INTRODUCTION
Circulation and perfusion of individual tissues is a basic physiological process that is necessary to sustain oxygenation and nutrition at a cellular level. Ischemia, or the insufficiency of perfusion, is a common mechanism for tissue death or degeneration, and at a lower threshold, a mechanism for the generation of sensory signals including pain. Ischemia is a common cause of pain from the myocardium in coronary heart disease, ranging from reversible changes in angina pectoris to acute myocardial infarction with its multitude of complications. Myocardial ischemia is usually due to stenoses of the larger epicardial arteries; but, microvascular changes, such as those commonly seen in patients with diabetes mellitus, can cause ischemia and biochemical changes triggering pain signalling from the tissues. Ischemia can similarly cause pain from abdominal organs, including the intestines, when stenoses of the proximal mesenteric arteries limit perfusion in the distal vascular bed. It is also possible that abnormalities in smaller vessels, and in their regulation of blood flow may cause similar biochemical changes in the intestinal wall and be an integral part of the pathogenesis of disease[1].
It is of considerable interest to study perfusion of peripheral abdominal tissues in a variety of circumstances. When studying the pathogenesis of various diseases, measurements of perfusion of the tissues affected may be essential to assess the relative contribution of ischemia to disease pathogenesis. Furthermore, in the surgical treatment of disease, assessment of perfusion may be important for assuring that anastomoses are established in well perfused segments of the gut. The beneficial or adverse effects of drug therapy might also be evaluated by monitoring perfusion of a segment of the gut.
MICROCIRCULATION IN THE GASTROINTESTINAL TRACT
Our knowledge of the peculiarities of the vascular bed of the gastrointestinal (GI) tract is still limited. There is considerable inter-individual variation in the anatomy of larger vessels, and the extent of collateral circulation, which may also explain differences in susceptibility to local ischemia. Perfusion is dependent on the arterial supply from the celiac, superior mesenteric and inferior mesenteric arteries. The watershed areas between these major arteries are likely to suffer from ischemia during acute or chronic arterial insufficiency.
The tissue volume occupied by moving blood cells is small; the average density of capillaries is about 50 capillaries per mm2 of mucosal area, and on average 20% of capillaries are open under resting conditions, perfusion being mainly regulated by the opening and closing of precapillary sphincters. This autoregulation of perfusion is well established in several studies, including studies employing laser Doppler perfusion monitoring (LDPM) and is highly dependent on endothelial cell function[2].
MICROCIRCULATION AND THE SPECTRUM OF GASTROINTESTINAL DISEASE
Microvascular disease of the abdominal organs has been implicated in the pathogenesis of a variety of disorders, including peptic ulcer disease and inflammatory bowel disease (including both ulcerative colitis and Crohn’s disease of the intestines). It has been suggested that apart from immunological and bacterial effects on the intestinal wall, changes in the microvasculature are essential for developing such key elements as mononuclear cell infiltration and fibrosis. Typically, the early stages of colitis show increased perfusion, whereas the later chronic stages of this disease show hypoperfusion of the mucosa. This was first shown in various animal models of acute inflammatory bowel disease (IBD), but also convincingly in patients with chronic disease with fibrosis[2]. Importantly, it has been found that the capacity for vasodilatation is decreased in chronic IBD, suggesting a mechanism for ischemia and pain[3]. It has been argued that in patients with Crohn’s disease, chronic vascular changes may result in areas of microinfarction in the gut wall, leading to granulomatous inflammation and fibrosis[4].
LASER DOPPLER PERFUSION MONITORING (LDPM)
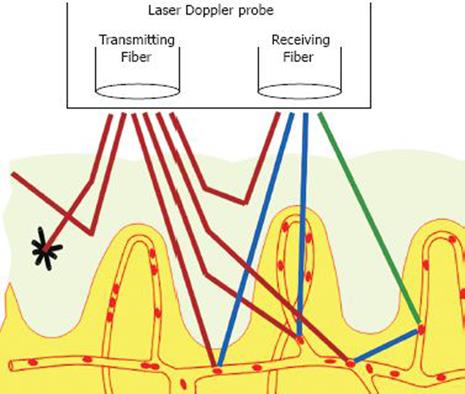
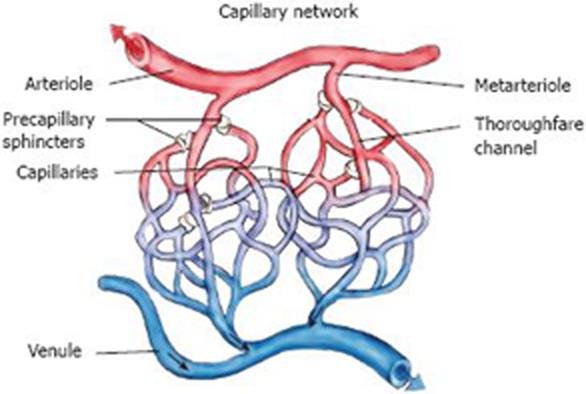
The basic principle of laser Doppler perfusion monitoring (LDPM; laser Doppler velocimetry, or laser Doppler flowmetry) is to analyse changes in the spectrum of light reflected from living tissues as a response to a beam of monochromatic laser light emitted (Figure 1). LDPM reflects the total local microcirculatory blood perfusion including perfusion in the capillaries (nutritive flow), arterioles (thermoregulatory flow - such as in the skin), venules and shunts (Figure 2).
Figure 1 A schematic depiction of laser Doppler perfusion monitoring showing the probe with its emitting fibre bundle which applies monochro-matic laser light to the tissue, and its receiving fibre bundle which returns reflected light for analysis.
The light that has undergone a doppler shift due to moving blood cells in the tissues reflects the microcirculatory perfusion at a given time. Reproduced by permission of Perimed AB.
Figure 2 The capillary network showing also precapillary sphincters which regulate perfusion locally in response to metabolic needs, and shunts which participate in thermoregulation.
One of the earliest papers concerning this issue was a report by Stern et al[5] in 1975. They performed an experiment to determine the feasibility of the method of coherent light scattering, in their case from a fingertip. They were able to demonstrate rapid microvascular reflexes which no other method was able to demonstrate at that time.
When a beam of light, carried by the fibre-optic probe, enters the tissues and hits moving blood cells in a random order, it undergoes changes in wavelength - a Doppler shift[6] - while the wavelength of light hitting static tissue structures is unchanged. The magnitude and frequency distribution of these changes in wavelength are directly related to the number of moving blood cells, but relatively unrelated to their direction of movement.
The tissue volume occupied by moving blood cells is generally small; the average capillary density is about 50 capillaries per mm2 mucosal area, and most photons do not undergo a frequency shift, but are backscattered or absorbed[7]. The backscattered and Doppler broadened (extended) light carries information about the speed and concentration of blood cells traversing the scattering volume[8].
The quantity that is measured in LDPM is generally referred to as perfusion, and expressed in Perfusion Units (PU) which are arbitrarily chosen. In general it is not possible to change PU values into blood flow expressed as mL/min per g tissue; but, it can be done in specific preparations when calibration can be done. Perfusion is defined as the product of local velocity and concentration of blood cells[8]. Speed refers only to the magnitude (mm/s) of the velocity vector, and even though the majority (99%) of blood cells in the undisturbed microcirculation are red cells, LDPM does not selectively measure red cells.
Penetration into the tissue explored depends on the wavelength of the emitted light, and is regulated by differences in fibre diameter/separation. Penetration depth is also influenced, to a great extent, by factors such as structure and density of the capillary bed. The measuring depth is often defined as the depth below the tissue to which approximately 2/3 of the surface light penetrates, and returns back to the tissue surface. A typical probe today is designed using a solid-state laser with a wavelength of 780 nm, one transmitting and one receiving fibre and a fibre separation of 0.25 mm. This could lead to a sample depth of about 0.5-1.0 mm, and the sample volume could be estimated to 1 mm3. This rather shallow measuring depth was the conclusion of several different studies published in the eighties[9–13]; but, other studies executed during the same period suggested that LDPM had a capacity for transmural measuring in the GI tract[14–17]. During the nineties a consensus was reached that LDPM monitors the microcirculation only in the mucosa and the upper submucosa of the GI tract.
Calibration generally has several purposes: to check the stability of the instrument; to establish the linearity of the instrument’s response to blood flow; to establish a relationship between different instruments; and to relate the reading of the instrument to true perfusion, if possible. A gold standard for calibration of LDPM does not exist, and because the optical properties and distribution of blood vessels in the tissue are heterogeneous it is not realistic to calibrate the instrument to measure absolute blood flow. Therefore, the manufacturers of these instruments have provided a more simple calibration protocol, based on a two-point calibration, which makes it easy to calibrate the probes in a clinical or experimental situation. The motility standard is an aqueous suspension of polystyrene microspheres in Brownian motion. The method has some major shortcomings[8] regarding its dynamic properties, and the suspension induces Doppler shifts which give rise to a homodyne measurement. However, in living tissues, the opposite situation is seen and a heterodyne spectrum is produced because the majority of photons do not undergo a Doppler shift.
During the early years of LDPM, the method was validated against well established methods for measuring blood flow, such as the electromagnetic method. However, this method clearly measures total blood flow, not just blood flow in the microcirculation[9]. Later validation was performed using alternative methods known to selectively measure perfusion of the mucosal or muscularis layers i.e. local isotope washout[12], radioactive labelled microspheres[11], and H2-clearance[1113]. Generally, it is not easy to evaluate these validation studies because the single point laser Doppler probe is measuring from a different and much smaller tissue volume. However, carefully executed experiments performed on preparations of canine stomach and intestinal wall showed excellent linear correlation between the LDPM signal obtained and total blood flow measured by the electromagnetic technique[910141518]. One of these studies was also the first one to show that the gastric mucosa can autoregulate its blood flow, independent of other layers of the wall[10].
LDPM has emerged as a research and clinical tool in the absence of other methods, because it is a continuous, non-invasive and real time method for measuring microvascular blood flow, and it is also sensitive for detecting rapid changes in perfusion in the capillary circulation. LDPM is easily used in the clinical setting; but, to do so, one must be aware of its limitations. It is very important to ensure that the normal action and physiological responses of the microcirculation are not ignored when using this method. To get an optimal result there are both environmental and physical factors to take into consideration. These should be limited or accounted for when doing an investigation, in order to obtain reproducible data. It is also important to realise that it is impossible to say what the exact blood flow for any tissue is, and to remember that the optical properties and microvascular architecture cannot be determined in advance.
Physiological factors to be considered are temperature (thermoregulation has a significant effect on the microcirculation), the position and motion of the probe relative to the tissue surface, anatomical site and mental stress. Food and drugs also have effects on the microcirculation.
Technical limitations such as motion artifacts, multiple sequential Doppler shifts, variations in the specification of instruments from different manufacturers, lack of exact knowledge of the depth of measurements, the instrument zero and/or biological zero[19] all have to be taken into consideration when analysing an investigation. There are several review articles published in recent literature describing these phenomena in more detail[82021].
The laser Doppler probe is a sensitive motion detector, and many extraneous sources of noise e.g. respiratory movements cause mechanical vibrations in the same frequency range as the laser Doppler shifts produced by moving cells in the tissues (mucosa). Muscle fasciculation, vasomotion, respiration or any tissue movement relative to the laser Doppler probe may add noise to the laser Doppler signal. The GI organs are inherently motile, and motility-induced artefacts always occur during LDPM. One could argue that it is just noise which is recorded from the GI tract; but, Kiel et al[10] showed that this is not the case, and that true, perfusion can be measured from the GI tract.
Currently available instruments for LDPM generally also measure and display total backscattered light, of which Doppler shifted light makes up just a small fraction. The unit of measurement of backscattered light is EV, whereas that of Doppler shifted light is mEV. The significance of total backscattered light is that when this is detected as stable; it is an indication of minimal motion artifacts between probe and tissue. One can argue that only when backscatter is stable can we assume that LDPM actually measures perfusion in the adjacent tissues and is not simply dominated by artefacts.
THE APPLICATION OF LASER DOPPLER PERFUSION MONITORING TO STUDY DISEASE PROCESSES IN GASTROINTESTINAL TISSUES
During the last 20-25 years numerous studies have been done in different parts of the GI tract using LDPM. The majority of studies have been done on animals or humans during anaesthesia or surgery. This gives much better control of factors which potentially might influence the measurements. In a fully awake human, it is much more complicated to do LDPM, especially in the upper GI tract. A survey of the literature indicates that research employing LDPM has focussed on a limited number of questions, primarily those evaluating the influence of drugs or surgical procedures on mucosal perfusion, especially in the upper GI tract[22] or cardiovascular system[23], and the influence of septic shock[24], portal hypertensive gastropathy[2526], or hepatic cirrhosis[2728]. LDPM has certainly been used in some other clinical settings, but less systematically.
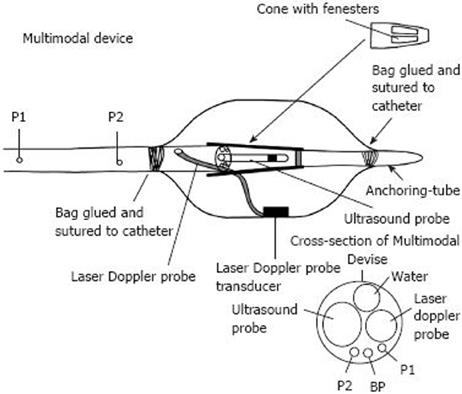
During recent years, we have been working with a multi-modal device (Figure 3) incorporating a laser Doppler probe, developing this device primarily in order to investigate patients suffering from functional chest pain of presumed oesophageal origin[29], an illness which is incompletely understood. Distending a bag in the oesophageal body typically reproduces the painful sensation in such patients but also elicits pain in a subset of healthy subjects[30–33]. The exact mechanism is unknown. We hypothesised that chest pain of presumed oesophageal origin could be due to a mechanical or an ischemic mechanism, leading to excitation of afferent nerves in the oesophageal wall.
Figure 3 The multi-lumen PVC catheter (Outer Diameter = 6.
0 mm) and a distal bag for acoustic coupling and symptom provocation. A water perfused manometric system measures pressures inside the bag (BP) and proximal to the bag at locations P1 and P2. The end of the multi-lumen catheter was attached to a fenestrated cone of polyethylene. The distal end of the cone was attached to a smaller end mounted catheter (anchoring-tube) for distal attachment to the bag. A 20 MHz ultrasound probe was placed in the centre of the bag and the transducer of the laser Doppler probe (780 nm) was fixed with double-sided tape to the inner surface of the bag. Modified from Hoff et al[34], 2006.
The multimodal catheter concept in gastroenterology was introduced in 2002 by Drewes and coworkers[32] who integrated technology for inducing electrical, mechanical, cold and heat stimuli into the same catheter device. We have developed the concept and technology further to include real time imaging with ultrasonography and LDPM[34]. As summarized in Figure 3, the device has a specially designed multi-luminal catheter as the central core, and a bag attached at its distal end. Inside the bag, as well as a sensor for measuring bag pressure, there is a radial 20 MHz miniature ultrasound probe (UM-3R, Olympus Corp, Tokyo, Japan) and a laser Doppler probe (LDP-415-253 (Perimed AB, Stockholm, Sweden). Its small size (10 mm × 6 mm × 4.5 mm, fibre diameter 140 &mgr;m, separation 250 &mgr;m, wavelength 780 nm) has facilitated its inclusion in the device. This is connected to a PF 5001 main unit with a PF 5010 LDPM Unit (Perimed AB).
In tests, high quality signals from manometry, LDPM and endosongraphy were obtained. The LDPM signal decreased moderately during bag distensions. Contractions characterized by high amplitudes and long duration were associated with a decrease in mucosal perfusion; but, minor fluctuations were also observed without contractile activity. During injection of 20 mg butylscopolamine bromide, fewer contractions were recorded and the LDPM signal fluctuated less.
LDPM can be obtained up to a depth of 1 mm with the type of equipment selected for our studies[8]. Hence, presumably at all degrees of bag distension, signals will originate primarily from the mucosa. Animal experiments have demonstrated residual compressive stresses in the mucosa-submucosa and tensile stresses in the muscle layers. This indicates more evenly distributed stress and strain throughout the oesophageal wall as also demonstrated in a study of the multilayered composite oesophagus by Liao and co-workers[35]. It is, therefore, likely that changes in perfusion throughout the wall are also evenly distributed. No current method can provide reliable flow data from the entire human oesophageal wall, and LDPM seems to be the best available choice, particularly for the multimodal device.
This is, to our knowledge, the first time LDPM has been included in a multimodal device for measuring perfusion in the oesophageal wall. Preliminary studies show the feasibility of the method; but, obviously the present material does not allow firm conclusions about whether GI pain is primarily of mechanical or ischemic origin. Future studies to look into ischemic- or strain-dependent pain mechanisms, may need to employ advanced distension protocols such as strain softening protocols.
CONCLUSION
Laser Doppler perfusion monitoring has emerged as a research and clinical tool in preference to other methods because it is non-invasive, and yields continuous and real-time measurements of microvascular blood flow. Furthermore, it is sensitive to rapid changes in perfusion in the capillary circulation. LDPM is easily used in the clinical setting; but, users have to be aware of its limitations and account for them when reporting results. LDPM can be included in multimodal devices, and we have demonstrated that simultaneous measurements of pressure, perfusion and ultrasound can be obtained from the oesophagus, when combined with bag distension. The quality of the data indicates that new insights can be obtained from studies in healthy volunteers and patients with functional chest pain. There are still some major challenges to face due to the fact that the method is highly motion-sensitive, and we cannot give the exact depth location from where perfusion measurements are obtained.
Peer reviewers: Yuji Naito, Professor, Kyoto Prefectural University of Medicine, Kamigyo-ku, Kyoto 602-8566, Japan; Shingo Tsuji, MD, PhD, AGAF, Professor, Department of Internal Medicine and Therapeutics, Osaka University Graduate School of Medicine (A8), 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan
S- Editor Li LF L- Editor O’Neill M E- Editor Ma WH