Published online Jun 14, 2009. doi: 10.3748/wjg.15.2739
Revised: August 26, 2008
Accepted: September 3, 2008
Published online: June 14, 2009
AIM: To identify multi-detector computed tomography (MDCT) features most predictive of serous cystadenomas (SCAs), correlating with histopathology, and to study the impact of cyst size and MDCT technique on reader performance.
METHODS: The MDCT scans of 164 patients with surgically verified pancreatic cystic lesions were reviewed by two readers to study the predictive value of various morphological features for establishing a diagnosis of SCAs. Accuracy in lesion characterization and reader confidence were correlated with lesion size (≤ 3 cm or ≥ 3 cm) and scanning protocols (dedicated vs routine).
RESULTS: 28/164 cysts (mean size, 39 mm; range, 8-92 mm) were diagnosed as SCA on pathology. The MDCT features predictive of diagnosis of SCA were microcystic appearance (22/28, 78.6%), surface lobulations (25/28, 89.3%) and central scar (9/28, 32.4%). Stepwise logistic regression analysis showed that only microcystic appearance was significant for CT diagnosis of SCA (P = 0.0001). The sensitivity, specificity and PPV of central scar and of combined microcystic appearance and lobulations were 32.4%/100%/100% and 68%/100%/100%, respectively. The reader confidence was higher for lesions > 3 cm (P = 0.02) and for MDCT scans performed using thin collimation (1.25-2.5 mm) compared to routine 5 mm collimation exams (P > 0.05).
CONCLUSION: Central scar on MDCT is diagnostic of SCA but is seen in only one third of SCAs. Microcystic morphology is the most significant CT feature in diagnosis of SCA. A combination of microcystic appearance and surface lobulations offers accuracy comparable to central scar with higher sensitivity.
- Citation: Shah AA, Sainani NI, Ramesh AK, Shah ZK, Deshpande V, Hahn PF, Sahani DV. Predictive value of multi-detector computed tomography for accurate diagnosis of serous cystadenoma: Radiologic-pathologic correlation. World J Gastroenterol 2009; 15(22): 2739-2747
- URL: https://www.wjgnet.com/1007-9327/full/v15/i22/2739.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2739
Pancreatic serous cystadenomas (SCAs) are rare lesions that are almost always benign and usually asymptomatic[1]. SCAs comprise 1%-2% of pancreatic neoplasms and 10%-15% of cystic lesions[2]. While SCAs are relatively uncommon in comparison to pseudocysts and solid tumors of the pancreas, their clinical importance is indisputable. Though generally regarded as benign, 3% of SCAs have malignant potential with local infiltration and distant metastases[13–5]. These are slow growing tumors; however the growth rate varies depending on tumor size. When the tumor is under 4 cm in diameter, the growth rate is only 0.12 cm/year whereas when the tumors ≥ 4 cm in diameter they can grow at a remarkable 1.98 cm/year[6]. Due to the benign nature and the morbidity associated with pancreatic surgery, a follow up imaging for surveillance is recommended for these tumors[37]. Complete resection is considered curative, and is recommended when the lesion is symptomatic, when it increases in size upon follow-up, and when confident non-invasive differentiation from a more aggressive lesion is impossible[6]. Tumors that are resected have a good prognosis, with no requirement for postoperative surveillance[36].
Due to increased utilization of high-resolution imaging such as multi-detector computed tomography (MDCT), magnetic resonance imaging (MRI) and MR cholangiopancreatography (MRCP), SCAs are now more frequently identified, and often incidentally[89]. This high rate of detection of incidental pancreatic cystic lesions has generated great interest regarding the appropriate management of these lesions[6]. However, there is some overlap in imaging appearance among cystic pancreatic lesions, and it can be difficult to differentiate SCAs from other types of pancreatic cysts, such as pseudocysts, mucinous cystic neoplasms (MCNs) and intraductal papillary mucinous neoplasms (IPMNs). Thus the diagnosis of serous cystadenomas assumes particular significance because they need to be differentiated from other cystic neoplasms like MCNs, which are known to be premalignant or malignant[10]. The differentiation is vital to avoid unnecessary pancreatic surgery, which although increasingly safe in experienced hands continues to cause significant postoperative morbidity[6]. Thus accurate preoperative lesion characterization is crucial in determining appropriate action.
MDCT is the initial imaging modality of choice for evaluation of cystic lesions of the pancreas. Although the imaging features of SCAs on MDCT have been described before, few studies have compared the accuracy of various CT features for distinguishing SCAs from other lesions. With this purpose in mind, we undertook this study to examine the different features of SCAs on MDCT and identify the common and uncommon imaging features of SCAs that enable a confident diagnosis. Other commonly occurring cystic lesions were also studied from a large cohort of cystic lesions to identify the specific features that characterize SCAs.
This is a retrospective study that was approved by the Institutional Review Board and follows the Health Insurance Portability and Accountability Act regulations. Informed patient consent forms were waived. We searched the electronic database of hospital medical records for patients who had pancreatic surgery for cystic lesions from January 1999 to August 2007. The inclusion criteria required the patients to have had the MDCT exam prior to surgical resection. Out of a total of 180 patients with MDCT studies prior to surgical resection, 16 patients who had clinical and laboratory evidence of acute pancreatitis were excluded from the study. Criteria for diagnosis of pancreatitis were elevated serum amylase or lipase levels and/or imaging evidence of pancreatic inflammation. The process resulted in an initial cohort of 164 patients. These patients were evaluated for clinical presentation, imaging features, and pathological and surgical findings. Of the group of 164 patients, a subset consisting of 28 patients (17 women, 11 men; age range, 29-90 years; mean age, 62 years) with pathologically verified SCAs were studied to analyze their characteristic imaging features and constituted the main population for the present study. However, data from the other 136 patients in the cohort of 164 was also analyzed to study the accuracy of each imaging feature studied.
All patients had undergone CT examinations on MDCT (GE Health Care, Milwaukee, WI) with four, eight or 16 detector rows. The acquisition protocol for the CT exam was either dedicated pancreatic protocol CT (91/164, 14 SCAs) or a routine contrast enhanced CT study (73/164, 14 SCAs). The initial scan was a non-enhanced CT acquisition of 5 mm thickness through the liver and pancreas. For routine abdominal CT scanning, 120-150 mL of nonionic contrast material (300-370 mg/mL) was injected at a rate of 3.0 mL/s, and 5 mm thick images were acquired after a 65-70 s delay.
Pancreatic protocol consisted of two phase acquisition after administration of 120-150 mL of nonionic contrast material (370 mg of iodine per milliliter) at a rate of 4-5 mL/s. Pancreatic phase imaging was performed 45 s after starting contrast material injection by obtaining 1.25-mm/2.5-mm targeted reconstructed sections through the pancreas. Portal venous phase imaging followed at 65-70 s after contrast material injection with 5-mm-thick sections. For pancreatic phase imaging, the field of view was 28 cm; for the other phases, the field of view was adjusted according to the size of the patient.
Coronal reformatted images of 2.5-3 mm thickness were obtained in all of the patients. Additional reformatting techniques used included oblique coronal multiplanar reformations (MPRs, 5 mm) parallel to the pancreatic head or tail, 1-2 curved planar reformations along the course of the pancreatic duct (1.25 mm) and thin slab (5 mm) coronal maximum intensity projections (MIP) to display vessel involvement. These reformations were performed by one of the trained technologists on a work station (ADW 4.0, GE).
The CT images were retrospectively reviewed by consensus by two radiologists with 14 and 7 years of experience, respectively, who were kept blinded to patients’ clinical details and histopathology of the cystic lesion. The analysis was done on picture archiving and communication system (PACS version 4.0, Agfa, Richmond, VA). For the image analysis, a template was created to evaluate features of a pancreatic cyst. The following features were assessed: cyst size, presence or absence of septations, lesion margins, solid components, lobulations, central scar, calcification, pancreatic duct communication, duct obstruction, lymphadenopathy and vascular involvement. The pancreas was evaluated for presence or absence of duct dilatation, parenchymal/ductal calcification and parenchymal atrophy.
The largest dimensions of the cyst were measured on axial scans and mean size calculated. The septations were evaluated for thickness and enhancement. The margins of the lesion were considered either well defined or ill defined. The shape of the cysts was defined as either smooth, simple lobulated or complex lobulated. Simple lobulation was defined as the shape of a simple closed curve with bosselated surface whose borders could not be described within the same circle[10]. A complex lobulated shape was defined as one containing a conglomeration of two or more cysts either round, oval or tubular (pleomorphic in shape)[10]. Central scar was defined as a central stellate area of soft tissue density with or without calcification and with or without radiating surrounding linear strands. Attenuation values were obtained for the cysts by placing a ROI (mean size, 20 mm2) on the unenhanced scan. Care was taken to exclude septations, calcifications and solid component within the ROI. The attenuation values measured from the various types of lesions (SCA, MCN, etc) were then averaged for comparison. Cyst morphology was classified as unilocular, microcystic, macrocystic or oligocystic and cyst with solid component[8]. Simple unilocular cysts included pancreatic cysts without internal septa, a solid component or central-cyst wall calcification. Unilocular cysts with mural enhancement or calcification were categorized as complicated. Microcystic lesion was defined as consisting of collection of cysts (> 6) ranging in size from a few millimeters up to 2 cm in size[8]. Macrocystic or oligocystic lesions were defined as lesions with more than one but < 6 cysts with at least one of them > 2 cm in size.
Using these features, the cysts were categorized as a simple cyst/pseudocyst, SCA, mucinous cystadenoma (MCN) and intraductal papillary mucinous cystadenoma (IPMN) or else solid neoplasms with cystic degeneration (Adenocarcinoma, endocrine tumors and solid papillary epithelial neoplasm) on imaging. The criteria considered for diagnosis of SCAs included: lobulations, microcystic pattern, presence or absence of central scar, well defined margins and lack of enhancement. Readers’ diagnostic confidence for the diagnosis of serous cystadenoma was rated on a 5-point scale (with 1 being least confident and 5 being most) along with confidence as to whether the lesion was benign or malignant using a similar 5-point scale. Cystic lesions which did not have any specific attributable features to the above categories or those with a reader confidence less than three were considered indeterminate.
The type of surgical procedure performed was recorded. Histopathological analysis had been performed by a single gastrointestinal pathologist with ten years of specialized experience in pancreatic pathology. The gross and microscopic descriptions of the resected specimens described in the pathology reports were reviewed. A predefined template for pathology was filled in. The gross specimens were reviewed for cyst morphology, which included lesion size, septations and intralesional solid components. The final histopathological diagnosis was recorded as: SCA, IPMN, MCN, adenocarcinoma, endocrine tumors or solid papillary epithelial neoplasm. Those cysts without specific identifiable features where a conclusive diagnosis was not rendered on histopathology were categorized as unclassified cysts. The features on MDCT were then correlated with pathological reports.
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy for the various MDCT findings of SCA were calculated in comparison to other cystic lesions by using histopathology as the gold standard. T-test was used to calculate the statistical significance for various values and P < 0.05 was considered to suggest statistically significant difference. Statistical significance between the size of SCA and the occurrence of scar was evaluated using two sided Fisher’s exact test. Stepwise logistic regression was used to identify the significance of each CT feature for diagnosis of SCA using SAS software (SAS system release 8.2). The CT feature was considered significant for the diagnosis whenever a tail probability of P < 0.05 was reached.
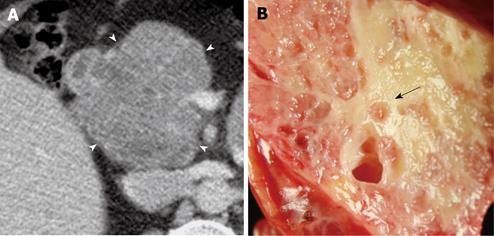
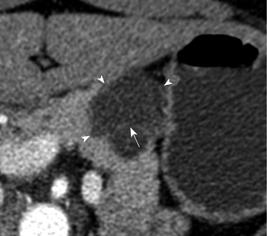
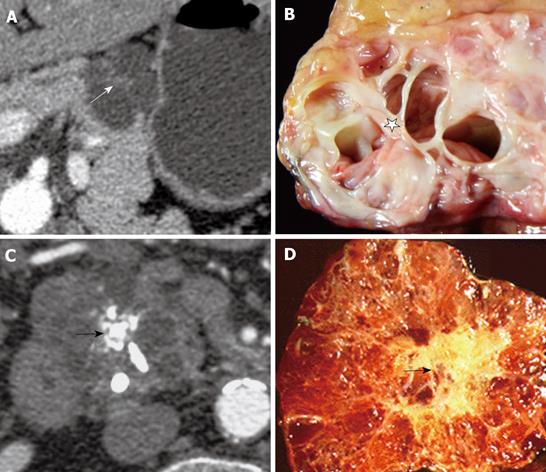
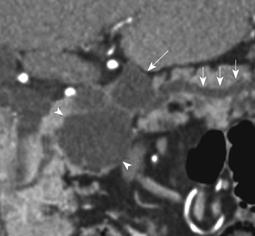
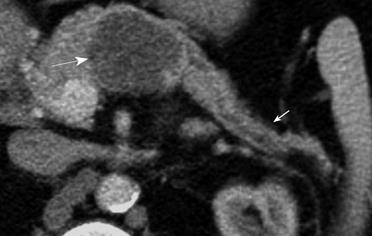
Of the 28 SCA (mean size, 39 mm; range, 8-92 mm), 11 lesions were located in the head, 11 in the body, 3 in the tail and 3 in body and tail (Table 1). 22/28 (78.6%) SCAs were observed to have microcystic morphology (Figure 1) and 6/28 (21.4%) of SCAs had macrocystic or oligocystic morphology (Figure 2). 25/28 (89.3%) of SCAs were lobulated whereas 3/28 (10.7%) presented with smooth wall. 9/28 (32.1%) SCAs showed central scar with calcification seen in 3 scars (Figure 3). 8/9 (88.9%) of SCAs with central scar measured at least 2 cm (mean size, 4.1 cm; range, 1.9-8.4 cm). This finding was statistically significant for lesion size and occurrence of central scar (P = 0.02, Fisher’s exact test).
| SCA (n = 28) | IPMN (n = 42) | MCN (n = 37) | Unclassified Cysts (n = 45) | Endocrine tumors (n = 6) | Adenocarcinoma (n = 4) | Lymphan-gioma (n = 1) | SPE (n = 1) | |
| Size (cm) mean (range) | 3.9 (0.8-9.2) | 2.7 (0.9-5.2) | 4.3 (2.2-11) | 2.4 (0.5-7.4) | 4.6 (1.6-8.9) | 3 (1.4-5.2) | 2.3 | 2.3 |
| Location | ||||||||
| Head | 11 | 26 | 12 | 20 | 2 | 2 | 1 | |
| Body | 11 | 7 | 7 | 8 | 0 | 1 | 1 | |
| Tail | 3 | 7 | 17 | 12 | 4 | 1 | ||
| Body/Tail | 3 | 3 | 1 | 5 | 0 | 0 | ||
| Lobulations (n = 35/164) | 25 | 3 | 1 | 5 | 1 | 0 | 0 | 0 |
| Microcystic (n = 25/164) | 22 | 1 | 2 | 0 | 0 | 0 | 0 | 0 |
| Central Scar (n = 9/164) | 92 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| L + M1 (n = 19/164) | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Septa | 27 | 32 | 25 | 28 | 2 | 2 | 0 | 0 |
| Wall | ||||||||
| Thin | 28 | 40 | 30 | 31 | 0 | 2 | 1 | 1 |
| Thick | 0 | 2 | 7 | 14 | 6 | 2 | 0 | 0 |
| Calcifications | ||||||||
| Wall | 0 | 4 | 1 | 5 | 0 | 0 | 0 | 0 |
| Septal | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Parenchymal | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mural nodules | 0 | 3 | 6 | 1 | 3 | 4 | 0 | 1 |
| Dilatation of PD | 3 | 14 | 11 | 2 | 0 | 3 | 0 | 0 |
| Vascular involvement | 0 | 0 | 1 | 2 | 0 | 2 | 0 | 0 |
| Lymphadenopathy | 0 | 3 | 6 | 3 | 0 | 2 | 0 | 0 |
Of the 136 other cystic lesions included for comparison of the morphological features with SCAs, 42 were IPMNs (mean size, 2.7 cm; range 0.9-5.2 cm), 37 were MCNs (mean size, 4.3; range, 2.2-11 cm), 45 were unclassified cysts (mean size, 2.4 cm; range 0.5-7.4 cm), 6 endocrine tumors (mean size, 4.6 cm; range, 1.6-8.9 cm), 4 adenocarcinomas (mean size, 3 cm; range, 1.4-5.2 cm), 1 was lymphangioma (2.3 cm) and 1 was solid papillary epithelial neoplasm (2.3 cm). Among these lesions microcystic pattern on CT was observed in 1 IPMN (2.3%) and 2 MCNs (5.4%) whereas the lobulated pattern was observed in 5 unclassified cysts (11.1%), 3 IPMNs (7.7%) and 1 MCN (3%). Central scar was not demonstrable in any of these lesions.
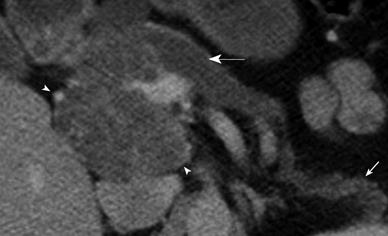
Upstream pancreatic ductal dilatation was observed in 3 patients. In one patient this was attributed to mass effect from a large SCA in the head of pancreas (Figure 4). However in two other patients with SCA, the pancreatic duct dilatation was considered due to background chronic pancreatitis changes in one and concurrent combined IPMN in another (Figure 5). Furthermore, five patients showed atrophy of the pancreatic parenchyma distal to the site of the lesion (Figures 4 and 6). None of the SCAs showed solid component, ductal communication or vascular encasement. The mean HU values for SCA were 19 ± 9 HU which was higher for microcystic variants (21 ± 8 HU) than the macrocystic variants (13 ± 4 HU). Though the average attenuation value for SCAs was higher than other types of cysts [mucinous lesions (MCNs and IPMNS): 10 ± 6 HU, pseudocysts: 12 ± 4 HU] it was not statistically significant (P > 0.05).
Twenty four (85.7%) of the 28 SCAs were correctly characterized as such by CT. Of the four SCAs incorrectly identified on CT, two were classified as MCNs, one lesion as a possible IPMN, and one lesion was considered an indeterminate cystic lesion. The two classified as mucinous lesions had a macrocystic appearance and no central scar. One of these was classified as a benign mucinous lesion based on its macrocystic pattern and lack of central scar on CT. The second patient had two cysts, of which one was classified as mucinous and second as IPMN of side branch variety. However, histopathological analysis showed that the former was a SCA with a side branch IPMN in the vicinity (Figure 5). The lesion characterized as IPMN was microcystic, but confidence for diagnosis of SCA was low. The lesion classified as indeterminate cystic lesion was lobulated with thin septations and though the morphology was identified as macrocystic and benign, the reader confidence for diagnosis of SCA was less than 3.
When the imaging data of SCA were compared with pathological analysis, the majority of the observations remained constant (Table 2). MDCT features were consistent with the histological data for microcystic appearance (27/28, 96.4%) and surface lobulations (28/28, 100%). The 25 SCAs with pathologically confirmed lobulated morphology and the 3 with pathologically smooth morphology were correctly identified as such by CT. Of the 21 SCAs with pathologically confirmed microcystic morphology and 6 SCAs with pathologically confirmed macrocystic pattern, all were confidently identified as such on CT. One SCA which was characterized as microcystic on CT was found to be unilocular on pathology. This lesion measured 2 cm in diameter and there was substantial background noise on CT images.
| MDCT features | Pathology findings | |
| Morphology | ||
| Microcystic | 22 | 21 |
| Macrocystic/Oligocystic | 6 | 6 |
| Unilocular | - | 1 |
| Shape | ||
| Lobulated | 25 | 25 |
| Smooth | 3 | 3 |
| Central scar | 9 | 10 |
| Wall | ||
| Thin | 28 | 28 |
| Thick | 0 | 0 |
| Mural nodules | 0 | 0 |
| Septa | 27 | 27 |
Of the 10 central scars discovered on pathology, CT correctly identified them in 8 lesions. Central scars were missed on CT in 2, while in another SCA the central scar recorded on CT was found to represent converging septa on histopathology. In two lesions where CT failed to detect central scar, the SCA measured < 3 cm in size (average, 2.4 cm) and were evaluated using a routine protocol (5 mm). It is conceivable that these factors could have contributed to the reduced accuracy in depiction of SCA morphology.
For cyst characterization into benign and malignant, 27/28 lesions were confidently diagnosed as benign except for one lesion that was considered indeterminate on CT and was found to be benign on histopathology. This lesion measured 5.4 cm in diameter and presented with pancreatic duct dilatation and parenchymal atrophy, which raised the suspicion for an aggressive biology.
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of each of these features in the diagnosis of SCA are shown in Table 3. Based on pathologic verification, microcystic morphology on MDCT was demonstrated in 21/28 (75%) of SCAs. Microcystic pattern was also considered in other cystic lesions on MDCT that included 1 IPMN (n = 42) and 2 MCNs (n = 37), however on histopathology the CT appearance was not corroborated. The presence of microcystic morphology alone thus had a PPV of 88% and a specificity of 97.79% for the diagnosis of SCA. Lobulations were seen in 25/28 (89.3%) of SCAs, 5/45 unclassified cysts (11.1%), 3/42 IPMNs (7.1%) and 1/37 MCNs (2.7%). Lobulations had a PPV of 71.4% and specificity of 92.6% in the diagnosis of SCAs. When both lobulations and microcystic morphology were used for the identification of cystic lesions there was a PPV and specificity of 100% for identification of SCAs. It was found that central scar, microcystic pattern and combined presence of microcystic pattern and lobulations had the highest accuracy in the diagnosis of SCA. Stepwise logistic regression analysis showed that only microcystic appearance was significant for the CT diagnosis of SCA (P = 0.0001).
| Lobulations (n = 35/164, %) | Microcystic (n = 25/164, %) | Central scar (n = 9/164, %) | L + M1 (n = 19/164, %) | |
| Sensitivity | 89.30 | 78.50 | 32.40 | 67.85 |
| Specificity | 92.64 | 97.79 | 100.00 | 100.00 |
| Positive predictive value | 71.42 | 88.00 | 100.00 | 100.00 |
| Negative Predictive value | 97.67 | 95.70 | 87.74 | 93.80 |
| Accuracy | 92.00 | 94.50 | 88.40 | 94.50 |
When comparing the diagnostic confidence for diagnosis of SCAs with reference to the lesion size, the confidence was higher for lesions ≥ 3 cm (4.6) compared to lesions < 3 cm (3.9, P = 0.02). The CT pathologic concordance was better for lesions scanned with dedicated pancreatic protocol CT. The diagnostic confidence of readers in identifying the morphology of SCAs was better in those lesions scanned with dedicated pancreatic protocol (1.25-2.5 mm) compared to those scanned with routine abdomino-pelvic CT (5 mm) though this was not statistically significant (confidence level, 4.4 vs 4, P > 0.05).
High rate of incidental detection of cystic pancreatic lesions, including serous cystadenoma and the overlapping imaging features of SCAs with other more aggressive cystic neoplasms makes management of these lesions challenging[791112]. Accurate characterization is therefore imperative because of the low malignant potential of SCA and to determine the treatment options and to differentiate them from more aggressive lesions. Several studies have described the spectrum of imaging appearances of SCA on CT which include microcystic, macrocystic, unilocular and mixed morphologic patterns[7911–14]. Microcystic pattern which is the most predominant pattern has been described in 70%-80% of SCAs[111214]. Surface lobulations considered to be specific for the diagnosis of SCA have been studied with reference to oligocystic SCA (SOAs)[101516]. Central scar which is considered pathognomonic for diagnosis of SCA has a low sensitivity and reliance on this imaging feature will not permit diagnosis of most SCAs[101117]. The accuracy of CT in diagnosis of SCA in comparison to other cystic neoplasms has been reported in several previous studies which found accuracy ranging from 27%-93%[17–19]. More recent studies have compared SOAs with MCNs and IPMNs and have emphasized the importance of patient demographics, lesion location and shape of the cyst in characterization of the various lesions[41015]. However, our study is unique as we have assessed the predictive value of specific features of SCA on MDCT in a large cohort of patients with surgically verified pancreatic cystic lesions and have evaluated how well the CT features correlate with pathology.
In our study, microcystic appearance was the only significant CT feature in the diagnosis of SCA. The combined presence of microcystic appearance and surface lobulations were also the strong predictors of SCA on CT with high PPV and specificity compared to other cystic lesions. Microcystic appearance was evident in 78% of SCAs in our study and this was concordant with other reports[11–14]. However, occasionally other cystic lesions when small in size can also appear microcystic on CT. In our study, small lesion size (< 2 cm) and background image noise potentially contributed to erroneous morphological depiction. Presence of surface lobulations within a cyst is a recognized feature of SCAs and can help to differentiate oligocystic SCAs from MCNs and IPMNs[1015]. We observed lobulated contour in 89% of SCAs despite different morphologic appearances (microcystic, macrocystic and unilocular), a finding that has also been reported in other studies[101115]. Although uncommon, surface lobulations can be encountered in a few MCNs (2.9%) and IPMNs (7.6%) as also reported by Kim et al[10].
The presence of a central scar is considered to be pathognomonic for SCAs, even when there is no distinct microcystic appearance[17]. Finding a central scar was highly specific of SCA in our study. It was not observed in any other cystic lesion but was encountered in only in 32% of SCAs. Other studies have observed it in anywhere from 30% to 45% of microcystic serous adenomas[172021]. Eighty eight point nine percent of SCAs with central scar measured at least 2 cm indicating that larger the lesion size the more likelihood of occurrence of scar. Size of the lesion also determines the detectability of central scar since the two lesions in our study where the central scar was missed measured less than 3 cm in size.
The mean CT attenuation for all SCAs in our data set was higher than the values for other cystic lesions, more so for the microcystic variants though they were not statistically significant. The marginally elevated HU values could be accounted for by the presence of more stroma between the fluid filled sacs and the higher stroma: fluid ratio in SCA than other lesions. However, the attenuation values cannot be used as primary feature for differentiating between various pancreatic cystic lesions.
Lesion size itself can influence the reader confidence as the confidence for diagnosis of SCA was found to be higher for lesions ≥ 3 cm compared to those with size < 3 cm. In addition to size of the cystic lesions, the MDCT scanning technique also influences the diagnostic accuracy and readers’ confidence for lesion characterization.
Although the comparison of MDCT with MRI was not a part of this study, in our experience we have found that MRI might be beneficial when in doubt since the cyst morphology may be better appreciated on MRI. In our study, lesions which underwent dedicated pancreatic protocol examination with thin collimation (1.25-2.5 mm) had an improved reader confidence in the depiction of the morphological features compared to those which were scanned with routine protocol (5 mm). Two lesions where the central scars were missed on CT and the three cases of SCA which were misdiagnosed as mucinous lesions were evaluated by routine protocol, highlighting the need to perform a dedicated pancreatic protocol CT for superior lesion characterization of cystic pancreatic lesions. We propose that all pancreatic cystic lesions should have at least one pancreatic protocol CT for better lesion characterization which will not only help avoid additional imaging follow up, but also prevent unnecessary surgical interventions.
This study had several limitations. Firstly, only surgically resected tumors were included for evaluation, which introduces a possible selection bias. However, since the majorities of SCAs are benign and are usually not resected, the comparison of MDCT findings with the histopathology findings in surgically resected SCAs adds to the value of the study. Secondly, not all patients had a dedicated pancreatic protocol CT exam. Though this could have affected the evaluation of diagnostic accuracy, it also provided us with an opportunity to study the effect of dedicated pancreatic protocol for evaluation of SCA compared to routine CT.
MDCT allows reliable assessment of the morphological features of SCA as depicted on gross histopathology. Central scar, although pathognomonic for SCA, is uncommonly seen. Microcystic morphology is the most significant CT feature in the diagnosis of SCA. Combined presence of microcystic morphology and surface lobulations offers high accuracy comparable to central scar but with higher sensitivity, thus allowing reliable diagnosis of SCA. Use of a dedicated pancreatic protocol with thin collimation improves the diagnostic accuracy of MDCT and enhances reader confidence.
Pancreatic serous cystadenomas (SCAs) are rare lesions that are almost always benign and usually asymptomatic. Though surgical resection results in complete cure, follow up surveillance is usually recommended due to their benign nature and the morbidity associated with pancreatic surgery. There is increased incidental detection of pancreatic cysts including SCAs with the use of multi-detector computed tomography (MDCT) and MRI. Accurate characterization of SCAs assumes importance due to their overlapping imaging features with other pancreatic cystic lesions particularly mucinous cystic neoplasms (MCNs) which have a malignant potential. Though several articles have described the imaging features of SCAs, few studies have compared the accuracy of these CT features in distinguishing between SCAs and other lesions. Therefore, this study was undertaken to evaluate the predictive values of various CT features of SCA that allow in its accurate characterization with pathological correlation.
One of the important innovations in the imaging evaluation of pancreatic cysts is the development of MDCT which allows excellent characterization of the morphology of pancreatic cyst. Though several authors have described the CT features of SCA, very few articles have discussed the predictive value of each imaging feature. This articles shows that microcystic appearance is the most significant imaging feature of SCA and the combination of microcystic appearance and surface lobulations is very specific for SCA in comparison to other cystic lesions.
The most important clinical application of this study is in the diagnosis and characterization of serous cystadenomas when incidentally detected on a CT scan. The finding of superiority of dedicated pancreatic protocol CT over routine protocol helps in the planning of CT examination when encountered with a incidental pancreatic cyst. This article emphasizes the effect of cyst size on lesion characterization and diagnostic accuracy. This calls for further studies for improve characterization of pancreatic cysts by furthering development of high resolution CT and MRI.
The important part of the paper is that the study shows that microcystic appearance and lobulated pattern when present together are highly specific for diagnosis of SCA. This study highlights the value of performing a dedicated pancreatic protocol in the evaluation of pancreatic cystic lesions in improving diagnostic accuracy and reader confidence.
| 1. | Strobel O, Z’graggen K, Schmitz-Winnenthal FH, Friess H, Kappeler A, Zimmermann A, Uhl W, Büchler MW. Risk of malignancy in serous cystic neoplasms of the pancreas. Digestion. 2003;68:24-33. |
| 2. | Horvath KD, Chabot JA. An aggressive resectional approach to cystic neoplasms of the pancreas. Am J Surg. 1999;178:269-274. |
| 3. | Galanis C, Zamani A, Cameron JL, Campbell KA, Lillemoe KD, Caparrelli D, Chang D, Hruban RH, Yeo CJ. Resected serous cystic neoplasms of the pancreas: a review of 158 patients with recommendations for treatment. J Gastrointest Surg. 2007;11:820-826. |
| 4. | Goh BK, Tan YM, Yap WM, Cheow PC, Chow PK, Chung YF, Wong WK, Ooi LL. Pancreatic serous oligocystic adenomas: clinicopathologic features and a comparison with serous microcystic adenomas and mucinous cystic neoplasms. World J Surg. 2006;30:1553-1559. |
| 5. | Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230:152-161. |
| 6. | Tseng JF, Warshaw AL, Sahani DV, Lauwers GY, Rattner DW, Fernandez-del Castillo C. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413-419; discussion 419-421. |
| 7. | Bassi C, Salvia R, Molinari E, Biasutti C, Falconi M, Pederzoli P. Management of 100 consecutive cases of pancreatic serous cystadenoma: wait for symptoms and see at imaging or vice versa? World J Surg. 2003;27:319-323. |
| 8. | Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005;25:1471-1484. |
| 9. | Megibow AJ, Lombardo FP, Guarise A, Carbognin G, Scholes J, Rofsky NM, Macari M, Balthazar EJ, Procacci C. Cystic pancreatic masses: cross-sectional imaging observations and serial follow-up. Abdom Imaging. 2001;26:640-647. |
| 10. | Kim SY, Lee JM, Kim SH, Shin KS, Kim YJ, An SK, Han CJ, Han JK, Choi BI. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol. 2006;187:1192-1198. |
| 11. | Procacci C, Graziani R, Bicego E, Bergamo-Andreis IA, Guarise A, Valdo M, Bogina G, Solarino U, Pistolesi GF. Serous cystadenoma of the pancreas: report of 30 cases with emphasis on the imaging findings. J Comput Assist Tomogr. 1997;21:373-382. |
| 12. | Kim HJ, Lee DH, Ko YT, Lim JW, Kim HC, Kim KW. CT of serous cystadenoma of the pancreas and mimicking masses. AJR Am J Roentgenol. 2008;190:406-412. |
| 13. | Procacci C, Biasiutti C, Carbognin G, Accordini S, Bicego E, Guarise A, Spoto E, Andreis IA, De Marco R, Megibow AJ. Characterization of cystic tumors of the pancreas: CT accuracy. J Comput Assist Tomogr. 1999;23:906-912. |
| 14. | Chaudhari VV, Raman SS, Vuong NL, Zimmerman P, Farrell J, Reber H, Sayre J, Lu DS. Pancreatic cystic lesions: discrimination accuracy based on clinical data and high resolution CT features. J Comput Assist Tomogr. 2007;31:860-867. |
| 15. | Cohen-Scali F, Vilgrain V, Brancatelli G, Hammel P, Vullierme MP, Sauvanet A, Menu Y. Discrimination of unilocular macrocystic serous cystadenoma from pancreatic pseudocyst and mucinous cystadenoma with CT: initial observations. Radiology. 2003;228:727-733. |
| 16. | Khurana B, Mortelé KJ, Glickman J, Silverman SG, Ros PR. Macrocystic serous adenoma of the pancreas: radiologic-pathologic correlation. AJR Am J Roentgenol. 2003;181:119-123. |
| 17. | Curry CA, Eng J, Horton KM, Urban B, Siegelman S, Kuszyk BS, Fishman EK. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol. 2000;175:99-103. |
| 18. | Johnson CD, Stephens DH, Charboneau JW, Carpenter HA, Welch TJ. Cystic pancreatic tumors: CT and sonographic assessment. AJR Am J Roentgenol. 1988;151:1133-1138. |
| 19. | Yuan D, Yu W, Ren XB, Pan WD, Zhang LH. [Characterization and diagnostic accuracy of serous cystadenomas and mucinous neoplasms of the pancreas with multi-slice helical computed tomography]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2007;29:232-237. |
| 20. | Yasuhara Y, Sakaida N, Uemura Y, Senzaki H, Shikata N, Tsubura A. Serous microcystic adenoma (glycogen-rich cystadenoma) of the pancreas: study of 11 cases showing clinicopathological and immunohistochemical correlations. Pathol Int. 2002;52:307-312. |