INTRODUCTION
Ewing’s sarcoma, or primitive neuroectodermal tumour (ES/PNET), is a neoplasm of undifferentiated small round cells that occurs in children, adolescents and young adults[12]. Although predominantly affecting the bones and deep soft tissues[2], these sarcomas are also being described as affecting the visceral organs, with increasing frequency. These extraskeletal ES/PNET sarcomas are histologically indistinguishable from the bony type[3], and have been documented in the pancreas[45], vagina[6], rectovaginal septum[7], small bowel[89], prostate[10], ovaries[11], esophagus[12], and kidney[13]. Two cases of gastric ES/PNET have been previously reported: the first in a 14-year-old boy[14] and the second in a 66-year-old woman[2]. We describe the third ever case of gastric ES.
CASE REPORT
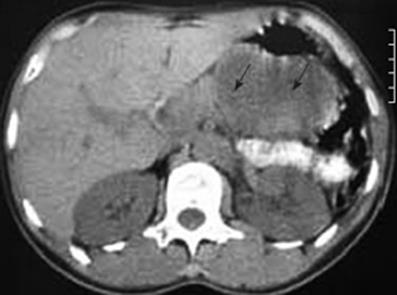
In January 2006, a 44-year-old obese woman presented with a 6-mo history of epigastric pain and weight loss of 5 kg. On examination, she had only mild tenderness in the epigastrium. All standard laboratory data were within normal limits, though fasting cholesterol was elevated at 6.80 &mgr;mol/L (normal range 3.1-6.4 &mgr;mol/L), and fasting triglyceride was 2.30 mmol/L (normal < 1.95 mmol/L); erythrocyte sedimentation rate was 14 mm/h. Ultrasonography (US) and computed tomography (CT) scans (Figure 1) confirmed a solid/cystic mass measuring 66 mm × 46 mm in the tail of the pancreas.
Figure 1 CT scan indicating a tumor at the tail of the pancreas (arrows).
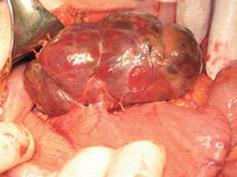
The patient underwent laparotomy for the tumor at the tail of the pancreas; but, at operation, the only pathology found within the abdomen was a mass along the posterior wall of the stomach (Figure 2). There was no lymphadenopathy in the vicinity of the tumor or the upper retroperitoneum. The tumor was dark-red in color, moderately soft, covered by a thin light-grey membrane, and adherent to the stomach along a surface of 3 cm × 3 cm. Intraoperative frozen section biopsy failed to clarify either the tumor type or the eventual malignant potential. Thus, when the tumor was completely excised, and no significant damage was seen on the stomach wall, the decision was made to not proceed to a gastric resection.
Figure 2 Photograph showing the tumor on the posterior wall of the stomach.
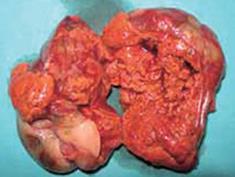
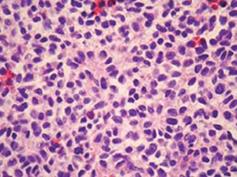
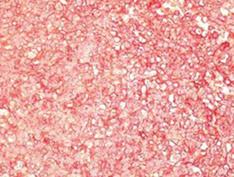
The pathological specimen measured 10 cm in its greatest diameter. On gross inspection, the cut surface showed large central hemorrhagic and necrotic changes and pseudocystic degeneration. The tumor tissue was largely light grey and solid, with some softer and more friable, reddish congested areas (Figure 3). Microscopic examination revealed a hypercellular diffuse, and rather monotonous proliferation of small round cells with clear cytoplasm and relative nuclear uniformity. However, there were also areas showing nuclear atypia and abortive pseudorosette formation (Figure 4). Microcystic and hemorrhagic changes were seen on most of the sections, as well as sharply demarcated borders frequently covered by intact serosa. Tumor necrosis was minimal, although atypical mitotic figures were seen with a mitotic index of 14 per 50 high power fields.
Figure 3 Photograph showing a cross section of the tumor.
Figure 4 Histological appearance of the diffuse neoplastic infiltration showing rather uniform diffuse small round cells and abortive pseudorosette formation (HE, × 112).
Immunohistochemical examination with monoclonal antibodies excluded most of the differential diagnoses. Immunostaining was negative for pancytokeratin AE1/AE3, epithelial membrane antigen (EMA), leukocyte common antigen, chromogranin A, synaptophysin, desmin, muscle-specific antigen, the &agr; subunit of smooth muscle antigen (SMA), glial fibrilar acidic protein, neurofilament protein, &agr;-inhibin, melanin A and CD34. The diagnosis of ES/PNET was established by the presence of strong membranous CD99 immunoexpression by the vast majority of tumor cells (Figure 5), in addition to clear, but patchy S-100 protein, vimentin, protein gene product (PGP) 9.5 and neuron-specific enolase (NSE) immunostaining, as well as weak focal CD117 cytoplasmic immunoreactivity in very few neoplastic cells. Fluorescent in situ hybridization (FISH) was not available to us.
Figure 5 Tumor cells showing strong diffuse membrane immuno-histochemical reactivity with CD99 antibodies [labeled streptavidin biotin (LSAB+) method, 3-amino-9-ethyl carbazole (AEC) visualization, × 112].
The patient did not receive any adjuvant chemotherapy or radiotherapy; her postoperative recovery was unevent-ful, and she remains symptom-free and without recurrence on US or CT scan 20 mo later.
DISCUSSION
Histologically, ES/PNET is composed of small round cells that are usually rich in glycogen, and the neuroepithelial morphologic differentiation is confirmed by pseudorosette formation. Immunohistochemically, the neuroendocrine phenotype is confirmed by positivity to CD99, and to NSE and S-100 to a lesser extent, as these are also found in a number of other small-round-cell tumors[2]. FISH testing for the presence of the t (11;12) translocation can be particularly useful when the tumor occurs in older patients or in an unusual site[215], as well as to differentiate from desmoplastic small round cell tumors (DSRCT)[15]. The ultimate diagnosis should be based on both histology and immunohistochemistry[16].
We considered several other small-round-cell tumors in our differential diagnoses. Mesenchymal chondrosarcoma, and small-cell osteosarcoma were excluded as no chondroid or osteoid differentiation was found. Embryonic rhabdomyosarcoma was excluded as all muscular markers were negative. Intra-abdominal desmoplastic round-cell tumor was excluded as cytokeratin and EMA markers were negative, and nodular dissemination was absent. Hemangiopericytoma, glomus and other perivascular tumors were excluded on the basis of CD34 and SMA negativity.
The results of surgery alone for extraskeletal ES are poor in most cases, while patients receiving multimodal chemotherapy and radiotherapy have a much better prognosis[17]. Through the combination of local surgical treatment and systemic chemotherapy, long-term survival has improved from 10% to 50%-60% or greater[1819], although the pathologist and oncologist will need to decide whether treatment regimens for tumors are better based on their phenotype or their genotype, when these two profiles are seemingly in conflict[16].
The two previously published cases of ES of the stomach both had poor outcomes. A 14-year-old boy with gastric ES was also found to have a diffuse metastatic lesion in the liver. He underwent a subtotal gastrectomy and lymphadenectomy followed by chemotherapy with a tyrosine kinase inhibitor because of intense expression of CD117 (c-kit), but died[14]. A 66-year-old woman with ES within the antropyloric area of the stomach underwent a distal gastrectomy and lymphadenectomy, but despite adjuvant chemotherapy, she also died 10 mo postoperatively[2].
Following postoperative pathological analysis, our patient was presented to the oncologists, who decided not to give any adjuvant chemotherapy. Despite this, she remains clinically well, and without recurrence to the present day (20 mo later).