastasis. A representative result of Western blotting for the expression of semaphorin 5A and plexin B3 is shown in Figure 2. After normalization with β-actin, the expression of Semaphorin 5A and plexin B3 protein gradually increased in non-neoplastic mucosa, primary gastric carcinoma and lymph node metastasis (P < 0.05), which was consistent with the result from RT-PCR analysis.
Semaphorin 5A is a member of class 5 semaphorins which are anchored to cell membranes and characterized by seven type 1 thrombospondin repeats. Plexin B3, belonging to class B plexin subfamily, was identified as a specific and functional receptor for semaphorin 5A. Semaphorin 5A and plexin B3 have been originally characterized as constituents of the complex regulatory system responsible for the wiring of neural networks during the development of the central nervous system, and subsequently found to participate in the activities outside of the nervous system such as migration of neural crest cells and heart development to name but a few examples[1112]. However, very little is known about the expression and role of semaphorin 5A and plexin B3 in human cancers including gastric carcinoma. A random p-element insertion screen has been used to identify genes that modulate tumor progression and tumorigenicity in Drosophila study[13]. One of the genes identified in this screen is the Drosophila homologue of semaphorin 5C, with which semaphorin 5A shares a high sequence similarity[13]. In addition, experiments performed by Paolo Conrotto and co-workers revealed that semaphorin 5A, through plexin B3, stimulates the tyrosine kinase activity of Met and RON which has been shown to play a role in tumor progression[1415]. Most importantly, there is cumulative evidence that certain semaphorins and their receptors implicated in axonal path finding in the developing nervous system are expressed in multiple types of cancer cells, modulate the behavior of cancer cells, promote tumor angiogenesis and progression by multiple mechanisms[16]. Taken together, these observations implicate that semaphorin 5A may play a role in the development and progression of human tumors by interacting with plexin B3.
To explore whether semaphorin 5A and plexin B3 are associated with the invasion and metastasis of gastric cancer and exert certain biological functions outside of the nervous system, we investigated the protein and mRNA expression of semaphorin 5A and plexin B3 in primary gastric carcinoma and its corresponding nonneoplastic mucosa as well as matched regional lymph node metastasis by RT-PCR and Western blotting assay. Our experimental results showed that the protein and mRNA expression level of semaphorin 5A was the lowest in normal gastric mucosa, moderate in primary gastric carcinoma, and the highest in lymph nodes with metastatic gastric carcinoma, respectively (P < 0.05). In contrast, plexin B3 and semaphorin 5A had a similar expression pattern, suggesting that the expression of plexin B3 is closely correlated with that of semaphorin 5A. These findings demonstrate that the expression of semaphorin 5A and its receptor plexin B3 increased gradually with gastric cancer procession, indicating that semaphorin 5A may play an important role in the invasion and metastasis of gastric carcinoma through plexin B3, displaying a novel expression and function of semaphorin 5A and plexin B3 outside of the nervous system. To our knowledge, this is the first report on the expression of semaphorin 5A and plexin B3 mRNA and protein in gastric carcinoma, and the functional role of semaphorin 5A and plexin B3 in the invasion and metastasis of gastric cancer.
In conclusion, semaphorin 5A and plexin B3 expression increases significantly with gastric carcinoma progression, and semaphorin 5A and plexin B3 may be involved in the processes of gastric cancer invasion and metastasis. Therefore, the novel expression and function of semaphorin 5A and plexin B3 outside of the nervous system not only add more knowledge about semaphorin 5A and plexin B3, but also shed some lights on the pathogenesis of gastric carcinoma, and probably represent a new therapeutic target for gastric carcinoma.
Gastric carcinoma is one of the most common malignant tumors in China. Invasion and metastasis are the main cause of cancer-related death. Therefore, it is necessary to investigate the mechanism underlying invasion and metastasis of malignant tumors. Semaphorin 5A and its receptor plexin B3 have been originally described in the nervous system, and are important in axon migration and proper central nervous system development. However, very little is known about the expression and role of semaphorin 5A and plexin B3 in human cancers including gastric carcinoma.
Experiments were performed to study the expression of semaphorin 5A and plexin B3 in gastric carcinoma and its relation with tumor invasion and metastasis. This study showed that the expression of semaphorin 5A and plexin B3 increased gradually in non-neoplastic mucosa, primary gastric carcinoma, and lymph node metastasis, suggesting that the expression of semaphorin 5A and plexin B3 is closely correlated to the invasion and metastasis of gastric cancer.
This is the first report on the expression of semaphorin 5A and plexin B3 in gastric carcinoma, and the relation of semaphorin 5A and plexin B3 with the invasion and metastasis of gastric cancer.
The authors studied the expression of semaphorin 5A and plexin B3 in gastric carcinoma and its relation with tumor invasion and metastasis, and showed that the expression level increased gradually in non-neoplastic mucosa, primary gastric carcinoma, and lymph node metastasis, and was positively related to tumor invasion and metastasis, which can be used in research of gastric carcinoma, and provide a new target for gastric carcinoma treatment.
| 1. | Gherardi E, Love CA, Esnouf RM, Jones EY. The sema domain. Curr Opin Struct Biol. 2004;14:669-678. |
| 2. | Yazdani U, Terman JR. The semaphorins. Genome Biol. 2006;7:211. |
| 3. | Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217-227. |
| 4. | Negishi M, Oinuma I, Katoh H. Plexins: axon guidance and signal transduction. Cell Mol Life Sci. 2005;62:1363-1371. |
| 5. | Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789-800. |
| 6. | Potiron VA, Roche J, Drabkin HA. Semaphorins and their receptors in lung cancer. Cancer Lett. 2009;273:1-14. |
| 7. | Sun Q, Nawabi-Ghasimi F, Basile JR. Semaphorins in vascular development and head and neck squamous cell carcinoma-induced angiogenesis. Oral Oncol. 2008;44:523-531. |
| 8. | Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632-645. |
| 9. | Roth L, Koncina E, Satkauskas S, Crémel G, Aunis D, Bagnard D. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci. 2009;66:649-666. |
| 10. | Artigiani S, Conrotto P, Fazzari P, Gilestro GF, Barberis D, Giordano S, Comoglio PM, Tamagnone L. Plexin-B3 is a functional receptor for semaphorin 5A. EMBO Rep. 2004;5:710-714. |
| 11. | Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961-975. |
| 12. | Goldberg JL, Vargas ME, Wang JT, Mandemakers W, Oster SF, Sretavan DW, Barres BA. An oligodendrocyte lineage-specific semaphorin, Sema5A, inhibits axon growth by retinal ganglion cells. J Neurosci. 2004;24:4989-4999. |
| 13. | Woodhouse EC, Fisher A, Bandle RW, Bryant-Greenwood B, Charboneau L, Petricoin EF 3rd, Liotta LA. Drosophila screening model for metastasis: Semaphorin 5c is required for l(2)gl cancer phenotype. Proc Natl Acad Sci USA. 2003;100:11463-11468. |
| 14. | Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene. 2004;23:5131-5137. |
| 15. | Giordano S, Corso S, Conrotto P, Artigiani S, Gilestro G, Barberis D, Tamagnone L, Comoglio PM. The semaphorin 4D receptor controls invasive growth by coupling with Met. Nat Cell Biol. 2002;4:720-724. |
| 16. | Neufeld G, Shraga-Heled N, Lange T, Guttmann-Raviv N, Herzog Y, Kessler O. Semaphorins in cancer. Front Biosci. 2005;10:751-760. |