INTRODUCTION
The term spondyloarthropathy (SpA) indicates a group of related diseases, including ankylosing spondylitis (AS), reactive and psoriatic arthritis (ReA and PsA), and undifferentiated spondyloarthritis (uSpA). All these forms of SpA share common clinical features which are sacroiliitis, inflammatory low back pain and oligoarticular asymmetric synovitis. SpA is a frequently observed extraintestinal manifestation in Crohn’s disease (CD) and ulcerative colitis (UC), the two major forms of inflammatory bowel diseases (IBD). Indeed, the prevalence of SpA associated with CD and UC was 45.7% and 9.9%, respectively in a recent series[1]. Moreover a subtle gut inflammation is present in 25%-75% of patients with documented SpA[2], depending on the subtype, and among these, 6%-13% may evolve to overt IBD, suggesting common pathogenetic mechanisms between these two clinical entities[3–5].
The observation that SpA may occur during IBD has led to the hypothesis that IBD-related SpA originates from extraintestinal spreading of the immunologic process originating in the gut. Results from several studies suggest that the activation of the intestinal immune system may indeed lead, in certain conditions, to the generation of T cell clones which leave the gut compartment to home into the joints. These T cell clones would be able to replicate, in this site, the inflammatory process observed in the gut. However, to render this model plausible some conditions need to be met. Indeed the idea that joint inflammation is driven by T cell clones originating in the gut implies that these cells are able to leave the gut and to transfer into the joints. Secondly, gut-derived T cells need to encounter in the joint environment an adequate antigenic stimulus to allow the reactivation of these cells. Finally, the immune response shaped in the gut must be responsible for the inflammation-related tissue damage observed in SpA.
FROM THE GUT TO THE JOINT: THE T CELL HOMING
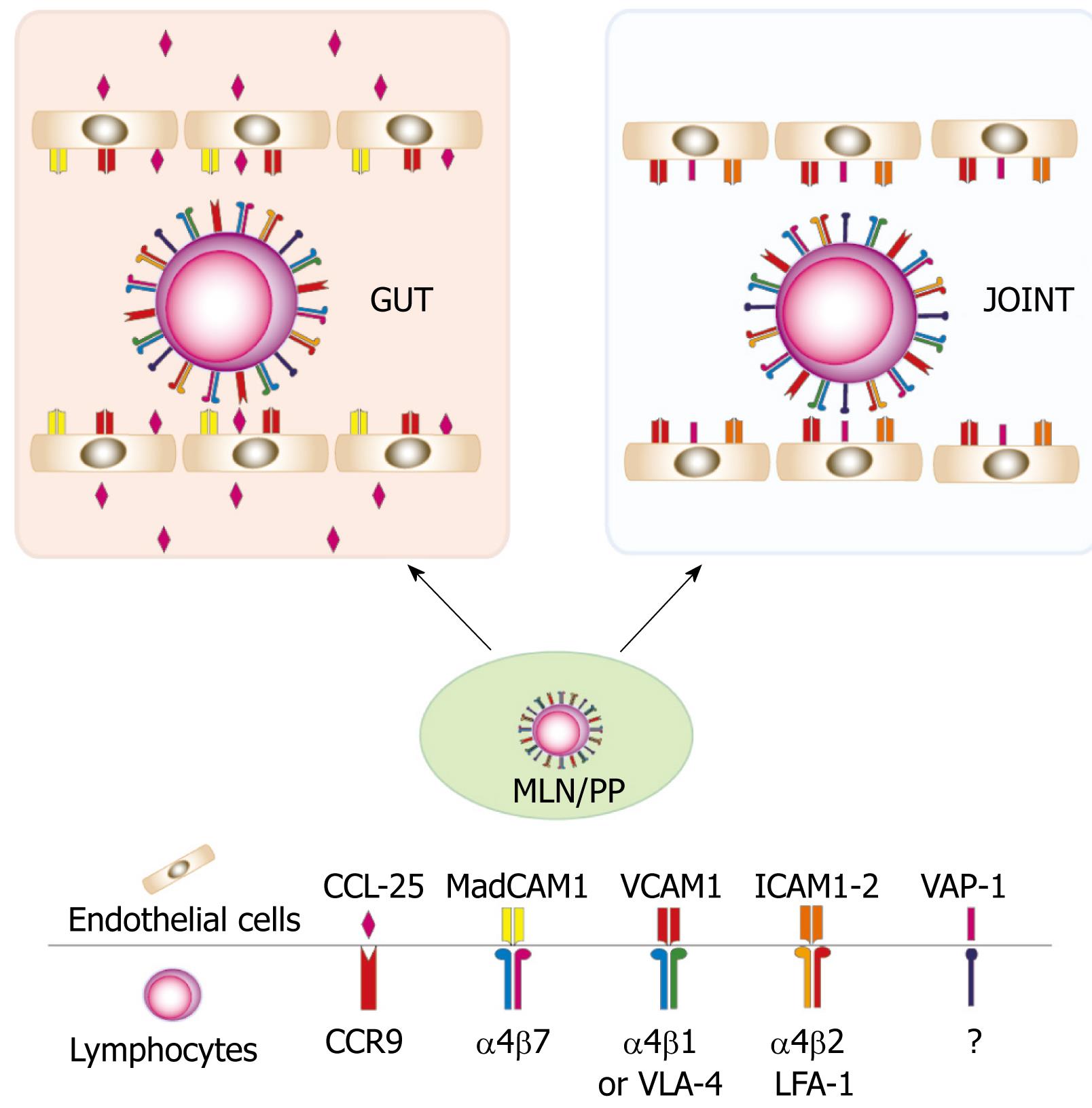
A critical point for transfer of the inflammatory process from the gut to the joints is the possibility of redirecting the tissue-specific homing of inflammatory cells, mainly T cells, into the synovial compartment. In IBD, the abnormal reactivity of T cells against harmless antigens expressed by the commensal flora is thought to cause chronic intestinal inflammation[67]. In the gut-associated lymphoid tissue (i.e. Peyer’s patches and lymphoid follicles) and mesenteric lymph nodes, professional antigen presenting cells (i.e. dendritic cells, DC) migrate from the intestinal lamina propria, prime naïve T cells which in turn differentiate into specialized T helper cells (e.g. Th1, Th2, Th17), thus acquiring the capacity to sustain a specific immune response. The profound changes observed in differentiated T cells in the secondary lymphoid organs include the expression of cell surface adhesion molecules and chemokine receptors which are responsible for the gut-specific T cell homing. Indeed, T cells activated in the Peyer’s patches and mesenteric lymph nodes express the gut-addressing integrin α4/β7 and the chemokine receptor CCR9[8]. Once activated, these cells reach the bloodstream through the efferent lymphatics and the thoracic duct. In the gut mucosa, the interaction between α4/β7 integrin and its ligand, the mucosal addressin cell adhesion molecule 1 (MadCAM-1) expressed on the venular endothelial sheet[910] causes the initial rolling and subsequent arrest of activated T cells. MadCAM-1 is normally expressed on the intestinal mucosa and its expression is further enhanced during inflammation[11]. Once arrested on the surface of the intestinal venules, activated T cells transmigrate through the endothelial layer and move into the lamina propria following the gradient formed by the CCR-9-specific ligand CCL-25[1213]. Therefore, the specific interaction between α4/β7 integrin with MadCAM-1 and CCR9 with CCL-25 is pivotal for T cell homing into the gut. However it is worth noting that other molecules mediate the cell-to-cell interaction in this process. For instance CD44, the very late antigen-4 (VLA-4, α4β1) and the lymphocytes function associated antigen-1 (LFA-1, αLβ2) expressed by activated T cells play a role in the recruitment of T cells into the gut. Moreover the expression of the vascular activated peptide-1 (VAP-1), intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and different subgroups of selectins (i.e. P- and E-selectins) which bind P-selectin glycoprotein-1 (PSGL-1) on T cells, is enhanced in endothelial cells of the inflamed intestine[14]. However, these molecules are not gut specific and they seem to contribute marginally to the specificity of T cell homing into the intestine. Nevertheless, many lines of evidence indicate that these molecules may be involved in the homing of gut-activated T cells in other organs. Initial studies indicated that lamina propria lymphocytes (LPL) isolated from uninflamed gut were able to bind to uninflamed synovial vessels and that this interaction was critically mediated by CD44, VLA-4 and LFA-1[15]. In contrast, the adherence of lamina propria T cells to inflamed synovial vessels was dependent on VAP-1 and CD44[16]. When LPL were isolated from the inflamed gut of IBD patients, these cells bound more efficiently to synovial vessels than cells isolated from uninflamed gut[17]. This higher affinity could be explained by the observation that, in contrast with cells isolated from uninflamed gut, the binding of small, memory-like, T cells from IBD patients was, in addition to VLA-4, α4β7-dependent, thus indicating a more varied use of adhesion molecules by IBD lamina propria T cells. By contrast, immunoblasts isolated from the lamina propria of IBD patients relied on CD44, LFA-1 and VAP-1 for their adhesion to synovial vessels, similar to immunoblasts from uninflamed gut.
Overall these data indicate that T cells primed in the gut-draining secondary lymphoid organs express a pattern of adhesion molecules that in part are responsible for the intestinal specific homing but that might, in particular conditions, mediate the entrance of activated T cells into extraintestinal compartments such as synovial tissue (Figure 1).
Figure 1 The heterogeneous expression of adhesion molecules allows T cells activated in the gut to home into the joints.
CCR9: Chemokine receptor-9; CCL-25: Chemokine ligand-25; MadCAM1: Mucosal addressin cell-adhesion molecule-1; VCAM1: Vascular cell adhesion molecule-1; ICAM :Intracellular adhesion molecule; VLA-4: Very late antigen-4; LFA-1: Lymphocyte function associated antigen-1; VAP-1: Vascular adhesion protein-1.
ROLE OF ANTIGEN MIMICRY AND HLA-B27 IN THE ANTIGENIC STIMULATION
As previously mentioned, intestinal commensal bacteria are thought to sustain intestinal inflammation in IBD. Analogously, IBD-related SpA is thought to depend on the interaction between the host and the gut microbiota. This hypothesis is supported by the observation made in rat models of colitis and colitis-associated SpA. These rats, characterized by the over-expression of human HLA-B27, spontaneously develop intestinal inflammation and SpA. However, these animals did not develop inflammation when grown in germ-free conditions[18]. In contrast, both intestinal inflammation and SpA reappeared when they were transferred to a specific pathogen free environment[1920], thus indicating that the intestinal and articular inflammatory processes depend on the presence of bacteria into the gut lumen.
Although many attempts to isolate living pathogens from the joint fluid during reactive arthritis failed, the presence of Yersinia enterocolitica-, Salmonella enteritidis- and typhimurium-, Yersinia- and Shigella-related antigens have been detected in the joints of these patients[21–23]. This observation suggests that the enteric antigens may be transported into the joints by monocytes. Accordingly, macrophages from the lamina propria of IBD patients were shown to adhere to the endothelial cells of synovial tissue[17]. It is therefore possible that recirculation of antigen-loaded macrophages may provide the antigenic stimulus necessary to sustain T cell activation and inflammation in the joints.
The crucial role of antigen stimulation in the pathogenesis of IBD-related SpA is also supported by the strong genetic association between SpA and the human leukocyte antigen (HLA) class I B-27 (HLA-B27). HLA-B27 was found in 75%-95% of patients affected by SpA[2425] and in 25%-78% of IBD patients without SpA who developed this extraintestinal manifestation at a later stage of the disease[2627]. Despite the strong genetic association, the pathogenetic role of HLA-B27 are still poorly understood. Activation of CD8+ T cells involved in the arthritic process by specific bacterial antigens exposed on HLA-B27 has been proposed[2829]. Moreover, it has been shown that CD4+ T cells isolated from patients with reactive arthritis are activated by bacterial peptides presented in the context of HLA-B27[30]. These data draw a possible scenario in which activated CD4+ T cells migrate into the joints from the gut, in response to bacterial antigens presented by HLA-B27-expressing macrophages. Not necessarily in contrast with this hypothesis are data demonstrating the homology between HLA-B27 sequences and antigens derived from virus and enterobacteria. Indeed a certain level of antigen mimicry may contribute to the onset and/or maintenance of the inflammatory process initially induced by bacterial antigens. For instance, the nonapeptide, LRRYLENGK, derived from HLA-B27 (residues 168-176) shares the same sequence as peptides from enteric organisms (i.e. Pseudomonas aeruginosa, Klebsiella nitrogenase, Escherichia coli, Bacillus megaterium, Salmonella typhimurium). The same peptides originating from either bacteria or HLA-B27 endogenous turnover can be presented by HLA-B27, determining the activation of the same T cell repertoire[31]. Moreover, a dodecapeptide contained in the intracytoplasmic tail of HLA-B27 shows a strong homology with the sequence contained in the DNA primase of the arthritogenic bacteria Chlamydia trachomatis[32]. This fits well with the observation that high titers of antibodies anti-Saccharomyces cerevisiae (ASCA) and anti-neutrophils (pANCA) antibodies are present in IBD-related SpA[33]. Indeed pANCAs have been shown to cross-react with both neutrophil nuclear membrane and a E. coli proteins[34], while no self-antigens have been so far identified for ASCA. These data indicate that in the presence of HLA-B27 but also independently of this HLA antigen, an immune response initially evoked by a bacterial antigen may be further sustained by the cross-reactivity with self-antigens.
Recently, the process of folding and expression of the HLA-B27 heavy chain has received increasing attention as a potential mechanism involved in the pathogenesis of SpA. Under normal conditions, the peptide-loaded HLA class I heavy chain binds the β2-microglobulin (β2m). This assembling process takes place in the endoplasmic reticulum[35]. The folding process of the HLA-B27 heavy chain is slower than that of other HLA alleles thus leading to the generation of misfolded chains[36]. Misfolded chains are usually removed in the endoplasmic reticulum, but in certain conditions, such as viral infection, they accumulate thus generating a cascade of intracellular events including the activation of the protein BiP, the endoplasmic reticulum-unfolded-protein-response (UPR) and the activation of nuclear factor κB (NFκB) which plays a critical role in the induction of inflammation[37]. In contrast with this theory, it has been recently demonstrated that in HLA-B27 over-expressing rats, which normally develop colitis and SpA, the increased expression of β2m prevented colitis but not SpA[38]. In this study over-expression of β2m caused a reduction of HLA-B27 misfolding and an unfolded protein response, suggesting that HLA-B27 heavy chain misfolding may be critical in the development of colitis but not of SpA. In addition to misfolding, data suggest that HLA-B27 heavy chains preferentially form homodimers which bind immunoglobulin-like receptors expressed on the cell surface of natural killer (NK) cells, T cells and monocytes[39]. However, the actual role played by the interaction between HLA-B27 homodimer and its paired receptor in the inflammatory process is still poorly understood. Finally, data suggest that deposition of β2m, caused by the high dissociation rate between HLA-B27 heavy chain and β2m, occurring within synovial tissue, may lead to the initiation of chronic inflammation[40].
COMMON INFLAMMATORY MECHANISMS: FROM THE GUT TO THE JOINTS
The concept that immune cells, activated in the inflamed gut and migrating into the joints, may be able to reproduce in this tissue a similar immune response, is sustained by the observation that common immunological processes operate at both these sites. Attention has been recently focused on the role of T helper 17 cells (Th17) in IBDs and IBD-related SpA. Th17 cells form a novel class of T helper cells characterized by the expression of the proinflammatory cytokines interleukin (IL)-17A, from which comes the name Th17, IL-17F, IL-22 and TNFα. IL-6 and TGFβ have been shown to be crucial for the differentiation of these cells while IL-23, another proinflammatory cytokine, is thought to be important for their maintenance and expansion[4142]. Several lines of evidence indicate that Th17 cells may play a role in the induction and maintenance of gut inflammation in CD while their role in UC is still uncertain. Indeed, IL-17A and IL-17F are highly expressed in the gut of patients affected by CD, and Th17 cells have been shown to induce intestinal inflammation in different mouse models of colitis[43–46]. Analogously, high expression of IL-17 was found in the synovial fluids of SpA-affected patients and an increased number of circulating Th17 memory-like T cells has been recently reported in these patients[4748]. An association between Th17 cells and IBD is further supported by the observation that mutations of IL-23 receptor reduce the risk of developing IBD[49] and the same mutations were found to protect against SpA[50]. Although the functional role of IL-23R mutations remains unclear, the fact that IL-23 signaling plays a critical role in the Th17-mediated inflammation, implicates that Th17 cells may represent a common pathogenetic mechanism in both IBD and SpA.
Tumor necrosis factor-α (TNFα) is a proinflammatory cytokine largely expressed in the lamina propria of patients affected by IBD (mainly CD and to a lesser extent UC), rheumatoid arthritis (RA) and SpA. The role of TNFα in IBD-related SpA has been investigated in the TnfΔARE mouse model which is characterized by the high expression of TNFα. These mice develop a phenotype dominated by IBD-like intestinal inflammation and arthritis thus implicating TNFα as a required factor for the induction of inflammation in both IBD and IBD-related SpA[51]. In this model, intact TNFα signaling in radiation-resistant mesenchymal cells was found to be required for the induction of SpA as shown by the absence of SpA observed in lethally irradiated TNFαRI knockout mice reconstituted with TnfΔARE bone marrow cells[52]. Moreover, selective expression of TNFαRI in intestinal myofibroblasts (IMF) and synovial fibroblasts (SF) was sufficient to re-establish intestinal inflammation and SpA in TnfΔARE-TNFαRI knockout mice. MIF and SF expressed high levels of extracellular matrix-degrading metalloproteinase (MMP)-9 and -3 accompanied by reduced levels of the tissue inhibitor of MMPs-1 (TIMP-1) in response to TNFα stimulation which were in part responsible for the tissue damage observed in both the gut and the joints.
COMMON TREATMENT OPTIONS
These studies provide a rationale for developing new strategies for the therapy of IBD-related SpA. However, only the neutralization of TNFα has so far found clinical application in the therapy of IBD-related SpA. An early report showed that two patients affected by CD-associated AS refractory to conventional therapy, experienced amelioration of the axial symptoms after anti-TNFα therapy with infliximab 5 mg/kg intravenously[53]. The efficacy of infliximab in the therapy of SpA was later confirmed by two randomized controlled trials. In a first randomized, double-blind trial 40 patients affected by SpA were randomly assigned to receive either infliximab 5 mg/kg (weeks 0, 2, and 6) or placebo. At 12 wk there was a significant improvement of both the BASDAI (Bath Ankylosing Spondylitis Disease Activity Index) and BASFI (Bath Ankylosing Spondylitis Functional Index) in the infliximab group in comparison to controls[54]. Similar results were obtained by Braun et al[55] in a randomized, controlled, multicenter trial in which 53% (18 of 34) patients affected by AS treated with infliximab 5 mg/kg (weeks 0, 2, 6) vs 9% (3 of 35) treated with placebo showed a reduction of at least 50% of the BASDAI, BASFI and BASMI (Bath Ankylosing Spondylitis Metrology Index) compared to baseline. However the presence of concomitant IBD in the patients participating in both these trials was unknown. The only trial evaluating the efficacy of anti-TNFα in a cohort of patients affected by CD-related SpA is an open-label study comparing infliximab vs conventional therapy[56]. In this study 21 patients with active SpA were enrolled. Sixteen patients with active CD were treated with infliximab (5 mg/kg) at 0, 2, and 6 wk. If remission was achieved patients were treated with a maintenance dose of 3 mg/kg every 6-8 wk otherwise 5 mg/kg was administered. Eight CD-affected patients were in clinical remission at the beginning of the study. These patients were treated with a dose of 3 mg/kg following the same schedule. Twelve additional patients affected by active CD and SpA underwent conventional therapies. Results from this study showed a significant reduction of the BASDAI and spinal pain in the group treated with infliximab in comparison to patients undergoing conventional therapy. Finally, it has been recently suggested that treatment of SpA with infliximab but not etanercept (another anti-TNFα agent) prevents new onset or flares of IBD[57].
The use of adalimumab, a fully humanized anti-TNFα has achieved similar results. A multicenter, randomized, placebo-controlled, trial aimed at assessing the efficacy and safety of 40 mg adalimumab administered subcutaneously for 12 and 24 wk, found that adalimumab was significantly more effective in inducing ASAS20 (20% response according to the ASsessment in Ankylosing Spondylitis International Working Group criteria) than placebo[58]. Moreover the long term efficacy of adalimumab regimen in the treatment of IBD-related SpA has been recently confirmed in a 2-year follow-up study after the initial treatment[59].
CONCLUSION
SpA is a common extraintestinal manifestation of IBD. However, the immunological mechanisms linking gut and joint inflammation are still poorly characterized. The observation that in most of the cases intestinal inflammation precedes SpA has led to the hypothesis that the inflammatory process initially localized in the gut may be “relocated” to a different site. Indeed most of the data summarized here support this concept, providing evidence that T cells and monocytes/macrophages activated by gut-related antigens may be able to home in to the synovial tissue as a result of the expression of adhesion molecules which partially overlap with those expressed by endothelial cells in the gut. In synovial tissue, activation of inflammatory cells may be sustained by several mechanisms including the presence of bacterial antigens and/or by the altered expression of HLA-B27. Finally Th17 cells and high expression of TNFα may play a crucial role in the inflammation-related tissue damage in both the gut and the joints by inducing the expression of extracellular matrix metalloproteinases. However, it is important to note that in some cases, SpA has been shown to precede IBD thus indicating that the illustrated mechanism may not be always applicable and that other immunological processes may link gut inflammation to inflammatory processes localized in different extra-intestinal sites.
Peer reviewers: Elke Cario, MD, Division of Gastroenterology and Hepatology, University Hospital of Essen, Institutsgruppe I, Virchowstr. 171, Essen D-45147, Germany; Emiko Mizoguchi, MD, PhD, Department of Medicine, Gastrointestinal Unit, GRJ 702, Massachusetts General Hospital, Boston, MA 02114, United States