Published online May 21, 2009. doi: 10.3748/wjg.15.2414
Revised: April 10, 2009
Accepted: April 17, 2009
Published online: May 21, 2009
Taking herbal-extracts to lose weight is an underestimated health hazard. Often, these products contain active agents that can cause acute liver damage. In this case report, a 22-year-old female patient, who presented with a feature of cholestatic syndrome, was so sure that the “natural products” were not dangerous that she did not inform her physicians that she had taken them, making their task that much more challenging. Clinical presentation mimicked acute cholecystitis and the patient underwent a cholecystectomy. Surgery was without any consequences and complications, although it did not completely cure the illness. She later admitted to having taken herbal remedies and this led to the correct diagnosis of phytotherapy-related hepatotoxicity and a successful therapeutic approach. The true incidence of phytotherapy-related hepatotoxicity and its pathogenic mechanisms are largely unknown. It is important to increase the awareness of both clinicians and patients about the potential dangers of herbal remedies.
- Citation: Tarantino G, Pezzullo MG, Dario di Minno MN, Milone F, Pezzullo LS, Milone M, Capone D. Drug-induced liver injury due to “natural products” used for weight loss: A case report. World J Gastroenterol 2009; 15(19): 2414-2417
- URL: https://www.wjgnet.com/1007-9327/full/v15/i19/2414.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2414
The desire to lose weight using “natural products” and the availability of these items can induce pathologies, the causes of which are often overlooked. The main problem with “natural products” is that the exact quantity and purity of a given ingredient contained in extracts of vegetable origin (mainly herbs) are largely unknown. It can happen that patients deny taking these products, thinking that they are “safe” because they are “natural” and thus physicians do not have the key facts to interpret dangerous pathologies. Here we report a case of a patient who experienced jaundice and pruritus, not assuming the “natural products” was at fault, with a clinical presentation highly suggestive of cholecystitis whose final diagnosis turned out to be drug-induced liver injury (DILI).
A 22-year-old obese (BMI 32) woman presented to hospital in May 2007 with a cholestatic syndrome of unknown origin. Her only declared pre-existing medication was paracetamol (500 mg daily), used as an analgesic for menstrual pain. Pre-admission blood tests were normal.
Symptoms included jaundice, pruritus, right upper quadrant pain and epigastric tenderness, accompanied by fever of low grade, nausea, vomiting, dark urine and pale stools. At the time of admission routine liver enzyme tests revealed bilirubin 128 &mgr;mol/L (< 20 &mgr;mol/L), alkaline phosphatase (ALP) 1229 U/L (40-110 U/L), γ-glutamyltransferase (GGT) 293 U/L (< 50 U/L), Aspartic-aminotransferase (AST) 1378 U/L (< 45 U/L) and Alanine-aminotransferase (ALT) 1686 U/L (< 40 U/L). She had never consumed alcohol and there was no recent travel history. Viral serology for hepatitis and HIV were negative. An infection screen was carried out because our country has an increasing incidence of exotic illnesses due to recent immigration that can cause transient liver enzyme derangement. This panel included cytomegalovirus, Epstein-Barr virus, Flavivirus, Dengue virus, Ross River virus, Barmah Forest virus, Spotted fever virus, Scrub Typhus, and Leptospirosis serology; all of them were unremarkable. Serum copper and caeruloplasmin, α-fetoprotein, α-1 antitrypsin, iron deposits were in the normal range. Anti-nuclear antibodies, perinuclear antineutrophil cytoplasmic antibodies, antibodies to liver kidney microsomal antigen type-1, anti-mitochondrial and anti-smooth muscle antibodies were negative. Her complete blood count was slightly abnormal. In fact, a modest increase in WBCs without left shift was present on admission, concurrent with an increase in eosinophils (7%) and a decrease in lymphocyte counts.
Abdominal ultrasound revealed the presence of microcalculi in the gallbladder. No clear dilatation of the common bile duct was seen, but the exam was performed in the presence of marked abdominal meteorism.
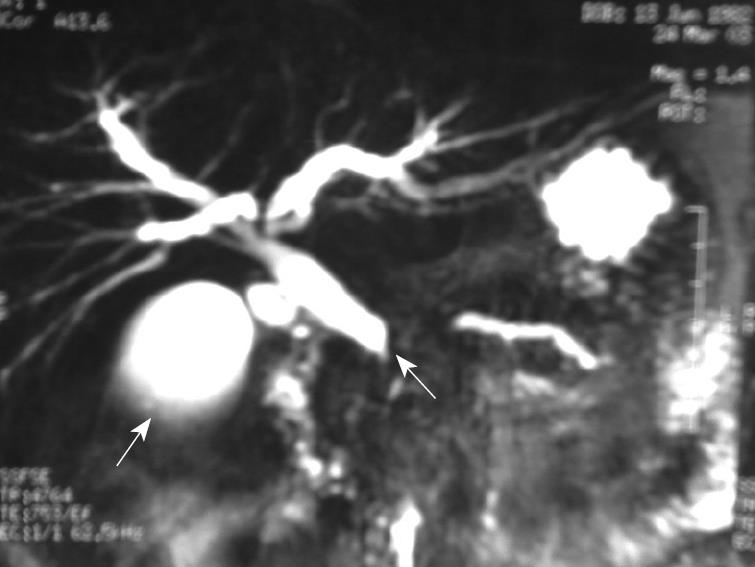
A magnetic resonance cholangio-pancreatography (MRC) indicated a dilatation of the choledocus with a likely interruption of its terminal tract, with some evidence of microstones in the gallbladder (Figure 1). Consequently, physicians empirically treated this illness by imipenem/cilastatin (500/500 mg every 6 h) for seven days.
A negative history of drug use, the physical findings (i.e. right upper quadrant and epigastric tenderness in the absence of peritoneal findings), laboratory data (i.e. elevated levels of bilirubin, alkaline phosphatase, ALT, and γ-glutamyltransferase) were consistent with extrahepatic obstruction, suggesting stones complicated by acute cholecystitis. Therefore, to gain access to and/or remove impacted common bile duct (CBD) stones at the ampulla of Vater, the patient was submitted to a preoperative endoscopic retrograde cholangiopancreatography (ERCP) with endoscopic sphincterotomy and extraction of sand-like stones.
Using this standard therapy, her laboratory data improved, with AST and ALT values of 1110 U/L and 1225 U/L, respectively. At this point, a laparoscopic operative CBD exploration with mini-invasive technique was planned.
The abdominal inspection showed the presence of extensive visceral adherences, as well as a general aspect of diffuse bowel inflammation. The gallbladder was reddish-colored with a thick wall and bled easily. This was suggestive of a complex abdominal pathology, therefore a conversion to an open procedure was chosen.
Abdominal exploration and biliary manometry caused the surgeons to utilize an ante-grade trans-ampullary intra-operative endobiliary stenting. The macroscopic examination showed acute cholecystitis with moderate wall-thickening containing very dense bile and some small stones.
The postoperative course was pain-free and the patient was prematurely discharged with drastically reduced (though elevated compared to normal) enzymatic values of AST 108 U/L and ALT 156 U/L.
Four days later the patient underwent liver laboratory tests that underlined an elevation of the transaminases, with an AST level of 644 U/L, ALT of 810 U/L, AP of 806 U/L and bilirubin of 64 &mgr;mol/L, mainly unconjugated with a persistently draining T-tube.
An interrogation of the woman’s relatives highlighted that the patient had taken several doses of a phyto-preparation in solution, bought from a store run by an herbalist, labeled as “herbal therapy for losing weight”. Fortunately, the relatives found this preparation, containing “Lycopodium serratum and Chelidonium maius” at home. The Roussel Uclaf Causality Assessment Method, also known as Danan’s international consensus criteria[1], was developed to quantify the strength of association between liver injury and herbal remedies and implicated the phyto-preparation as causing the injury. The case was adjudicated by three reviewers (DC, GT, MND) working independently and the patient was diagnosed as likely to be suffering from DILI. One month after given up taking the herbal remedy, all the liver parameters returned to normal values, without any apparent consequences. The case was re-adjudicated after a four-week follow-up by the same reviewers and the diagnosis was confirmed.
The “chelidonium majus” belongs to the family of papaveraceae; its roots contain the biologically active components chelerythrine and sanguinarine. The active “principia” are similar to those of opium, and have well-known hepatotoxic effects[2–4], although in animals an average daily oral dose of alkaloids up to 5 mg/kg has been proven to be safe[5].
The herb “Lycopodium serratum” has several[6] active agents that can cause hepatotoxicity[7]. The hepatic damage caused by these agents, generally of a cholestatic type, is possibly mediated by an idiosyncratic or hypersensitivity reaction. Recently, a different hypothesis was proposed involving an impairment of mitochondrial respiration[8].
Although this is not the first case reported in the literature, its importance lies in the atypical presentation. Indeed, was this a case of misdiagnosis, the co-existence of two diseases or an uncommon manifestation of DILI with a clinical presentation mimicking an other disease? The results of liver laboratory tests and imaging studies were attributed to an earlier combination of symptomatic gallstones and cholangitis and the patient was treated accordingly. Unfortunately, a liver biopsy, which would have indicated the presence of canalicular cholestasis with bile plugs in dilated canaliculi, occasional portal tracts containing a prominent lymphocytic infiltrate with mild piecemeal necrosis, was not performed and consequently the opportunity for a definitive diagnosis was lost.
Gallstone disease remains one of the most common medical problems. The risk factors predisposing to gallstone formation include obesity, diabetes mellitus, estrogen and pregnancy, hemolytic diseases, and cirrhosis. Acute cholecystitis can carry the risk of complications, including empyema, perforation, abscess, peritonitis and sepsis. Acute cholecystitis also causes acute pain in the right upper quadrant (RUQ). However, cross-sectional imaging is essential, because more than one-third of patients with acute RUQ pain do not have acute cholecystitis. Today, laparoscopic cholecystectomy, laparoscopic common bile duct exploration, and endoscopic retrograde management of CBD stones play important roles in the treatment of gallstones, even though the treatment of choice remains cholecystectomy. However, when asymptomatic gallstones are detected during the evaluation of a patient, a prophylactic cholecystectomy is normally not indicated because of several factors. Only about 30% of patients with asymptomatic cholelithiasis will warrant surgery during their lifetime, suggesting that cholelithiasis is a relatively benign condition in some people.
The main question we should ask ourselves is: was surgery the right choice? Although the patient’s symptoms and signs were extremely atypical for establishing the diagnosis of acute cholecystitis in this young immune-competent patient, her declaration of not having taken any other medications, including over-the-counter medications, herbal or traditional medicines, definitely misled physicians. The late admission by her parents allowed a correct diagnosis of DILI and not acute cholecystitis. Given the diagnosis of DILI, the patient took a further risk with anesthesia.
Should physicians have performed further studies before surgery? A CT cholangiogram would have shown contrast material being excreted by the renal tract, suggesting that the pathology concerned hepatocellular damage rather than a biliary obstruction.
Was the patient incautiously discharged from the surgery unit? The answer is probably yes, because the reduction of liver enzymatic activity caused surgeons to underestimate the pathology, with overconfidence in the previous diagnosis of cholecystitis.
There are many examples of hepatotoxicity induced by herbal remedies, which have been widely used in recent decades as weight loss agents. Germander (Teucrium chamaedrys) extracts cause DILI, probably mediated by furano neoclerodane diterpenoids[9]. Chaparral is a desert shrub traditionally used by Native Americans for treatment of several ailments. Recently, preparations of chaparral leaves have been marketed as weight loss agents. The mechanism of chaparral toxicity involves its active ingredient, nordihydroguaiaretic acid[10]. Kava (kava kava, awa, or kew), derived from the dried root and rhizome of Piper methysticum, has recently been marketed as an anxiolytic and mood enhancer. Recent studies from Europe have described cases of kava-associated hepatic injury. The mechanism of hepatic injury appears to be immune-mediated, with CYP2D6 deficiency perhaps being a risk factor[11]. Herba Ephedrae (from Ephedra sinica and other Ephedra species) is a traditional Chinese extract also used for treatment of asthma, nasal congestion, and fever. Although most adverse effects of Herba Ephedrae are cardiovascular or neurological, 4% of reports mentioned acute hepatitis. Herba Ephedrae contains phytochemicals, which are thought to strengthen its toxic activity[12].
In addition to the above supplements, liver injury has been attributed to other botanical agents. The pyrrolizidine alkaloids found in comfrey leaves and Heliotropium, Senecio, and Crotalaria species are known to cause veno-occlusive disease of the liver via a toxic effect[13]. Mixtures of valerian and skullcap (Valeriana officinalis and Scutellaria lateriflora) have induced hepatitis via alkylating agents. LipoKinetix was marketed as a dietary supplement for weight loss. Hepatic injury appears to be due to an idiosyncratic reaction, perhaps related to phenylpropanolamine[14]. Among other weight loss agents, Usnic acid should be suspected in case of severe hepatotoxicity[15].
In our case, the patient was sure the product was harmless and denied the use of a potentially dangerous product in her history, thus not allowing physicians to discover the etiology of the serious pathology from which she suffered. Only an accurate interrogation of relatives was able to discover the relationship between the herbal remedy and DILI.
The diagnostic approach was, in spite of the lack of a certain etiology, the most cautious possible; in fact, MRC and ERCP, perfectly framed into the clinical picture of this patient, are generally considered investigations of first level.
The prevalence of adverse drug reactions (ADR) in health care systems has generated immense interest in recent years. Some of these adverse events are completely unpredictable, but some result from medical errors, patient’s negligence or ignorance, and may occur anywhere and at anytime in the health care processes. However, a majority of them may be preventable. The consequences of these ADRs might vary, from little or no harm to ultimately being fatal to the patients.
Patient safety has received increased attention in recent years, but mostly with a focus on the epidemiology, rather than on practices that reduce (1) ADRs, (2) adverse events related to exposure to herbal remedies and dietary supplements and (3) invasive procedures in medical care involving a wide spectrum of diagnoses or conditions. Potential safety practices should be identified, based on preliminary surveys of the literature and expert consultation.
The misdiagnosis of DILI has many ramifications. These include medical and psychological implications for patients and their families, and financial and public health implications for health-care institutions.
The patient could have sued the health care practitioners (specifically the surgeons) if she felt she had been injured. However, successful medical malpractice lawsuits require proof of the following items: the care provided was below the ordinary standard of care that would be provided by similar health care practitioners under similar circumstances and the patient was harmed because of the deviation from the standard of care. In our case, concerns about lawsuits did not arise, because the physicians’ actions were in the best interests of the patient. In fact, a good defence against malpractice lawsuits is to provide excellent medical care and to build close, trusting, and collaborative relationships with patients.
As a final consideration, patients should be especially cautious about using drugs, and should inform their doctor about any drugs or other substances they are taking, including prescription and over-the-counter medications, recreational drugs, herbal remedies, and nutritional supplements. Health care professionals are encouraged to report all ADRs, especially hepatotoxicity and to pay much more attention in prescribing and administering drugs.
For the vegetal abstracts, it should be mandatory to correctly describe their contents, taking in account the active ingredients, the real quantity per unit of product contained with the preparation, and to make clear any possible side effects.
In conclusion, the authors believe that a detailed, painstaking and meticulous history could have unveiled the underlying condition and the patient would not have been subjected to invasive and potentially harmful interventions. This is probably the most important learning point that emerged from this case report.
| 1. | Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323-1330. |
| 2. | Conti E, De Checchi G, Mencarelli R, Pinato S, Rovere P. Lycopodium similiaplex-induced acute hepatitis: a case report. Eur J Gastroenterol Hepatol. 2008;20:469-471. |
| 3. | Stickel F, Pöschl G, Seitz HK, Waldherr R, Hahn EG, Schuppan D. Acute hepatitis induced by Greater Celandine (Chelidonium majus). Scand J Gastroenterol. 2003;38:565-568. |
| 4. | Crijns AP, de Smet PA, van den Heuvel M, Schot BW, Haagsma EB. [Acute hepatitis after use of a herbal preparation with greater celandine (Chelidonium majus)]. Ned Tijdschr Geneeskd. 2002;146:124-128. |
| 5. | Kosina P, Walterová D, Ulrichová J, Lichnovský V, Stiborová M, Rýdlová H, Vicar J, Krecman V, Brabec MJ, Simánek V. Sanguinarine and chelerythrine: assessment of safety on pigs in ninety days feeding experiment. Food Chem Toxicol. 2004;42:85-91. |
| 6. | Takayama H, Katakawa K, Kitajima M, Yamaguchi K, Aimi N. Ten new Lycopodium alkaloids having the lycopodane skeleton isolated from Lycopodium serratum Thunb. Chem Pharm Bull (Tokyo). 2003;51:1163-1169. |
| 7. | Woolf GM, Petrovic LM, Rojter SE, Wainwright S, Villamil FG, Katkov WN, Michieletti P, Wanless IR, Stermitz FR, Beck JJ. Acute hepatitis associated with the Chinese herbal product jin bu huan. Ann Intern Med. 1994;121:729-735. |
| 8. | Choy CS, Cheah KP, Chiou HY, Li JS, Liu YH, Yong SF, Chiu WT, Liao JW, Hu CM. Induction of hepatotoxicity by sanguinarine is associated with oxidation of protein thiols and disturbance of mitochondrial respiration. J Appl Toxicol. 2008;28:945-956. |
| 9. | Stickel F, Egerer G, Seitz HK. Hepatotoxicity of botanicals. Public Health Nutr. 2000;3:113-124. |
| 10. | Gordon DW, Rosenthal G, Hart J, Sirota R, Baker AL. Chaparral ingestion. The broadening spectrum of liver injury caused by herbal medications. JAMA. 1995;273:489-490. |
| 11. | Russmann S, Lauterburg BH, Helbling A. Kava hepatotoxicity. Ann Intern Med. 2001;135:68-69. |
| 12. | Lee MK, Cheng BW, Che CT, Hsieh DP. Cytotoxicity assessment of Ma-huang (Ephedra) under different conditions of preparation. Toxicol Sci. 2000;56:424-430. |
| 13. | Whiting PW, Clouston A, Kerlin P. Black cohosh and other herbal remedies associated with acute hepatitis. Med J Aust. 2002;177:440-443. |
| 14. | Lake CR, Gallant S, Masson E, Miller P. Adverse drug effects attributed to phenylpropanolamine: a review of 142 case reports. Am J Med. 1990;89:195-208. |
| 15. | Sanchez W, Maple JT, Burgart LJ, Kamath PS. Severe hepatotoxicity associated with use of a dietary supplement containing usnic acid. Mayo Clin Proc. 2006;81:541-544. |