Published online May 28, 2009. doi: 10.3748/wjg.15.2512
Revised: April 23, 2009
Accepted: April 30, 2009
Published online: May 28, 2009
AIM: To measure circulating angiotensins at different stages of human cirrhosis and to further evaluate a possible relationship between renin angiotensin system (RAS) components and hemodynamic changes.
METHODS: Patients were allocated into 4 groups: mild-to-moderate liver disease (MLD), advanced liver disease (ALD), patients undergoing liver transplantation, and healthy controls. Blood was collected to determine plasma renin activity (PRA), angiotensin (Ang) I, Ang II, and Ang-(1-7) levels using radioimmunoassays. During liver transplantation, hemodynamic parameters were determined and blood was simultaneously obtained from the portal vein and radial artery in order to measure RAS components.
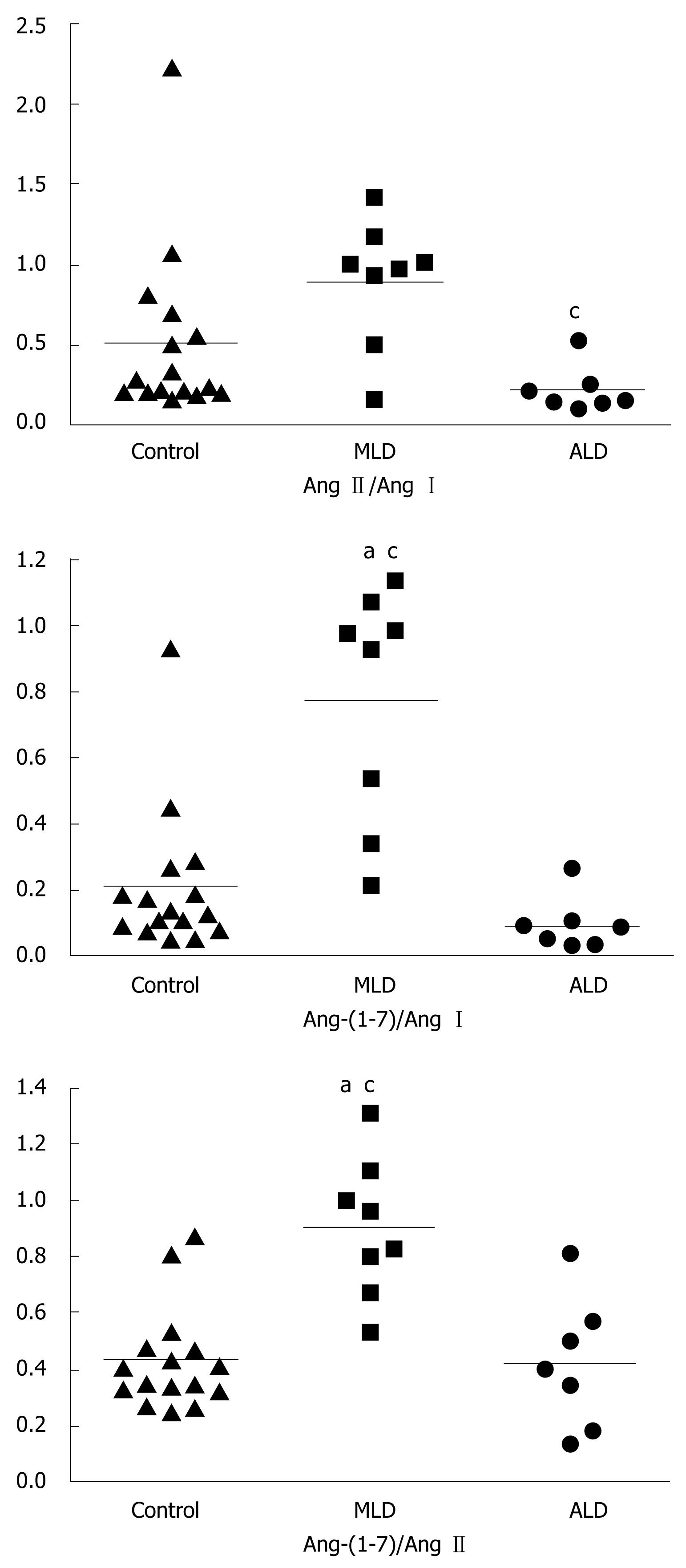
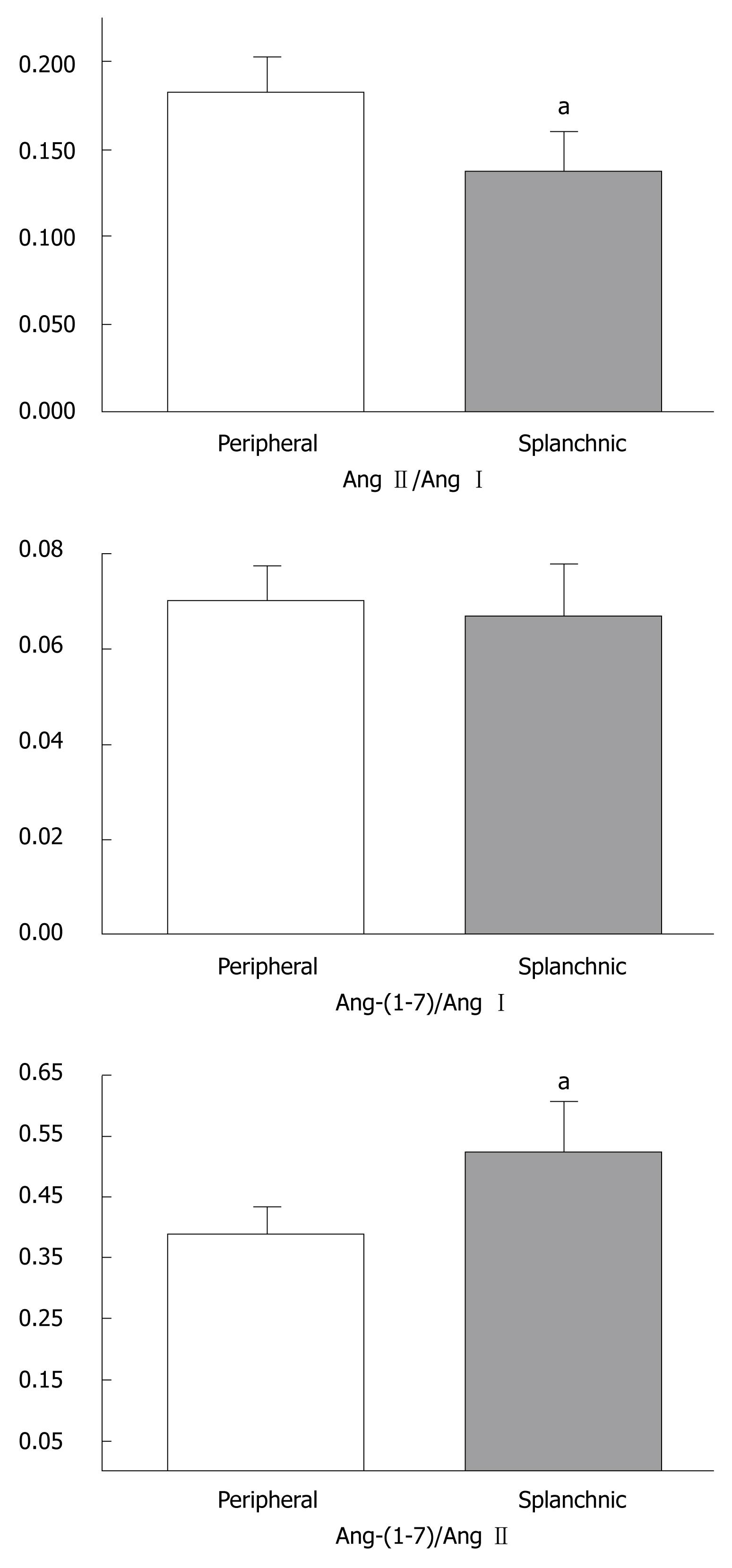
RESULTS: PRA and angiotensins were elevated in ALD when compared to MLD and controls (P < 0.05). In contrast, Ang II was significantly reduced in MLD. Ang-(1-7)/Ang II ratios were increased in MLD when compared to controls and ALD. During transplantation, Ang II levels were lower and Ang-(1-7)/Ang II ratios were higher in the splanchnic circulation than in the peripheral circulation (0.52 ± 0.08 vs 0.38 ± 0.04, P < 0.02), whereas the peripheral circulating Ang II/Ang I ratio was elevated in comparison to splanchnic levels (0.18 ± 0.02 vs 0.13 ± 0.02, P < 0.04). Ang-(1-7)/Ang II ratios positively correlated with cardiac output (r = 0.66) and negatively correlated with systemic vascular resistance (r = -0.70).
CONCLUSION: Our findings suggest that the relationship between Ang-(1-7) and Ang II may play a role in the hemodynamic changes of human cirrhosis.
- Citation: Vilas-Boas WW, Ribeiro-Oliveira Jr A, Pereira RM, Ribeiro RDC, Almeida J, Nadu AP, Simões e Silva AC, Santos RASD. Relationship between angiotensin-(1-7) and angiotensin II correlates with hemodynamic changes in human liver cirrhosis. World J Gastroenterol 2009; 15(20): 2512-2519
- URL: https://www.wjgnet.com/1007-9327/full/v15/i20/2512.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2512
The renin angiotensin system (RAS) is now viewed as a dual system composed of 2 arms: vasoconstriction encompassing angiotensin converting enzyme (ACE)-angiotensin (Ang) II-Ang II type 1 (AT1) receptor and vasodilation encompassing ACE2-Ang-(1-7)-Mas receptor. The ACE2-Ang-(1-7)-Mas receptor arm mainly acts as a counter-regulatory mechanism to the vasoconstrictor arm[1]. According to this novel concept, the final functional effect of the RAS may reflect a balance between these 2 arms[2–4]. Apart from the circulating RAS, the existence of local systems has been described in a number of organs, including the liver[5]. These local systems act in response to various physiological and pathophysiological stimuli, and the locally generated angiotensins have been implicated in the modulation of cell growth and proliferation, generation of reactive oxygen species, hormone secretion, and in the control of local inflammation and fibrosis[6]. The circulating RAS is well recognized for its role in hemodynamic regulation through Ang II, a potent vasoconstrictor, and the counter-regulatory peptide, Ang-(1-7), a vasodilator. The local and circulating RAS can interact with each other and with other regulatory systems[7].
Liver cirrhosis has 2 major circulatory dysfunctions: portal hypertension and a hyperdynamic circulation characterized by elevated cardiac output and low systemic vascular resistance[8]. It is well established that Ang II plays a role in the pathogenesis of portal hypertension by increasing intrahepatic vascular resistance and also by contributing to liver fibrosis[5]. The development of portal hypertension in cirrhosis is associated with arterial vasodilation in the splanchnic circulation, leading to a decrease in systemic vascular resistance[9]. Early in the course of the disease, the decrease in systemic vascular resistance is compensated by the development of a hyperdynamic circulation[10]. Despite a reduction in systemic vascular resistance, the effective arterial blood volume remains normal, as does the circulating RAS components and antidiuretic hormone[8]. However, as the disease progresses and arterial vasodilation increases, the hyperdynamic circulation is insufficient to correct the effective arterial hypovolemia[8]. Arterial hypotension develops, leading to activation of the circulating RAS, sympathetic nervous system and secretion of antidiuretic hormone[8]. The splanchnic circulation is resistant to the effect of Ang II, noradrenaline and vasopressin, and the maintenance of arterial pressure is a result of vasoconstriction in extra-splanchnic vascular areas[8]. Liver cirrhosis has been studied recently in light of the new view of the RAS. It is becoming clear that the RAS can influence liver cirrhosis through its 2 main arms. While the ACE-Ang II-AT1 receptor arm contributes to liver tissue injury and fibrosis[6] and the maintenance of basal vascular tonus in non-compensated cirrhosis[11], the activation of the ACE2-Ang-(1-7)-Mas arm exerts anti-fibrotic actions[61213]. In addition, it has been speculated that this counter-regulatory arm also has a role in the arterial vasodilation in liver cirrhosis[14]. In this regard, we have recently shown that chronic treatment with propranolol in cirrhotic patients was characterized by marked changes in the precursors of the RAS cascade (renin and Ang I), with repercussions on the 2 main RAS components, Ang II and Ang-(1-7), in the splanchnic and peripheral circulations[15]. Our previous data suggested that a possible therapeutic approach for advanced human cirrhosis could be the combination of a beta-blocker with an AT1 receptor blocker or ACE inhibitor[15].
Therefore, the aim of the present study was to evaluate circulating levels of angiotensins in mild-to-moderate and advanced stages of human cirrhosis without the interference of any kind of RAS blockade such as beta-blockers, ACE inhibitors and AT1 receptor blockers. In addition, we also evaluated a correlation between RAS peptides and hemodynamic parameters in systemic and splanchnic circulations of cirrhotic patients during liver transplantation.
This cross-sectional study used a convenience sample recruited from the Alfa Institute of Hepatology/Liver Transplantation and the Clinical Primary Care Center of our institution.
Inclusion criteria: Patients diagnosed with hepatic cirrhosis (n = 24) defined through liver histopathology and/or ultrasonography findings were included in this study. Table 1 displays the Child-Pugh scores[16] of our patients. The primary etiology of the liver disease was established in 21 subjects (87.5%), and included alcoholism, hepatitis C virus, hepatitis B virus and biliary cirrhosis. All cirrhotic patients showed portal hypertension at the time criteria were set for each protocol group. Cirrhotic patients were then allocated to one of 3 study groups based on the presence or absence of ascites and values of Child-Pugh score[16]: the first group was composed of patients who had mild-to-moderate liver disease (MLD, n = 8), the second group included patients with advanced liver disease (ALD, n = 7), which were seen in an outpatient clinic and the third group was composed of liver transplant recipients during surgery (LT, n = 9).
| Characteristics and measurements | Mild to moderate liver disease (MLD) | Advanced liver disease (ALD) | ALD during Liver transplantation (LT) |
| n= 8 | n= 7 | n= 9 | |
| Age (yr) | 55.5 ± 1.8 | 54 ± 5 | 50 ± 3 |
| Sex, male/female | 3 (37.5%)/5 (62.5%) | 4 (57%)/3 (43%) | 7 (78%)/2 (22%) |
| Child Pugh Score | 6.7 ± 0.2 | 11 ± 0.8a | 11 ± 1.8a |
| Albumin, g/dL | 2.9 ± 0.15 | 2.4 ± 0.3 | 2.6 ± 0.2 |
| Bilirubin, mg/dL | 2.4 (1.7-5.9) | 2.5 (1.2-7.1) | 3.3 (2.0-5.5) |
| Creatinine, mg/dL | 0.70 (0.70-0.80) | 1.0 (1.0-1.45)a | 1.0 (0.75-1.50) |
| INR (International normalization ratio) | 1.2 ± 0.07 | 1.7 ± 0.3 | 1.8 ± 0.2 |
| Prothrombin activity | 68.3% ± 7.5% | 50% ± 12% | 42% ± 7%a |
| Serum Na+, mEq/L | 139 ± 2 | 126 ± 3a | 130 ± 2a |
The MLD patients did not receive any medication and did not exhibit ascites at the time of blood collection. However, they had other endoscopic or ultrasonographic signs of portal hypertension (small varices < 5 mm, collateral vessels, abnormalities in portal flow direction).
The ALD group comprised outpatients with ascites and extra-hepatic complications such as encephalopathy and moderate to large esophageal varices (> 5 mm) with risk of bleeding. These patients were using diuretics (furosemide 40-80 mg/d associated with spironolactone 25-100 mg/d).
The LT group included hospitalized cirrhotic patients with the same severity of liver disease as the ALD group based on Child Pugh and MELD scores (Child Pugh: 11.0 ± 0.8 in LD vs 11.2 ± 1.2 in LT and MELD: 29.3 ± 2.1 in LD vs 29.8 ± 3.2 in LT, P > 0.05 for both comparisons). These patients also presented the same clinical and laboratory features as the ALD group and received the same diuretic treatment. The only difference between both groups was that LT patients had been submitted for liver transplantation.
The control group (n = 16) consisted of healthy age-matched subjects from our Clinical Primary Care Center. Health status was determined through the subjects’ medical history to rule out the presence of chronic or acute diseases. All subjects were subjected to a complete physical examination prior to blood sampling as part of our study protocol.
Exclusion criteria: Co-morbidities such as diabetes, heart, pulmonary, autoimmune and neurological diseases automatically excluded subjects from the study. Patients receiving chronic treatment with ACE inhibitors, angiotensin receptor blockers, renin inhibitors, beta-blockers and corticosteroids were also in our exclusion criteria. During liver transplantation, blood collection was performed whenever the subject showed acute hemodynamic derangement demanding vasoconstrictor use.
Ethical aspects: The Ethics Committee of the Federal University of Minas Gerais approved the study. Informed consent was obtained from all included subjects. The research protocol did not interfere with any medical recommendations or prescriptions. Subject follow-up was guaranteed even in cases of refusal to participate in the study.
The study was performed in outpatients at different clinical stages of cirrhosis (MLD and ALD groups) and in hospitalized patients during liver transplantation (LT group). Circulating RAS components levels were measured in all groups; only in LT patients were measurements of RAS components also obtained from the splanchnic circulation simultaneously with the hemodynamic parameters.
Protocol 1-Evaluation of circulating RAS in controls and patients with mild-to-moderate and advanced cirrhosis: Blood samples for measurement of plasma renin activity (PRA) and angiotensins were obtained from healthy subjects, MLD and ALD patients on a single occasion taking into account the inclusion and exclusion criteria for each group. For ethical reasons, no changes to the clinical approach were made for study purposes. Blood samples (10 mL) were collected through peripheral venipuncture in the morning after a fasting period of 8 h. All subjects rested in the supine position for at least 30 minutes before blood sampling.
Protocol 2-Evaluation of peripheral and splanchnic RAS components during the pre-anhepatic stage of liver transplantation: Anesthesia for liver transplantation was induced by a rapid sequence of etomidate, fentanyl and succinylcholine and maintained by isoflurane (CAM about 1.0) and atracurium until blood sampling. In the LT group, blood sampling was performed during the pre-anhepatic stage of liver transplantation and samples were obtained simultaneously from the radial artery (10 mL) and portal vein (10 mL) to evaluate RAS components before and after the enteric circulation, respectively. Furthermore, the portal vein is part of the splanchnic circulation, which is the original source of hyperdynamic circulation.
Protocol 3-Evaluation of hemodynamic parameters during the pre-anhepatic stage of liver transplantation: Hemodynamic parameters (cardiac output, cardiac index, systemic vascular resistance and systemic vascular resistance index) were determined simultaneously with blood sampling to measure the RAS components. These measurements were obtained through invasive continuous monitoring via a Swan-Ganz catheter (CCOMBO/SvO2, 110 cm/7.5F, Edwards Lifesciences, Irvine, CA, USA), using Dixtal (DX 2020, Dixtal Biomedical, São Paulo, Brazil) and Vigilance (CEDV, Edwards Lifesciences, Irvine, CA, USA) monitors.
For all blood collection, samples were drawn into 2 sets of ice-cooled tubes-one containing 7.5% EDTA for PRA determination and the other containing a cocktail of protease inhibitors for angiotensin measurements[17]. Blood samples were centrifuged at 2000 g for 20 min at 4°C and plasma was stored at -20°C[17]. Plasma samples were extracted using Bond-Elut cartridges (Analytichem International, Harbor City, CA), as described elsewhere[17].
PRA as well as Ang I, Ang II and Ang-(1-7) concentrations were determined through radioimmunoassays, as detailed elsewhere[17]. The recovery of 125I-labeled Ang I, Ang II, and Ang-(1-7) was 79.2% ± 2.3%, 86.9% ± 0.8% and 83.5% ± 0.9%, respectively. Results were expressed as nanograms of Ang I generated per milliliter of plasma per hour (ng Ang I/mL per hour) for PRA and pg/mL of plasma for Ang measurements).
The software Graphpad PRISM, version 4.03, was used for the statistical analyses. The Gaussian distribution of the variables was evaluated by the Shapiro normality test. Results were reported as mean ± SE or median, when appropriate. Analysis of variance followed by the Bonferroni test was used for the comparison of means between groups. Mann-Whitney or Kruskal-Wallis followed by the Dunn test was used to compare non-parametric data. The paired Student t-test was used to compare means from variables of the LT group before and after the enteric circulation. The level of significance was set at P < 0.05.
The primary etiology of liver disease of the compensated cirrhotic patients (CLD) included: hepatitis C virus in 3, alcoholism in 4 and hepatitis B virus in 1 patient. Laboratory data, Child-Pugh and MELD scores confirmed the mild to moderate stage of liver disease (Table 1).
The causes of liver disease in the ALD group were: alcoholism in 2, bile cirrhosis in 1, hepatitis C virus in 1 and idiopathic disease in 3 patients. Laboratorial data, Child-Pugh and MELD scores revealed the advanced stage of liver disease (Table 1).
The etiologies of hepatic disease in the LT group included hepatitis C virus in 3, alcoholism in 3, bile cirrhosis in 2 and hepatitis B virus in 1 patient. Laboratory findings, Child-Pugh and MELD scores were very similar to those of the ALD group and also showed an advanced stage of liver disease (Table 1).
Healthy controls comprised 16 subjects, including 7 males and 9 females from 40 to 65 (49.7 ± 2.6) years.
As displayed in Table 2, PRA was significantly higher in the ALD group in comparison to MLD patients and healthy controls (P < 0.05). The same profile was observed for Ang I, which also presented a significant increase in plasma levels in ALD patients when compared to controls and the MLD group (Table 2). On the other hand, statistical differences were detected when comparing Ang I measurements obtained from MLD patients and healthy controls (Table 2).
| RAS components | Healthy controls | MLD | ALD |
| PRA (ng Ang I/ mL per hour) | 0.10 (0.03-0.23) | 0.10 (0.01-0.22) | 2.7 (0.43-6.61)ac |
| Ang I (pg/mL) | 179.8 (86.9-220.8) | 28.9 (23-65.2)a | 412.3 (326.5-1123)ac |
| Ang II (pg/mL) | 47.0 (41.8-61.7) | 27.5 (23.9-35.9)a | 84.4 (60.9-154.6)ac |
| Ang-(1-7) (pg/mL) | 20.1 (17.1-25.5) | 24.9 (21.1-27.4) | 32.6 (27.8-61.6)a |
ALD patients also exhibited a significant elevation in Ang II and Ang-(1-7) when compared to healthy controls (P < 0.05, Table 2). In contrast, Ang II levels were significantly lower in patients with mild-to-moderate cirrhosis even when compared with healthy controls, whereas plasma Ang-(1-7) in this group did not differ from that of healthy subjects or of the ALD group (Table 2).
Ratios of Ang-(1-7) and Ang I levels, of Ang II and Ang I, and of Ang-(1-7) and Ang II in all groups are displayed in Figure 1 as medians. The Ang-(1-7)/Ang I ratio was significantly higher in MLD patients [0.95 (0.43-1.02)] than in the ALD and control groups. On the other hand, healthy controls and ALD patients exhibited similar Ang-(1-7)/Ang I ratios [controls: 0.13 (0.08-0.22) vs NLD: 0.08 (0.02-0.10), Figure 1]. The Ang II/Ang I ratio was significantly reduced in the ALD group [0.15 (0.13-0.25)] when compared to MLD patients [0.98 (0.71-1.09)], but not in comparison to healthy controls [0.25 (0.20-0.63), Figure 1]. More importantly, the Ang-(1-7)/ Ang II ratio, which could represent the final functional relationship between RAS components, was significantly increased in MLD patients [0.89 (0.73-1.04)] in comparison to ALD patients [0.40 (0.17-0.57)] and controls [0.38 (0.32-0.47)], whose median values were similar (Figure 1).
As displayed in Table 3, the comparisons of PRA, Ang I and Ang-(1-7) levels revealed no difference between the peripheral and splanchnic circulations. However, Ang II levels were significantly reduced in the splanchnic circulation when compared to the peripheral circulation (P < 0.05, Table 3). The ratios between angiotensins were also different in the peripheral and splanchnic circulation (Figure 2). The Ang-(1-7)/Ang II ratio was higher in the splanchnic circulation than in the peripheral circulation (0.52 ± 0.08 vs 0.38 ± 0.04, P < 0.05, Figure 2), whereas the peripheral circulating Ang II/Ang I ratio was elevated in comparison to splanchnic levels (0.18 ± 0.02 vs 0.13 ± 0.02, P < 0.05, Figure 2). No differences were detected in the Ang-(1-7)/Ang I ratio between both sites (Figure 2).
| RAS components | Peripheral measurements | Splanchnic measurements |
| PRA (ng Ang I/mL per hour) | 2.4 ± 0.6 | 2.5 ± 0.4 |
| Ang I (pg/mL) | 764 ± 115 | 765 ± 103 |
| Ang II (pg/mL) | 138 ± 23 | 97 ± 13a |
| Ang-(1-7) (pg/mL) | 48 ± 5 | 48 ± 7 |
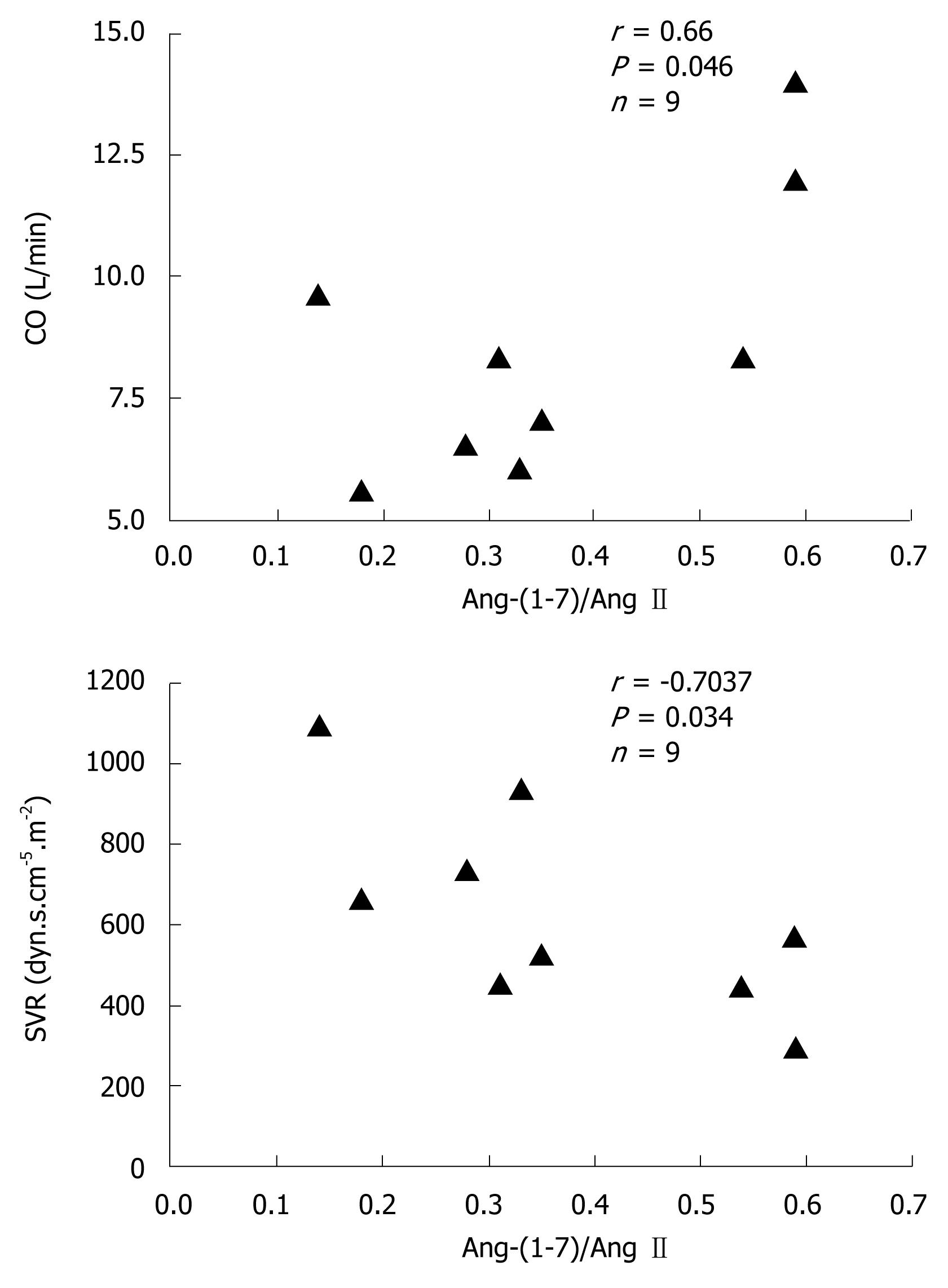
In general, hemodynamic parameters from patients with cirrhosis (LT group) were different from the reference values. Systemic vascular resistance (555 ± 57 dyn.s.cm-5vs 1200-1500 dyn.s.cm-5) and respective index (1029 ± 95 dyn.s.cm-5.m-2vs 2400-2900 dyn.s.cm-5.m-2) were below the reference range, whereas cardiac output (9.5 ± 1.1 L/min vs 3.0-7.0 L/min) and its index (5.0 ± 0.5 L.min-1.m-2vs 2.5-4.0 L.min-1.m-2) were above the reference range. There was no significant correlation between Ang-(1-7) or Ang II concentrations and hemodynamic parameters. However, the Ang-(1-7)/Ang II ratio was positively correlated with cardiac output (r = 0.66, P < 0.05) and negatively correlated with systemic vascular resistance (r = -0.70, P < 0.05) (Figure 3).
The present study supports the concept that RAS may contribute to circulatory dysfunction in human cirrhosis. In general, our data showed that the progression of liver dysfunction is characterized by marked changes in circulating Ang-(1-7) and Ang II levels. In the initial stages, the circulating RAS is not activated, although there is a predominance of Ang-(1-7), the vasodilator, rather than Ang II. On the other hand, the advanced stages of cirrhosis show an activation of peripheral and splanchnic RAS, and a metabolic deviation toward the RAS vasodilator axis in the splanchnic circulation. Furthermore, we observed a positive correlation between the Ang-(1-7)/Ang II ratio and cardiac output as well as a negative correlation between this ratio and systemic vascular resistance, indicating that the final functional effects of the RAS may reflect a balance between these 2 opposing axes. Taken together, these findings suggest a dynamic change in RAS profile according to disease stage (mild-to-moderate vs advanced) and vascular bed (peripheral vs splanchnic circulation), which could interfere with hemodynamic parameters in human cirrhosis.
The first part of this study evaluated circulating RAS components according to the stage of liver disease and without the interference of any kind of RAS blockade. The RAS profile was completely different in mild-to-moderate cirrhosis when compared to advanced liver disease. The upstream RAS components (PRA and Ang I), which are common to both RAS axes and indicate the level of system activation, were increased in ALD patients compared to MLD and healthy controls. Ang I and Ang II levels were also reduced in the MDL group even in comparison to healthy controls, whereas the ALD group exhibited an overall elevation of PRA and angiotensins when compared to other groups. Other studies have also detected increased PRA and Ang II levels in ALD patients[818]. In the initial stages of human cirrhosis, PRA measurements revealed a non-activated or even suppressed circulating RAS[1618]. In this regard, we have recently found that chronic treatment with propranolol in advanced cirrhotic patients is characterized by marked changes in the precursors of the RAS cascade (renin and Ang I) with repercussions in the 2 main components of the RAS [Ang II and Ang-(1-7)] in the splanchnic and peripheral circulation[15]. Additionally, treatment with propranolol seemed to be able to control the hyperdynamic circulation of cirrhotic patients probably resulting from an overall RAS inhibition, but without changes in the balance between the 2 RAS arms: ACE-Ang-AT1 receptor (vasoconstrictor) vs ACE2-Ang-(1-7)-Mas receptor (vasodilator)[15]. Taken together, our previous and present data further support the idea that a possible therapeutic approach for advanced human cirrhosis could be the combination of beta blockade with AT1 receptor antagonism or ACE inhibition.
Experimental studies have recently evaluated Ang-(1-7) in bile duct-ligated (BDL) rats. Paizis et al[14] demonstrated that in both BDL rat and human livers there was an increased ACE2 expression and activity that might facilitate the conversion of Ang II to Ang-(1-7). Pereira et al[12] demonstrated that the progression of liver dysfunction in BDL rats was characterized by marked changes in Ang-(1-7) and Ang II levels and the overall activation of circulating RAS was associated with the progression of hepatic fibrosis. Subsequently, Herath et al[13] also found RAS activation in chronic liver injury to be associated with upregulation of ACE2, Mas receptor and hepatic conversion of Ang II to Ang-(1-7), leading to increased circulating Ang-(1-7). Taken together, these studies support the presence of an activated ACE2-Ang-(1-7)-Mas receptor axis in liver injury that may counteract the effects of Ang II[1213]. Indeed, blockade of Ang-(1-7) with A-779 worsened the cirrhosis evolution in BDL rats by increasing liver fibrosis[12].
As mentioned above, in the circulation, RAS seems to act through 2 opposing axes, the classic ACE-Ang II-AT1 receptor (vasoconstrictor) and the counter-regulatory ACE2-Ang-(1-7)-Mas receptor (vasodilator)[12]. The increase of the Ang-(1-7)/Ang II ratio suggests a deviation of RAS metabolism toward Ang-(1-7) formation in MLD patients when compared to the ALD group. The comparison between peripheral and splanchnic levels of angiotensins also revealed a differential regulation in LT patients. While an increased Ang-(1-7)/Ang II ratio was detected in the splanchnic circulation, the ratio between Ang II and Ang I (an indirect estimation of net ACE activity) predominated in the peripheral circulation. Arterial splanchnic vasodilation was consistently detected in human cirrhosis[819]. A large number of studies have demonstrated the vasodilatory effect of Ang-(1-7)[20–23]. Possible mechanisms for this effect include bradykinin potentiation[2122], enhanced prostacyclin (PGI2) release from vascular smooth muscle cells[23] and direct stimulation of nitric oxide (NO) synthesis[2022]. NO, PGI2, carbon monoxide, endocannabinoids and other vasodilators have been associated with arterial splanchnic vasodilation[24]. Our results suggest that the reduction of Ang II levels accompanied by an increased Ang-(1-7)/Ang II ratio in the splanchnic circulation may be, at least in part, responsible for changes in vascular splanchnic tone. A possible explanation for this finding is that the elevated expression and activity of ACE2[1314] during hepatic injury could promote the synthesis of Ang-(1-7). During initial stages of the disease, hyperdynamic circulation is responsible for the maintenance of cardiovascular system homeostasis. The increase of the Ang-(1-7)/Ang II ratio in the peripheral circulation in MLD patients suggests that a deviation of RAS metabolism toward the vasodilator axis could be responsible at least in part for the reduction of the systemic vascular resistance. However, as the disease progresses, the splanchnic vasodilation continues to increase and the hyperdynamic circulation is insufficient to compensate for the effective arterial hypovolemia, leading to a reduction in blood pressure, which, in turn, could stimulate the classic ACE-Ang II-AT1 receptor axis of the RAS in the peripheral circulation, the sympathetic nervous system and vasopressin release, as an attempt to restore blood volume and systemic perfusion pressure[9]. At this stage, in spite of the continued increase in splanchnic vasodilation, the systemic vascular resistance is kept low although constant. As the splanchnic circulation is resistant to the effect of Ang II, noradrenaline and vasopressin due to the local release of NO and other vasodilators[25], the maintenance of arterial pressure could be the result of vasoconstriction in extra-splanchnic vascular areas[26]. This is in accordance with the reduction in the Ang-(1-7)/Ang II ratio in the peripheral circulation from mild-to-moderate to advanced stages of cirrhosis, and from the splanchnic to the peripheral circulation in LT patients.
The correlation between the Ang-(1-7)/Ang II ratio and hemodynamic parameters (cardiac output and systemic vascular resistance) suggests that not only Ang II, but also an excessive formation of Ang-(1-7) may be involved in circulatory changes in human cirrhosis. Cardiac output and systemic vascular resistance depends on the balance between the 2 RAS axes. Besides vascular effects, the literature has also provided evidence of a role for Ang-(1-7) in the regulation of cardiac function[2728]. Indeed, Ang-(1-7) increases cardiac output and stroke volume in rats[28]. Moreover, the finding that isolated hearts from Mas-knockout mice exhibited an impaired function and an increase in coronary vascular resistance supports the importance of the Ang-(1-7)-Mas receptor axis in cardiovascular function[28]. In summary, the cardiovascular effects of Ang-(1-7) suggest a further mechanism by which RAS may contribute to altered vascular tone in cirrhosis.
It should be also pointed that we are aware of the limitations of our study design. First, peripheral blood samples generally represent the cumulative expression of RAS in multiple tissues and may not reliably reflect molecular activity in the splanchnic circulation. For this reason, we did manage to collect samples from the portal vein during liver transplantation. However, it is still difficult to compare these findings to the samples collected in the peripheral blood from outpatients. Another concern is the use of diuretics in ALD patients which could produce relative hypovolemia and activation of the ACE-Ang II-AT1 receptor axis. However, the diuretics are probably not the sole cause of this activation, since their interruption normally reduces but does not normalize PRA[29]. Nevertheless, some aspects of this study may increase the strength of our findings, such as the utilization of strictly defined inclusion and exclusion criteria and the well-established protocol for the measurements of PRA and angiotensins.
In conclusion, based on our previous[15] and present findings, the relationship of Ang-(1-7) and Ang II may play a role in hemodynamic changes of human cirrhosis. We hypothesize that the ACE2-Ang-(1-7)-Mas receptor axis predominates in the peripheral circulation in the initial stages of disease (MLD patients) and in the splanchnic circulation in the advanced stages of cirrhosis (LT group), both contributing to a reduction in vascular resistance and consequently to hyperdynamic circulation. In the peripheral circulation of ALD patients, when compared to the splanchnic circulation, the ACE-Ang II-AT1 receptor arm predominates, probably leading to extra-splanchnic vasoconstriction that occurs at this stage. However, further studies with a larger number of patients should address the precise role of RAS in human cirrhosis. If these preliminary data are confirmed, future therapies interfering with 2 RAS axes in both the systemic and splanchnic circulations should lead to more success in the management of reversible fibrosis[30] and hemodynamic changes in human cirrhosis.
Liver cirrhosis has been recently studied in the light of the new view of the renin angiotensin system (RAS). It is becoming clear that the RAS can influence liver cirrhosis through its 2 main arms. While the angiotensin converting enzyme (ACE)-angiotensin (Ang) II-AT1 receptor arm contributes to liver tissue injury and fibrosis and the maintenance of basal vascular tonus in non-compensated cirrhosis, the activation of the ACE2-Ang-(1-7)-Mas receptor arm exerts anti-fibrotic actions and probably has also a role in arterial vasodilation in liver cirrhosis.
This study represents an initial approach to understand how RAS mediators change according to the stage of human cirrhosis and, more importantly, how the relationship between Ang-(1-7) and Ang II may affect the hemodynamic parameters during liver transplantation.
The data showed that the progression of liver dysfunction is characterized by marked changes in circulating Ang-(1-7) and Ang II levels. At the initial stages, there is a predominance of Ang-(1-7) rather than Ang II. On the other hand, advanced stages of cirrhosis show an activation of peripheral and splanchnic RAS, and a deviation toward the formation of Ang-(1-7) in the splanchnic circulation. Furthermore, there was a positive correlation between the Ang-(1-7)/Ang II ratio and cardiac output and a negative correlation between this ratio and systemic vascular resistance, indicating that the final functional effects of the RAS may reflect a balance between these 2 opposing peptides.
According to this study, future therapies modifying the 2 RAS axes in both the systemic and splanchnic circulation should lead to more success in the management of reversible fibrosis and the hemodynamic changes in human cirrhosis.
The manuscript “The relationship between angiotensin (1-7) and angiotensin II correlates with hemodynamic changes in human liver cirrhosis” is a preliminary, well designed study with a small cohort of patients for each cirrhotic group studied, presented by an experienced research group that, on the basis of the results of this study and those obtained in a previous work (published in World J Gastroenterol, 2008), concluded that the relationship between Ang-(1-7) and Ang II may play a role in hemodynamic changes in human cirrhosis. If the results are corroborated in future studies, a novel therapy could be designed for management of reversible fibrosis and hemodynamic changes in human cirrhosis.
| 1. | Santos RA, Ferreira AJ. Angiotensin-(1-7) and the renin-angiotensin system. Curr Opin Nephrol Hypertens. 2007;16:122-128. |
| 2. | Simoes e Silva AC, Pinheiro SV, Pereira RM, Ferreira AJ, Santos RA. The therapeutic potential of Angiotensin-(1-7) as a novel Renin-Angiotensin System mediator. Mini Rev Med Chem. 2006;6:603-609. |
| 3. | Matsui T, Tamaya K, Matsumoto K, Osajima Y, Uezono K, Kawasaki T. Plasma concentrations of angiotensin metabolites in young male normotensive and mild hypertensive subjects. Hypertens Res. 1999;22:273-277. |
| 4. | Nogueira AI, Souza Santos RA, Simoes E Silva AC, Cabral AC, Vieira RL, Drumond TC, Machado LJ, Freire CM, Ribeiro-Oliveira A Jr. The pregnancy-induced increase of plasma angiotensin-(1-7) is blunted in gestational diabetes. Regul Pept. 2007;141:55-60. |
| 5. | Bataller R, Sancho-Bru P, Gines P, Lora JM, Al-Garawi A, Sole M, Colmenero J, Nicolas JM, Jimenez W, Weich N. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology. 2003;125:117-125. |
| 6. | Warner FJ, Lubel JS, McCaughan GW, Angus PW. Liver fibrosis: a balance of ACEs? Clin Sci (Lond). 2007;113:109-118. |
| 7. | Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005;43:19-29. |
| 8. | Arroyo V, Terra C, Gines P. Advances in the pathogenesis and treatment of type-1 and type-2 hepatorenal syndrome. J Hepatol. 2007;46:935-946. |
| 9. | Blendis L, Wong F. The hyperdynamic circulation in cirrhosis: an overview. Pharmacol Ther. 2001;89:221-231. |
| 10. | Benoit JN, Granger DN. Splanchnic hemodynamics in chronic portal hypertension. Semin Liver Dis. 1986;6:287-298. |
| 11. | Helmy A, Jalan R, Newby DE, Hayes PC, Webb DJ. Role of angiotensin II in regulation of basal and sympathetically stimulated vascular tone in early and advanced cirrhosis. Gastroenterology. 2000;118:565-572. |
| 12. | Pereira RM, Dos Santos RA, Teixeira MM, Leite VH, Costa LP, da Costa Dias FL, Barcelos LS, Collares GB, Simoes e Silva AC. The renin-angiotensin system in a rat model of hepatic fibrosis: evidence for a protective role of Angiotensin-(1-7). J Hepatol. 2007;46:674-681. |
| 13. | Herath CB, Warner FJ, Lubel JS, Dean RG, Jia Z, Lew RA, Smith AI, Burrell LM, Angus PW. Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1-7) levels in experimental biliary fibrosis. J Hepatol. 2007;47:387-395. |
| 14. | Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-1796. |
| 15. | Vilas-Boas WW, Ribeiro-Oliveira A Jr, Ribeiro Rda C, Vieira RL, Almeida J, Nadu AP, Simoes e Silva AC, Santos RA. Effect of propranolol on the splanchnic and peripheral renin angiotensin system in cirrhotic patients. World J Gastroenterol. 2008;14:6824-6830. |
| 16. | Samonakis DN, Triantos CK, Thalheimer U, Patch DW, Burroughs AK. Management of portal hypertension. Postgrad Med J. 2004;80:634-641. |
| 17. | Simoes E Silva AC, Diniz JS, Regueira Filho A, Santos RA. The renin angiotensin system in childhood hypertension: selective increase of angiotensin-(1-7) in essential hypertension. J Pediatr. 2004;145:93-98. |
| 18. | Arroyo V, Colmenero J. Ascites and hepatorenal syndrome in cirrhosis: pathophysiological basis of therapy and current management. J Hepatol. 2003;38 Suppl 1:S69-S89. |
| 19. | Gines P, Cardenas A, Arroyo V, Rodes J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646-1654. |
| 20. | Porsti I, Bara AT, Busse R, Hecker M. Release of nitric oxide by angiotensin-(1-7) from porcine coronary endothelium: implications for a novel angiotensin receptor. Br J Pharmacol. 1994;111:652-654. |
| 21. | Paula RD, Lima CV, Khosla MC, Santos RA. Angiotensin-(1-7) potentiates the hypotensive effect of bradykinin in conscious rats. Hypertension. 1995;26:1154-1159. |
| 22. | Brosnihan KB, Li P, Ferrario CM. Angiotensin-(1-7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27:523-528. |
| 23. | Muthalif MM, Benter IF, Uddin MR, Harper JL, Malik KU. Signal transduction mechanisms involved in angiotensin-(1-7)-stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J Pharmacol Exp Ther. 1998;284:388-398. |
| 24. | Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927-934. |
| 25. | Sieber CC, Lopez-Talavera JC, Groszmann RJ. Role of nitric oxide in the in vitro splanchnic vascular hyporeactivity in ascitic cirrhotic rats. Gastroenterology. 1993;104:1750-1754. |
| 26. | Maroto A, Gines P, Arroyo V, Gines A, Salo J, Claria J, Jimenez W, Bru C, Rivera F, Rodes J. Brachial and femoral artery blood flow in cirrhosis: relationship to kidney dysfunction. Hepatology. 1993;17:788-793. |
| 27. | Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1-7) in rats. Am J Physiol Heart Circ Physiol. 2003;284:H1985-H1994. |
| 28. | Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1-7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension. 2005;46:937-942. |
| 29. | Kalambokis G, Economou M, Kosta P, Papadimitriou K, Tsianos EV. The effects of treatment with octreotide, diuretics, or both on portal hemodynamics in nonazotemic cirrhotic patients with ascites. J Clin Gastroenterol. 2006;40:342-346. |
| 30. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. |