INTRODUCTION
Deep muscular and visceral pain is very frequent and causes major challenges in pain management[1]. Visceral pain is different from somatic pain because it is more diffuse, hard to localize, accompanied by autonomic reflexes, and very often described with an associated somatic referred pain. Pathophysiology and the mecha-nisms behind visceral pain conditions remain poorly understood. This lack of knowledge makes treatment of visceral pain challenging and often suboptimal.
Non-opioids are often insufficient in relieving pain to an acceptable level in patients suffering from severe pain originating from the gastrointestinal tract[2]. On the other hand, treatment with traditional &mgr;-opioid agonists may not be optimal. It often fails to relieve pain sufficiently, and at the same time causes side effects such as constipation, euphoria, sedation and nausea. These adverse effects are mainly mediated through &mgr;-receptors in the central nervous system (CNS). To encompass these problems, new therapeutic approaches have addressed opioids interacting with the peripheral κ-receptor, NMDA-antagonists and adjuvant analgesics (antidepressants and anticonvulsants)[3–7].
In experimental pain models, it is important to have a robust pain measure to obtain a reliable model and to detect the analgesic effect[8]. In standardized experimental human pain models, the investigator can control the induced pain (including modality, localization, intensity, frequency and duration), and provide quantitative measures of the responses. Hence, confounding factors such as sedation, nausea and general malaise that underlie clinical pain can, to a large extent, be controlled or avoided[9]. Different experimental stimulations can be used to induce visceral pain. Thermal, mechanical, electrical and chemical stimulations can be performed with a multi-modal approach, for which, different receptor types, pathways and mechanisms can be activated. This may act as a proxy for some of the mechanisms involved in clinical visceral pain conditions[10]. Such a multi-modal stimulus regimen can be used in conjunction with a multi-tissue approach in which skin, muscles and viscera are stimulated[9].
Pharmacokinetic and pharmacodynamic (PK-PD) modeling of analgesics can be used to identify and explain potential differences between the analgesic substances in their mechanism of alleviating pain. The effect depends on the concentration at the site of action. The site of action (biophase) for many analgesic substances is within the CNS, while most pharmacokinetic studies measure analgesic concentrations in plasma. When PK-PD modeling is performed, it is, therefore, important to account for a possible delay between plasma concentration and effect[11].
PK-PD MODELING
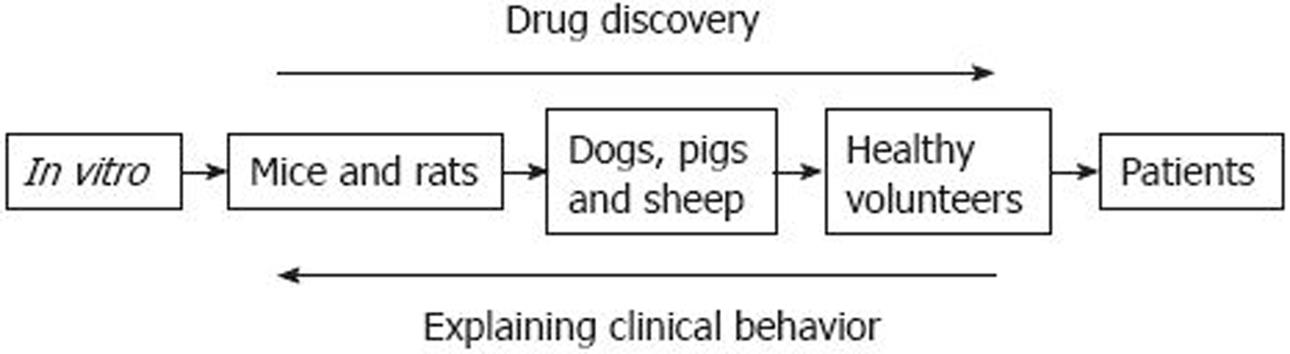
There are various approaches to the study of opioid pharmacokinetics and pharmacodynamics (Figure 1). These can be classified according to their relative advantages and disadvantages.
Figure 1 Understanding the effect of analgesics.
An overview of the different levels of investigation of analgesic effects. A good deal of attention has been focused on progressing from left to right in drug discovery. Less attention has been focused on progressing from right to left. Reproduced with permission from Upton et al[33].
Animal studies
These studies allow the investigation of fundamental mechanisms (such as cerebral equilibration rates) and the collection of arterial and venous blood concentration data. However, the dynamic information [e.g. tail flick times, changes in electroencephalography (EEG) or magnetic resonance imaging (MRI) signals] cannot be readily related to analgesia in humans. Representative studies include sheep studies performed at the University of Adelaide, Australia where a model to study the relationship between plasma and CNS concentration has been developed by Upton et al[1213]. They have previously used a sheep preparation to examine the cerebral kinetics and dynamics of analgesic drugs used in the perioperative period. Physiological PK-PD models developed in sheep have been adapted to assess the clinical profile of these drugs in humans[14]. One sheep study has shown that the faster analgesic onset with oxycodone compared to morphine might be explained by a faster equilibration between blood and brain for oxycodone[15].
Surgical patient studies
These studies are typically conducted in patients just before or during surgery when patients have an arterial cannula (often for patient management). As the patients are generally sedated (and cannot report pain) and the dose is high, the pharmacodynamic information is generally derived from changes in EEG. A representative study is that of Poyhia et al[16] in which EEG was used to quantify the CNS effects of oxycodone during anesthesia for primary coronary artery bypass grafting.
Volunteer and awake patient studies
For ethical reasons, these subjects usually only have a venous cannula placed in an arm vein. However, they can report highly relevant dynamic information such as pain and sedation scores, and can be studied using doses and routes that are directly relevant to clinical practice. Representative studies are the opioid study by Staahl et al[17], or the intranasal fentanyl studies of Christrup et al[18] and Foster et al[19]. Differences in the site of action for opioids may be reflected in the delay between opioid blood and CNS concentration and the analgesic effect. These differences might be more pronounced in diseases in which liver and kidney function are reduced or affected. Understanding these differences has implications for interpretation of PK-PD opioid studies, and provides insight into optimal clinical analgesic management of visceral pain[17]. A robust pain assessment is needed to obtain a reliable model of the PK-PD relationships for opioids. Experimental pain models in healthy volunteers provide less variable and less confounded pain measures, which are suitable for PK-PD modeling. A neurophysiological objective assessment of pain response and analgesic effect is EEG, which also can support the subjective findings in experimental pain studies. Previous investigations have shown that quantitative (spectral) analysis of the increase in delta frequency band of the resting EEG is a suitable biomarker for the PK-PD correlation of opioids[2021]. In a study of biophase kinetics within the PK-PD analysis of a wide range of opioids, morphine showed profound hysteresis between the blood pharmacokinetics and EEG effect[22]. Groenendaal et al[22] have concluded that within the wide range of opioids used in their study, only morphine displayed complex biophase distribution kinetics, which can be explained by its relatively low permeability of the blood-brain barrier and its interaction with active transporters present at the barrier.
TESTING OF ANALGESICS IN EXPERIMENTAL VISCERAL PAIN MODELS
Until now, only a few studies investigating the effect of analgesics in visceral experimental pain in healthy volunteers have been performed. Only two of these studies assessed the PK-PD relationship[1723].
Opioids
Morphine: Morphine is a highly potent opiate analgesic drug and is the principal active agent in opium, and is the prototypical opioid. Morphine is one of the few opioids to have been evaluated in human experimental visceral pain models[24]. Comparing somatic and visceral tissue, differences in opioid analgesia have been observed. Morphine does not affect somatic pain. Morphine analgesia is significantly better than placebo in attenuating mechanical and electrical esophageal pain, but not thermal esophageal pain.
Oxycodone: Oxycodone is a semi-synthetic opioid with analgesic effect and, as with morphine, has been evaluated in human experimental visceral pain models[24]. As for morphine, tissue differences in opioid analgesia have been observed. One study has shown that oxycodone is significantly better than placebo in attenuating mechanical, electrical and thermal esophageal pain. Furthermore, oxycodone has a superior effect on visceral pain compared to morphine[24]. This indicates that oxycodone may interact with other visceral opioid receptors more than morphine does. This reflects the clinical situation in which visceral pain, in contrast to somatic pain, can be difficult to treat with traditional &mgr;-opioid agonists[25].
Lalovic et al[23] have studied the pharmacokinetics and pharmacodynamics of oxycodone in healthy human volunteers; measurements included the time course of plasma concentrations and urinary excretion of metabolites, along with the time course of miosis, and subjective opioid side effects. The contribution of circulating metabolites to oxycodone pharmacodynamics has been analyzed by PK-PD modeling. The human study was complemented by in vitro measurements of opioid receptor binding and activation studies, as well as in vivo studies of the brain distribution of oxycodone and its metabolites in rats. Noroxycodone and noroxymorphone are the major metabolites in the circulation with elimination half-lives longer than that of oxycodone; but, their uptake into the rat brain is significantly lower compared with that of the parent drug. PK-PD modeling has indicated that the time course of pupil constriction in healthy volunteers is fully explained by the plasma concentration of the parent drug oxycodone. The metabolites do not contribute to the central effects, either because of their low potency or low abundance in circulation, or as a result of their poor uptake into the brain[23].
A pronounced delay between the plasma concentrations and analgesia will produce hysteresis when the analgesic effect is plotted against plasma concentration. This is characteristic for opioids and has been shown previously to be caused partially by the rate of transport of the opioid into the CNS (across the blood-brain barrier)[26], but also by receptor-mediated cascades[1327]. Traditionally, hysteresis is collapsed by the implementation of a theoretical effect compartment between the plasma compartment and effect (i.e. the effect is not delayed compared to the drug concentration in this compartment)[11].
In visceral pain assessments in healthy volunteers, obvious differences are seen between oxycodone and morphine PK-PD profiles. The effect of morphine is generally described through an effect compartment, whereas oxycodone tends to be more directly linked to the plasma concentration, because no hysteresis is produced[17]. This supports the results of Lalovic et al[23] and the theory that oxycodone acts partially at a peripherally located receptor. One hypothesis is that this peripherally located receptor is the κ receptor, since there is some evidence that oxycodone has a partial effect at the κ opioid receptor[2428]. In contrast to other opioid receptor types, for which central effects dominate, the peripheral κ receptor may also be important for visceral analgesia[32930]. Staahl et al[17] have confirmed that morphine and oxycodone have somewhat different PK-PD relationships in attenuation of visceral pain and, therefore, most likely act at receptors situated in different physiological compartments. These results partly address the question: to what extent do the cerebral pharmacokinetics of a drug contribute to its clinical behavior? Furthermore, they provide insight into optimal clinical analgesic management of visceral pain.
N-methyl-D-aspartic acid (NMDA)-antagonists
Ketamine is classified as an NMDA receptor antagonist. Ketamine has also been found to bind to opioid receptors. It has the added benefit of counteracting spinal sensitization or wind-up phenomena experienced with chronic pain. It is primarily used for the induction and maintenance of general anesthesia, usually in combination with some sedative drug, because otherwise unwanted psychological side effects can occur.
The analgesic effect of ketamine has been investigated in several experimental pain models. During visceral distension, pain and unpleasantness are decreased by ketamine[4]. Apparently, deep muscular or visceral pain is treated more successfully than superficial pain[431]. This supports the findings in other human studies in which deep pain activates central mechanisms (involving the NMDA receptor) such as summation more quickly than superficial pain does[32].
Hyperalgesia to electrical pain has been induced in the esophagus by acid infusion. It has been shown that ketamine prevents development of hyperalgesia and reverses induced hyperalgesia[5].
Antidepressants
Imipramine is a tricyclic antidepressant of the dibenza-zepine group. Imipramine is similar in structure to some muscle relaxants, and has a significant analgesic effect, and, therefore, is very useful in some pain conditions. As an example of experimental pain testing of the effect of imipramine, non-nociceptive sensation and pain to distension of the esophagus have been investigated by Peghini et al[6]. In this study, only stimulation within the painful range was affected, which shows a pain-specific action of imipramine.
Amitriptyline is a tricyclic antidepressant. In terms of its mechanism of action, amitriptyline inhibits serotonin and noradrenaline re-uptake almost equally. A pain model that involved esophageal and rectal distension was not sensitive to amitriptyline[7]. This study applied a rather uncontrolled stimulation paradigm in which there was a risk for bias in stimulus intensity[9]. Hence, more studies are necessary to determine if the stimulation paradigm caused the lack of sensitivity.
Regarding the studies of ketamine and the antidepressants, more knowledge could be obtained on their effects by combining these experimental studies with studies on pharmacokinetics. However, experimental human pain studies often lead to new information, and as such studies often consist of a very complicated protocol and setup, it is not always possible to study the pharmacokinetic profile in parallel.
CONCLUSION
To improve visceral pain treatment, it is important to study the underlying physiological mechanisms of the pain and the pharmacological mechanism of action of the different analgesics. Experimental human visceral pain research bridges the knowledge gap between animal studies and clinical studies in patients suffering from pain, making it an important tool in translational pain research, as illustrated in Figure 1. An experimental pain model activates different modalities and, therefore, explores the effect of analgesics. With further understanding of the cerebral pharmacokinetics and pharmacodynamics of analgesics, opportunities may emerge to improve the efficacy and safety of these drugs in clinical practice. In combination with PK-PD studies and objective assessments such as EEG, new information regarding a given drug, its dose regimen and its effects can be obtained. Thus, evaluation of pharmacokinetics and pharmacodynamics is needed in future drug research. It is of interest to study the effect of new drugs as well as drugs already on the market, as lack of knowledge on the pharmacokinetics and pharmacodynamics of analgesic agents makes treatment of visceral pain a difficult task, and often far from optimal.