Published online Jun 14, 2009. doi: 10.3748/wjg.15.2723
Revised: April 7, 2009
Accepted: April 14, 2009
Published online: June 14, 2009
AIM: To compare the features of biochemical metabolic changes detected by hepatic phosphorus-31 magnetic resonance spectroscopy (31P MRS) with the liver damage score (LDS) and pathologic changes in rabbits and to investigate the diagnostic value of 31P MRS in acute hepatic radiation injury.
METHODS: A total of 30 rabbits received different radiation doses (ranging 5-20 Gy) to establish acute hepatic injury models. Blood biochemical tests, 31P MRS and pathological examinations were carried out 24 h after irradiation. The degree of injury was evaluated according to LDS and pathology. Ten healthy rabbits served as controls. The MR examination was performed on a 1.5 T imager using a 1H/31P surface coil by the 2D chemical shift imaging technique. The relative quantities of phosphomonoesters (PME), phosphodiesters (PDE), inorganic phosphate (Pi) and adenosine triphosphate (ATP) were measured. The data were statistically analyzed.
RESULTS: (1) Relative quantification of phosphorus metabolites: (a) ATP: there were significant differences (P < 0.05) (LDS-groups: control group vs mild group vs moderate group vs severe group, 1.83 ± 0.33 vs 1.55 ± 0.24 vs 1.27 ± 0.09 vs 0.98 ± 0.18; pathological groups: control group vs mild group vs moderate group vs severe group, 1.83 ± 0.33 vs 1.58 ± 0.25 vs 1.32 ± 0.07 vs 1.02 ± 0.18) of ATP relative quantification among control group, mild injured group, moderate injured group, and severe injured group according to both LDS grading and pathological grading, respectively, and it decreased progressively with the increased degree of injury (r = -0.723, P = 0.000). (b) PME and Pi; the relative quantification of PME and Pi decreased significantly in the severe injured group, and the difference between the control group and severe injured group was significant (P < 0.05) (PME: LDS-control group vs LDS-severe group, 0.86 ± 0.23 vs 0.58 ± 0.22, P = 0.031; pathological control group vs pathological severe group, 0.86 ± 0.23 vs 0.60 ± 0.21, P = 0.037; Pi: LDS-control group vs LDS-severe group, 0.74 ± 0.18 vs 0.43 ± 0.14, P = 0.013; pathological control group vs pathological severe group, 0.74 ± 0.18 vs 0.43 ± 0.14, P = 0.005) according to LDS grading and pathological grading, respectively. (c) PDE; there were no significant differences among groups according to LDS grading, and no significant differences between the control group and experimental groups according to pathological grading. (2) The ratio of relative quantification of phosphorus metabolites: significant differences (P < 0.05) (LDS-moderate group and LDS-severe group vs LDS-control group and LDS-mild group, 1.94 ± 0.50 and 1.96 ± 0.72 vs 1.43 ± 0.31 and 1.40 ± 0.38) were only found in PDE/ATP between the moderate injured group, the severe injured group and the control group, the mild injured group. No significant difference was found in other ratios of relative quantification of phosphorus metabolites.
CONCLUSION: 31P MRS is a useful method to evaluate early acute hepatic radiation injury. The relative quantification of hepatic ATP levels, which can reflect the pathological severity of acute hepatic radiation injury, is correlated with LDS.
- Citation: Yu RS, Hao L, Dong F, Mao JS, Sun JZ, Chen Y, Lin M, Wang ZK, Ding WH. Biochemical metabolic changes assessed by 31P magnetic resonance spectroscopy after radiation-induced hepatic injury in rabbits. World J Gastroenterol 2009; 15(22): 2723-2730
- URL: https://www.wjgnet.com/1007-9327/full/v15/i22/2723.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2723
Acute hepatic radiation injury can lead to necrosis of hepatocytes, fatty degeneration and hepatic fibrosis. The current gold standard test is liver biopsy. This procedure is invasive, uncomfortable for the patient and sometimes leads to serious complications. These factors highlight the need for a noninvasive test to characterize diffuse liver disease. Already, it has been reported that phosphorus-31 magnetic resonance spectroscopy (31P MRS) not only complements liver biopsy but also is a possible replacement and, furthermore, 31P MRS has a particular value in assessing disease progression[1].
31P MRS has been used to study liver metabolism in vivo for several years, including clinical liver disease]1-5] and experimental studies[6–8]. It enables the observation of energy metabolism through the signals of phosphomonoesters (PME), phosphodiesters (PDE), inorganic phosphate (Pi) and adenosine triphosphate (ATP). The PME and PDE signals are multi-component, with phosphorylcholine and phosphorylethanolamine the main contributors to PME as well as glycerophosphorylcholine and glycerophosphorylethanolamine which are the main contributors to PDE[1]. The final typical signal of 31P MR spectra in vivo is phosphocreatine (PCr). Although it is a dominant signal in muscles, it is not readily observable in spectra of the liver because of its small contribution to hepatic metabolic processes. Its presence indicates some contribution of signals from abdominal wall muscles as a partial volume effect.
In this study, we investigated whether changes of 31P MRS in the liver with early acute radiation injury were related to the liver damage score (LDS) and pathologic changes, we determined the value of 31P MRS in detecting early acute hepatic radiation injury, and we identified the most valuable phosphorylated metabolite for detecting acute hepatic injury. This study set out to provide a rationale for clinical application of 31P MRS in diffuse liver disease.
This study was approved by the Animal Care Committee of Zhejiang University, School of Medicine. Forty healthy adult New Zealand white rabbits weighing 2.5-3.0 kg were used. These rabbits were randomly assigned into four groups of 10 rabbits. (1) Control group: without any treatment; (2) Group 1: the hepatic region of each rabbit received a single 5 Gy dose of radiation using an 8 MeV electron beam; (3) Group 2: the hepatic region of each rabbit received a single 10 Gy dose of radiation using an 8 MeV electron beam; (4) Group 3: the hepatic region of each rabbit received a single 20 Gy dose of radiation using an 8 MeV electron beam. The irradiation was confined to the whole liver by imaging-guidance. Blood biochemical tests and 31P MRS were carried out 24 h after irradiation. Following each MRS examination, animals were sacrificed, and the liver samples were collected for pathological examination.
MRS examination was performed on a Siemens Sonata (Erlangen, Germany) whole-body MR imager operating at 1.5 Tesla equipped with a commercial dual 1H/31P surface coil. Prior to MR examination, animals were fasted overnight. A skin mark in the center of the hepatic region was used in each rabbit at first examination to reduce error. All MR examinations were performed between 8:00 am and 12:00 noon and animals were anesthetized with pentothal sodium (the depth of anesthesia kept well under control) and placed in a prone position with the liver centered on the surface coil.
The basic MR images in all orientations were obtained with true fast imaging with steady precession (true FISP) sequence for the localization of voxels. 31P MR spectra were measured using a standard 2-dimensional chemical shift imaging (CSI) technique[9] in the transverse plane with the following parameters: TR = 440 ms, TE = 2.3 ms, matrix 8 × 8, viewing interpolation 16 × 16, field of view = 200 mm, mean number of times = 120, flip angle = 90 degrees, thickness = 4 cm, voxel volume 2.5 cm × 2.5 cm × 4 cm, acquisition time = 7.36 min. Respiration gating was not used.
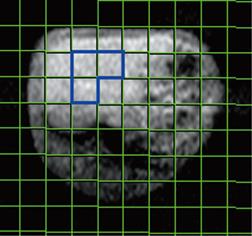
Spectra were evaluated using Siemens syngo 2004B software. Briefly, the free induction decay underwent 10 kHz exponential line broadening prior to Fourier transformation, and the resulting spectra were processed with manual phase and baseline correction. Peaks were registered relative to α-ATP resonance (-7.5 ppm), which served as an internal chemical shift reference. Finally, peak integrals were calculated by Gaussian curve fitting with all signals treated as singlets. Signal intensities of PME, Pi, PDE and β-ATP (α-ATP and γ-ATP signals were not used for the evaluation because of overlap with signals of other compounds), which are derived from the integral values of peaks on the spectra, were used for the measurement of relative quantification of metabolites. Volume of interest (VOI) for quantitative evaluation was selected in the center of the liver and the mean integral value of peaks of three voxels on the spectra was used for quantification of metabolites for a rabbit to reduce error (Figure 1).
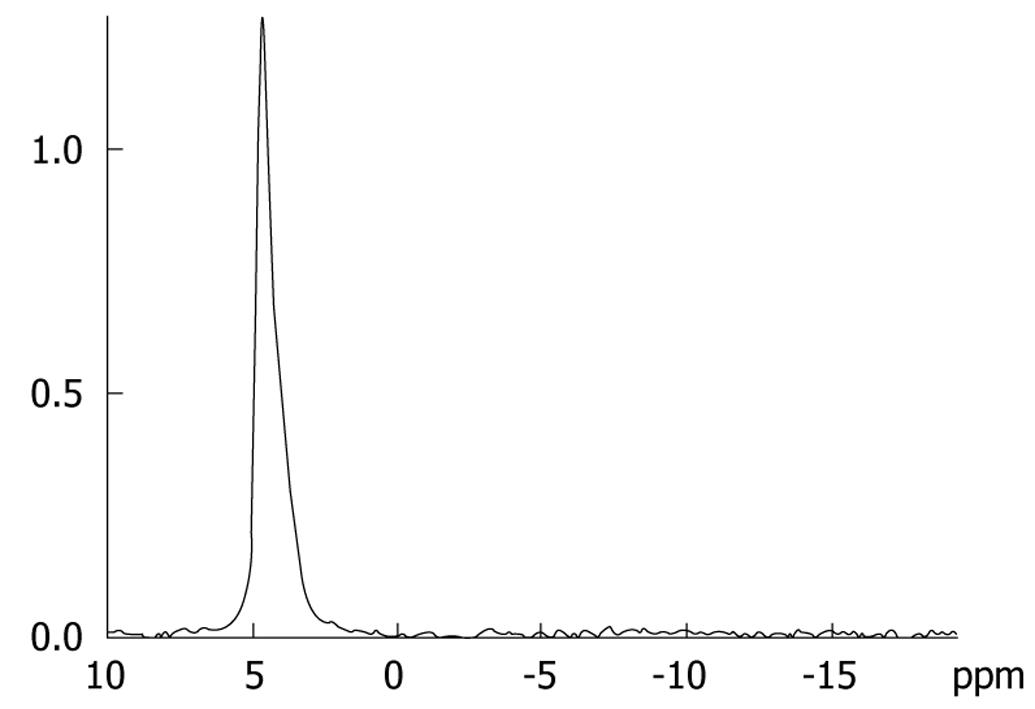
Phantom experiments were performed before the detection of relative quantification of hepatic metabolites in each rabbit to reduce error induced by the MR imager and environment factors[610]. A 500 mL phosphate (NaH2PO4) solution phantom with 0.05 mol/L concentration served as a phantom, on which identical MRS examinations were performed regularly throughout the experiment (Figure 2).
For the phantom, the relative quantification of phosphate was 16.6 ± 0.5, and the coefficient of variation was 3.03% (0.5/16.6). The ratio of relative quantification of phosphate between 2 d was conducted as the MRS correction factor (CF) of our MR imager, which was used to correct the relative quantification of hepatic metabolites in each rabbit. Therefore, all the relative quantification of phosphorus metabolites was corrected relative quantification. (corrected relative quantification = relative quantification × CF).
The corrected relative quantification might decrease the error made by the MR imager and the environmental factor in the room, and guarantee the comparability of various relative quantification of phosphorus metabolites.
This study adopted two methods to evaluate the degree of injury: LDS grading and pathological grading.
LDS grading: Sera were isolated from collected blood samples, and serum aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, r-glutamyl transpeptidase, albumin, globulin and albumin/globulin levels were measured by Olympus 2700 analyzer. Then the LDS was calculated for each rabbit according to Krastev’s standard (Table 1)[11]. The degree of injury of the liver was divided into mild (LDS ≤ 3 U), moderate (LDS 3-6 U) and severe (LDS > 6 U).
| Grade | Albumin (g/L) | γ-globulins (g/L) | AST (U/L) | Conjugated bilirubin (&mgr;mol/L) | Creatinine (&mgr;mol/L) |
| 0 | > 36.5 | < 19.9 | < 50 | < 6 | < 119 |
| 1 | 32.9-36.4 | 20-26 | 51-180 | 7-32 | 120-150 |
| 2 | 28.5-32.8 | 26.1-34.9 | 181-384 | 33-75 | 151-230 |
| 3 | 24.5-28.4 | > 35 | > 385 | > 76 | > 231 |
| 4 | 21.8-24.4 | ||||
| 5 | < 21.7 |
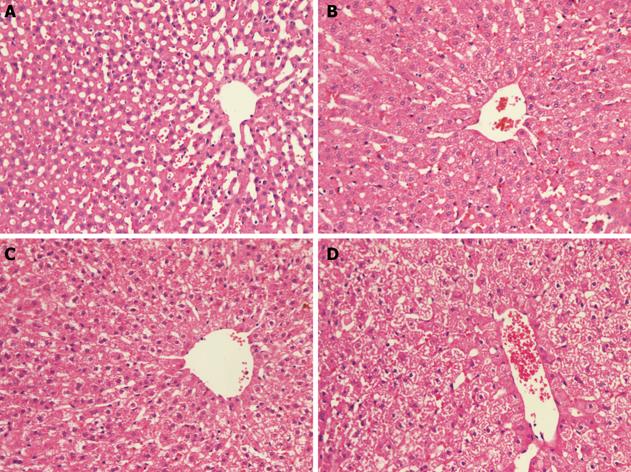
Pathological grading: The paraffin-section method and hematoxylin and eosin stain was applied to all liver samples, which were read by a single independent liver pathologist (Dr. Lin M) and assessed for swelling, degeneration, necrosis of hepatocytes, and hepatic hemorrhage. The pathologist was blinded to dose of radiation received and the results of 31P MRS. (1) Normal group (control group): normal hepatocytes, integrity of structure of hepatic lobules and regular arrangement of hepatic cord (Figure 3A). (2) Mild group: mild cellular swelling, fatty degeneration and (or) hydropic degeneration, without cell necrosis or hepatic hemorrhage (Figure 3B). (3) Moderate group: moderate cellular swelling and fatty degeneration accompanied by punctal necrosis and stray bleeding points (Figure 3C). (4) Severe group: diffuse cellular swelling and fatty degeneration, with constriction or emphraxis of hepatic sinuses, with or without local cell necrosis and (or) hepatic hemorrhage (Figure 3D).
The data were expressed as mean ± SD. Analysis of variance with SNK tests (the Student-Newman-Keuls post-hoc tests) of one-way ANOVA with SPSS 11.0 was used to examine differences between groups. Using Pearson’s correlation test, the correlation between relative quantification of ATP and LDS was examined. P < 0.05 were considered to indicate statistical significance.
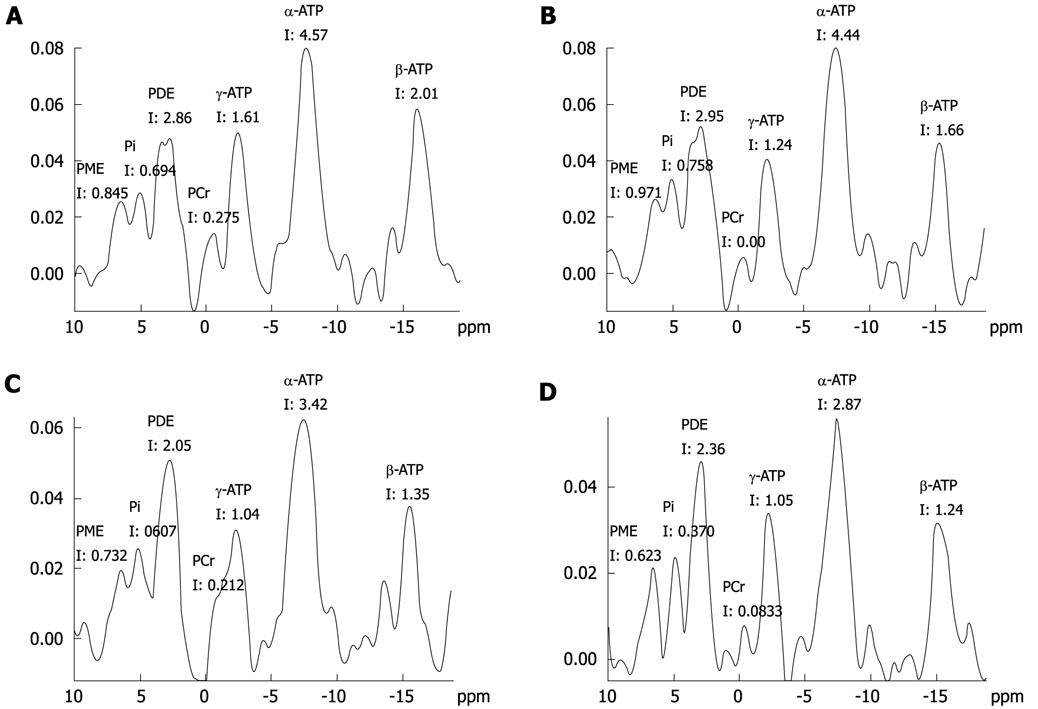
For the control group, all rabbits had normal hepatic micro-structure and a LDS of zero, while rabbits in the experimental groups varied between each other in pathology and LDS, depending on the degree of injury. The spectroscopic data of rabbits in the control group and rabbits with acute hepatic radiation injury, grouped by LDS are summarized in Table 2. The spectroscopic data of rabbits in the control group and rabbits with acute hepatic radiation injury, grouped by pathology are summarized in Table 3. The relative quantification of phosphorus metabolites detected by MRS included PME, PDE, Pi and ATP (Figure 4).
| Metabolite | Control group (n = 10) | LDS-mild group (n = 13) | LDS-moderate group (n = 9) | LDS-severe group (n = 8) | F | P |
| PME | 0.86 ± 0.23j | 0.79 ± 0.33 | 0.80 ± 0.22 | 0.58 ± 0.22a | 1.852 | 0.155 |
| PDE | 2.27 ± 0.62 | 2.14 ± 0.51 | 2.48 ± 0.63 | 1.99 ± 0.88 | 3.023 | 0.042 |
| Pi | 0.74 ± 0.18j | 0.63 ± 0.28 | 0.62 ± 0.29 | 0.43 ± 0.14a | 3.475 | 0.026 |
| ATP | 1.83 ± 0.33eil | 1.55 ± 0.24bhl | 1.27 ± 0.09cej | 0.98 ± 0.18cfg | 22.647 | 0.000 |
| PME/PDE | 0.36 ± 0.12 | 0.39 ± 0.18 | 0.35 ± 0.14 | 0.44 ± 0.54 | 0.462 | 0.710 |
| PME/ATP | 0.50 ± 0.11 | 0.50 ± 0.18 | 0.62 ± 0.15 | 0.61 ± 0.25 | 0.990 | 0.409 |
| PDE/ATP | 1.43 ± 0.31gj | 1.40 ± 0.38gj | 1.94 ± 0.50ad | 1.96 ± 0.72ad | 5.080 | 0.005 |
| Pi /ATP | 0.40 ± 0.09 | 0.41 ± 0.19 | 0.49 ± 0.24 | 0.46 ± 0.16 | 1.582 | 0.211 |
| PME/Pi | 1.47 ± 0.65 | 1.49 ± 0.92 | 1.76 ± 1.12 | 1.39 ± 0.58 | 0.081 | 0.970 |
| PDE/Pi | 3.46 ± 1.15 | 4.26 ± 2.58 | 4.91 ± 2.65 | 5.05 ± 3.18 | 0.902 | 0.450 |
| Metabolite | Control group (n = 10) | Pathological mild group (n = 12) | Pathological moderate group (n = 11) | Pathological severe group (n = 7) | F | P |
| PME | 0.86 ± 0.23j | 0.82 ± 0.35 | 0.79 ± 0.24 | 0.60 ± 0.21a | 1.861 | 0.154 |
| PDE | 2.27 ± 0.62 | 2.08 ± 0.47g | 2.67 ± 0.38dk | 1.92 ± 0.83h | 1.118 | 0.355 |
| Pi | 0.74 ± 0.18k | 0.61 ± 0.24 | 0.7 ± 0.33j | 0.43 ± 0.14bg | 2.334 | 0.090 |
| ATP | 1.83 ± 0.33dil | 1.58 ± 0.25agl | 1.32 ± 0.07cdk | 1.02 ± 0.18cfh | 22.878 | 0.000 |
| PME/PDE | 0.36 ± 0.12 | 0.40 ± 0.18 | 0.31 ± 0.13 | 0.45 ± 0.48 | 0.189 | 0.903 |
| PME/ATP | 0.50 ± 0.11h | 0.51 ± 0.19i | 0.60 ± 0.18bf | 0.59 ± 0.22 | 1.412 | 0.255 |
| PDE/ATP | 1.43 ± 0.31h | 1.34 ± 0.33ij | 2.04 ± 0.36bf | 1.83 ± 0.72d | 4.082 | 0.014 |
| Pi/ATP | 0.40 ± 0.09 | 0.39 ± 0.15 | 0.54 ± 0.26 | 0.43 ± 0.16 | 0.601 | 0.619 |
| PME/Pi | 1.47 ± 0.65 | 1.57 ± 0.97 | 1.44 ± 1.03 | 1.61 ± 0.75 | 0.316 | 0.814 |
| PDE/Pi | 3.46 ± 1.15 | 4.12 ± 2.36 | 4.87 ± 2.63 | 5.09 ± 3.24 | 0.810 | 0.497 |
The preceding two tables (Tables 2 and 3) showed the relation between biochemical index and relative quantification of phosphorus metabolites, as well as the relation between pathology and relative quantification of phosphorus metabolites. However, the results evaluated with LDS and pathology were of perfect consistency. The analysis is detailed below.
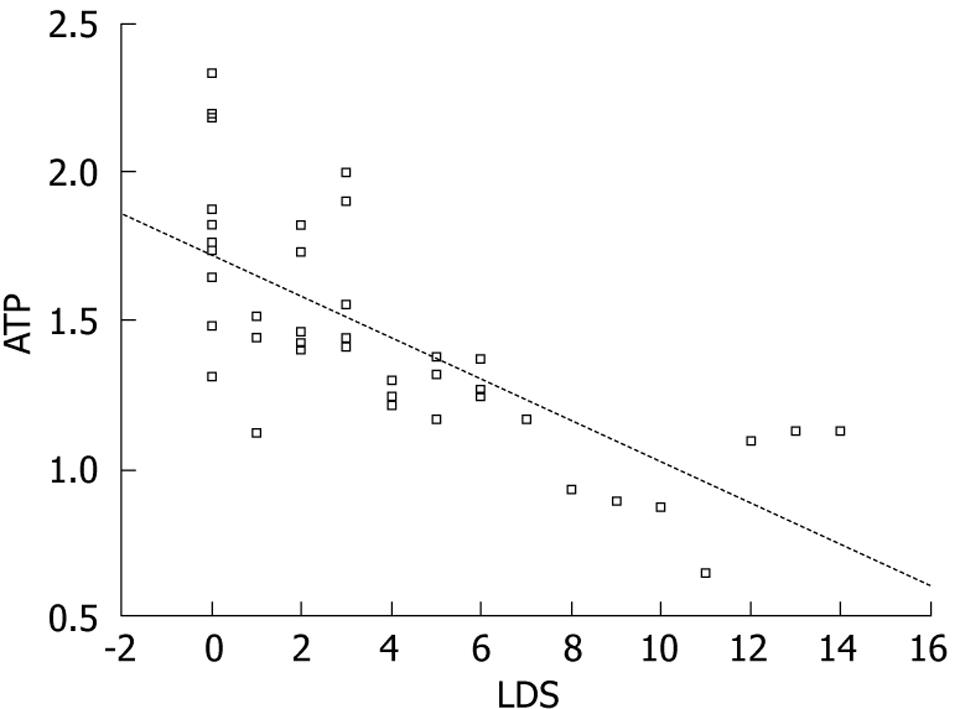
ATP: There were significant differences in ATP relative quantification among the control group, mild group, moderate group, and severe group according to both LDS grading and pathological grading (LDS groups: mild group, moderate group, severe group vs control group had P = 0.007, 0.000, 0.000, respectively; moderate group, severe group vs mild group had P = 0.008, 0.000, respectively; severe group vs moderate group had P = 0.013. Pathological groups: mild group, moderate group, severe group vs control group had P = 0.017, 0.000, 0.000, respectively; moderate group, severe group vs mild group had P = 0.000, 0.000, respectively; severe group vs moderate group had P = 0.008); it decreased progressively with the increased degree of injury, which is visually displayed by the correlation graph of ATP and LDS (Figure 5). These data illustrated that the hepatic ATP level may be the most sensitive criterion for reflecting hepatic injury in rabbits.
PME and Pi: The relative quantification of PME and Pi decreased significantly in the severe injured group, and the difference was significant compared with the control group (PME: LDS-severe group vs LDS-control group had P = 0.031, pathological-severe group vs pathological-control group had P = 0.037. Pi: LDS-severe group vs LDS-control group had P = 0.013, pathological-severe group vs pathological-control group had P = 0.005). Also, from the 31P MR spectra (Figure 4C and D), a significant decrease in the signal of phosphorylated metabolites could be seen. This indicated that if there was a significant difference in PME and Pi between normal data and test data, the tested liver was likely to be severely injured.
PDE: The relative quantification of various hepatic PDE levels changed irregularly, which indicated that the relative quantification levels of PDE may not be applied solely to assess acute hepatic radiation injury.
There were significant changes in the PDE/ATP ratio between control group and moderate group; mild group and moderate, severe group; moderate group and control group, mild group (LDS-groups: moderate group, severe group vs control group had P = 0.026, 0.025, respectively; moderate group, severe group vs control group had P = 0.014, 0.014, respectively; pathological-groups: moderate group, severe group vs control group had P = 0.076, 0.064, respectively; moderate group, severe group vs control group had P = 0.002, 0.020, respectively). There were no significant changes in the PDE/Pi ratio among all groups. Compared with the control group, no significant changes of PME/PDE, Pi/ATP and PME/Pi ratio were found in other groups.
The above results illustrated that there were few characteristic differences in the ratios of relative quantification of various hepatic phosphorus metabolites in hepatic radiation injury. Therefore, the ratios of relative quantification may not be used to evaluate acute mild hepatic radiation injury.
Acute liver diseases can result from various causes which operate through different pathophysiological pathways and which elicit distinct patterns of hepatic injury. Diagnosis of acute liver diseases including hepatic radiation injury is mainly based on invasive methods such as liver biopsy, laparoscopy, various radiological examinations and other clinical tests. On the other hand, signals from 31P MRS reflect in vivo intracellular and membrane metabolism non-invasively and they are objective parameters[212].
The evaluation of 31P MRS in the liver includes absolute or relative quantification of metabolite levels. Nowadays, absolute quantification of metabolites in mmol/L is hampered by the use of too short TR values and other technical complications[1314], but most studies with 31P MRS deal with the relative signal intensity for quantification. Also, here we measured the relative quantification of metabolites, and the relative signal ratios of the metabolites. The relative quantification evaluation had to satisfy 3 conditions for decreasing detection errors: (1) phantom experiments before relative quantification of hepatic metabolites in each rabbit to reduce errors induced by the MR imager and environment factors, etc; (2) a VOI selected in the largest section of liver and a small PCr signal characterizing the presence of abdominal muscles; (3) moderate anesthesia of rabbits and keeping the rabbits in the same position in the bed of the MR imager.
Many published studies of 31P MR spectra have shown that acute and chronic diffuse liver diseases are associated with a reduction in hepatic ATP levels[367], and some studies found that changes in hepatic ATP levels correlate with changes in liver histology[36]. Our findings, that the relative quantification of hepatic ATP levels displayed progressive reductions with increased hepatic injury, correspond to the results of a previous study[6], but we are the first to report that the changes in hepatic ATP levels correlate with the severity of acute hepatic radiation injury. Some reports have shown that, during the early phase of chronic diffuse liver diseases, only minor changes in hepatic ATP could be detected[1516]. Our study showed that the relative quantification of hepatic ATP levels obviously decreased, because there were significant differences between the control group and both the pathological-mild group and LDS-mild group. We also found that the changes in levels of other hepatic metabolites were less sensitive than the changes in ATP levels in mild hepatic injury, and the relative quantification of hepatic ATP levels could be well correlated with LDS. Thus, we suggest that the hepatic ATP level may be the most reliable criterion for evaluating acute hepatic injury in rabbits. The reason is that the β-ATP peak is unique, and quite different from other phosphorylated metabolite peaks, which overlap with signals of other metabolites in the 31P MRS map.
The mechanisms responsible for the decline in hepatic ATP levels include: gradual loss of viable hepatocytes, which is likely to be an important contributing factor-as the total amount of these cells per unit volume of liver decreases, MRS detectable signal from that volume will also decrease[6]; anoxemia of local liver tissue induced by injury of capillary vessels after hepatic radiation[17]; increased energy expenditure as liver disease progresses[18]. In addition, disturbed hepatic bioenergetics has also been ascribed to the capillarization of hepatic sinusoids during the development of cirrhosis[19].
PME, PDE and the correlation ratio: information about phospholipid membrane metabolism may also be obtained from the PME and PDE resonances in the 31P MR spectrum. Both resonances are multicomponent peaks containing contributions from several metabolites[20]. The significance of the changes in PME and PDE levels is not clear. Some previous studies have reported that an increase in PME levels is accompanied by a decrease in PDE levels or increased ratios of hepatic PME/ATP and PME/PDE in acute and chronic diffuse liver diseases[122122]. However, in other investigators, and our study, PME levels did not increase nor did PDE levels decrease in the same acute and chronic diffuse liver diseases[68]. The main reason for these conflicting findings may be the broad, overlapping characteristics of these peaks along with the multiple signals contributing to these resonances hindering accurate quantification of the PME and PDE peaks[8]. Another explanation is that hepatic phospholipid membrane activity may differ in animal models of liver diseases versus liver diseases in humans[6]. Therefore, either the changes in PME and PDE levels or the ratio PME/PDE cannot accurately reflect the liver diseases.
Pi and the correlation ratio: Pi is another marker of tissue bioenergetics. Increases in Pi have been observed by 31P MRS during high energy activities such as liver regeneration following partial hepatectomy[823]. The increase in hepatic Pi may result from the hydrolysis of high energy phosphate bonds which in turn liberates Pi species, increases hepatic uptake and accumulation of Pi due to enhanced metabolic activity and reduces recycling back to purine/pyrimidine moieties[8]. Some previous studies have reported a decrease or no difference in Pi levels in chronic diffuse liver diseases in humans and animals[16]. The reason for no changes in hepatic Pi is not clear, but the decrease in hepatic Pi may result from reduced hepatocyte mass[6]. In this study, significant decreases in Pi were only detected among the most seriously injured livers of the severe group. The lower levels of hepatic Pi likely result from reduced hepatocyte mass, as Corbin et al[6] reported. In addition, compared with the ATP peak, the Pi peak, which is located between the PME and PDE peaks, is more prone to being impacted by the overlapping characteristics of PME and PDE peaks, hindering accurate quantification of the Pi peak. However, some authors regarded the ratio ATP/Pi as the criterion of hepatic regenerative activity following partial hepatectomy, because the ATP levels decreased, the Pi levels increased, and the changes of the ratio were more sensitive than ATP or Pi alone[8]. However, the levels of ATP or Pi were decreased to different degrees after acute hepatic radiation injury in our study, so the ratio of ATP/Pi could not be used to evaluate acute hepatic radiation injury.
This study illustrated that 31P MRS of clinical 1.5T MRS could detect various changes of phosphorylated metabolite levels in early acute hepatic injury. In addition, the study also showed that though there were many hepatic phosphorylated metabolites and correlated ratios, the measurement of levels of hepatic ATP may be the most reliable criterion for reflecting both pathological hepatic injury and LDS in rabbits.
Acute hepatic radiation injury can lead to necrosis of hepatocytes, fatty degeneration and hepatic fibrosis. The current gold standard test is liver biopsy. This procedure is invasive, uncomfortable for the patient and sometimes has serious complications. These factors highlight the need for a noninvasive test to characterize diffuse liver disease. Already, it has been reported that phosphorus-31 magnetic resonance spectroscopy (31P MRS) not only complements liver biopsy but also is a possible replacement, and furthermore, 31P MRS has particular value in assessing disease progression.
31P MRS has been used to study liver metabolism in vivo for several years, including clinical liver disease studies and experimental studies. The research focus is how to observe the energy metabolism or pathological changes through the signals of phosphorus metabolites.
In this study the authors carefully used two different methods [liver damage score (LDS), and pathology] to evaluate the degree of injury, and then they studied the correlation between MRS and the degree of injury. Furthermore, they report that the changes in hepatic adenosine triphosphate (ATP) levels correlate with the severity of acute hepatic radiation injury measured by LDS.
This study may be particularly useful for allowing clinical detection of early acute hepatic injury with 31P MRS in the future.
MRS: Spectroscopic method for measuring the magnetic moment of elementary particles such as atomic nuclei, protons or electrons. It is employed in clinical applications such as NMR Tomography (magnetic resonance imaging).
31P MRS is a very interesting method especially to replace the gold standard biopsy, particularly in assessing disease progression.
| 1. | Lim AK, Patel N, Hamilton G, Hajnal JV, Goldin RD, Taylor-Robinson SD. The relationship of in vivo 31P MR spectroscopy to histology in chronic hepatitis C. Hepatology. 2003;37:788-794. |
| 2. | Munakata T, Griffiths RD, Martin PA, Jenkins SA, Shields R, Edwards RH. An in vivo 31P MRS study of patients with liver cirrhosis: progress towards a non-invasive assessment of disease severity. NMR Biomed. 1993;6:168-172. |
| 3. | Corbin IR, Ryner LN, Singh H, Minuk GY. Quantitative hepatic phosphorus-31 magnetic resonance spectroscopy in compensated and decompensated cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G379-G384. |
| 4. | Taylor-Robinson SD. Applications of magnetic resonance spectroscopy to chronic liver disease. Clin Med. 2001;1:54-60. |
| 5. | Mann DV, Lam WW, Hjelm NM, So NM, Yeung DK, Metreweli C, Lau WY. Human liver regeneration: hepatic energy economy is less efficient when the organ is diseased. Hepatology. 2001;34:557-565. |
| 6. | Corbin IR, Buist R, Peeling J, Zhang M, Uhanova J, Minuk GY. Hepatic 31P MRS in rat models of chronic liver disease: assessing the extent and progression of disease. Gut. 2003;52:1046-1053. |
| 7. | Corbin IR, Buist R, Peeling J, Zhang M, Uhanova J, Minuk GK. Utility of hepatic phosphorus-31 magnetic resonance spectroscopy in a rat model of acute liver failure. J Investig Med. 2003;51 (1):42-49. |
| 8. | Corbin IR, Buist R, Volotovskyy V, Peeling J, Zhang M, Minuk GY. Regenerative activity and liver function following partial hepatectomy in the rat using (31)P-MR spectroscopy. Hepatology. 2002;36:345-353. |
| 9. | Dezortova M, Taimr P, Skoch A, Spicak J, Hajek M. Etiology and functional status of liver cirrhosis by 31P MR spectroscopy. World J Gastroenterol. 2005;11:6926-6931. |
| 10. | Meyerhoff DJ, Karczmar GS, Matson GB, Boska MD, Weiner MW. Non-invasive quantitation of human liver metabolites using image-guided 31P magnetic resonance spectroscopy. NMR Biomed. 1990;3:17-22. |
| 11. | Krastev Z. Liver damage score--a new index for evaluation of the severity of chronic liver diseases. Hepatogastroenterology. 1998;45:160-169. |
| 12. | Taylor-Robinson SD, Sargentoni J, Bell JD, Saeed N, Changani KK, Davidson BR, Rolles K, Burroughs AK, Hodgson HJ, Foster CS. In vivo and in vitro hepatic 31P magnetic resonance spectroscopy and electron microscopy of the cirrhotic liver. Liver. 1997;17:198-209. |
| 13. | Murphy-Boesch J, Jiang H, Stoyanova R, Brown TR. Quantification of phosphorus metabolites from chemical shift imaging spectra with corrections for point spread effects and B1 inhomogeneity. Magn Reson Med. 1998;39:429-438. |
| 14. | Sijens PE, Dagnelie PC, Halfwerk S, van Dijk P, Wicklow K, Oudkerk M. Understanding the discrepancies between 31P MR spectroscopy assessed liver metabolite concentrations from different institutions. Magn Reson Imaging. 1998;16:205-211. |
| 15. | Kaita KD, Pettigrew N, Minuk GY. Hepatic regeneration in humans with various liver disease as assessed by Ki-67 staining of formalin-fixed paraffin-embedded liver tissue. Liver. 1997;17:13-16. |
| 16. | Kawakita N, Seki S, Yanai A, Sakaguchi H, Kuroki T, Mizoguchi Y, Kobayashi K, Monna T. Immunocytochemical identification of proliferative hepatocytes using monoclonal antibody to proliferating cell nuclear antigen (PCNA/cyclin). Comparison with immunocytochemical staining for DNA polymerase-alpha. Am J Clin Pathol. 1992;97:S14-S20. |
| 17. | Yin J, Gao Z, He Q, Zhou D, Guo Z, Ye J. Role of hypoxia in obesity-induced disorders of glucose and lipid metabolism in adipose tissue. Am J Physiol Endocrinol Metab. 2009;296:E333-E342. |
| 18. | Schneeweiss B, Graninger W, Ferenci P, Eichinger S, Grimm G, Schneider B, Laggner AN, Lenz K, Kleinberger G. Energy metabolism in patients with acute and chronic liver disease. Hepatology. 1990;11:387-393. |
| 19. | Harvey PJ, Gready JE, Hickey HM, Le Couteur DG, McLean AJ. 31P and 1H NMR spectroscopic studies of liver extracts of carbon tetrachloride-treated rats. NMR Biomed. 1999;12:395-401. |
| 20. | Morikawa S, Inubushi T, Kitoh K, Kido C, Nozaki M. Chemical assessment of phospholipid and phosphoenergetic metabolites in regenerating rat liver measured by in vivo and in vitro 31P-NMR. Biochim Biophys Acta. 1992;1117:251-257. |
| 21. | Schlemmer HP, Sawatzki T, Sammet S, Dornacher I, Bachert P, van Kaick G, Waldherr R, Seitz HK. Hepatic phospholipids in alcoholic liver disease assessed by proton-decoupled 31P magnetic resonance spectroscopy. J Hepatol. 2005;42:752-759. |
| 22. | Menon DK, Sargentoni J, Taylor-Robinson SD, Bell JD, Cox IJ, Bryant DJ, Coutts GA, Rolles K, Burroughs AK, Morgan MY. Effect of functional grade and etiology on in vivo hepatic phosphorus-31 magnetic resonance spectroscopy in cirrhosis: biochemical basis of spectral appearances. Hepatology. 1995;21:417-427. |
| 23. | Campbell KA, Wu YP, Chacko VP, Sitzmann JV. In vivo 31P NMR spectroscopic changes during liver regeneration. J Surg Res. 1990;49:244-247. |