Published online May 21, 2009. doi: 10.3748/wjg.15.2361
Revised: April 13, 2009
Accepted: April 20, 2009
Published online: May 21, 2009
AIM: To assess the ability of endoscopic ultrasonography (EUS) to differentiate neoplastic from non-neoplastic polypoid lesions of the gallbladder (PLGs).
METHODS: The uses of EUS and transabdominal ultrasonography (US) were retrospectively analyzed in 94 surgical cases of gallbladder polyps less than 20 mm in diameter.
RESULTS: The prevalence of neoplastic lesions with a diameter of 5-10 mm was 17.2% (10/58); 11-15 mm, 15.4% (4/26), and 16-20 mm, 50% (5/10). The overall diagnostic accuracies of EUS and US for small PLGs were 80.9% and 63.9% (P < 0.05), respectively. EUS correctly distinguished 12 (63.2%) of 19 neoplastic PLGs but was less accurate for polyps less than 1.0 cm (4/10, 40%) than for polyps greater than 1.0 cm (8/9, 88.9%) (P = 0.02).
CONCLUSION: Although EUS was more accurate than US, its accuracy for differentiating neoplastic from non-neoplastic PLGs less than 1.0 cm was low. Thus, EUS alone is not sufficient for determining a treatment strategy for PLGs of less than 1.0 cm.
- Citation: Cheon YK, Cho WY, Lee TH, Cho YD, Moon JH, Lee JS, Shim CS. Endoscopic ultrasonography does not differentiate neoplastic from non-neoplastic small gallbladder polyps. World J Gastroenterol 2009; 15(19): 2361-2366
- URL: https://www.wjgnet.com/1007-9327/full/v15/i19/2361.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2361
Polypoid lesions of the gallbladder (GB) are increasingly detected by ultrasonography (US). Indeed, 4%-7% of healthy individuals have been reported to have polyps of the GB[12]. The significance of these polypoid lesions is poorly understood, and the appropriate management of these lesions is controversial. Although most GB polyps are benign, some early carcinomas of the GB share the same appearance as benign polyps. Currently, GB polyps larger than 1 cm should be surgically removed because of the increased risk of malignancy[3]. On the other hand, patients with smaller polyps usually require repeated US and follow-up. Distinguishing between non-neoplastic, neoplastic, and potentially malignant lesions is a major diagnostic dilemma, and the therapeutic options for these lesions remain controversial.
Endoscopic ultrasonography (EUS) is considered to be superior to conventional US for imaging GB lesions, because EUS can provide high-resolution images of small lesions with higher ultrasound frequencies (7.5-12 MHz vs 3.5-5 MHz)[45]. The improved accuracy of EUS in imaging small GB lesions has been previously reported in a surgical series[5–7]. However, polyps with a maximum diameter of less than 10-15 mm are difficult to differentially diagnose in many cases. The present study assesses the predictive value of EUS in the differential diagnosis of small polypoid lesions (maximum diameter, ≤ 20 mm) of the GB in a surgical series.
Between 1996 and 2006, 365 patients underwent EUS for small (maximum diameter, ≤ 20 mm) polypoid lesions of the GB detected by transabdominal US. Of these 365 patients, 94 patients who underwent laparoscopic cholecystectomy for GB polyps were enrolled. US was performed as an abdominal screening test for asymptomatic patients or as a detailed examination for patients suspected of having a gastrointestinal disorder because of clinical symptoms. When US revealed polypoid lesions inside the GB, the patient then underwent EUS. In principle, EUS was indicated for polypoid lesions exceeding 5 mm or for potentially neoplastic polyps. Patients with localized adenomyomatosis or diffuse wall-thickening lesions resulting from inflammation were excluded from the study.
All patients with suspected neoplastic lesions based on EUS underwent surgery. Generally, surgery was not indicated for patients with a EUS diagnosis of non-neoplastic lesions, except for symptomatic cases or patients undergoing combined operations for other abdominal diseases. In our surgical series, the EUS diagnosis was compared with the histopathological diagnosis. Based on the pathological evaluation of specimens obtained upon cholecystectomy, the GB polyps were assigned into two groups: neoplastic (adenoma and carcinoma) and non-neoplastic (cholesterol, inflammatory, and fibrous). In patients with multiple polyps, the size of the largest polyp was measured.
Demographics and EUS findings were prospectively collected at the time of the procedure and were analyzed retrospectively. EUS was performed by one of the authors with knowledge of the ultrasonographic findings. In all cases, the differential diagnosis of polypoid lesions of the GB by EUS and US was made according to the criteria outlined below[56].
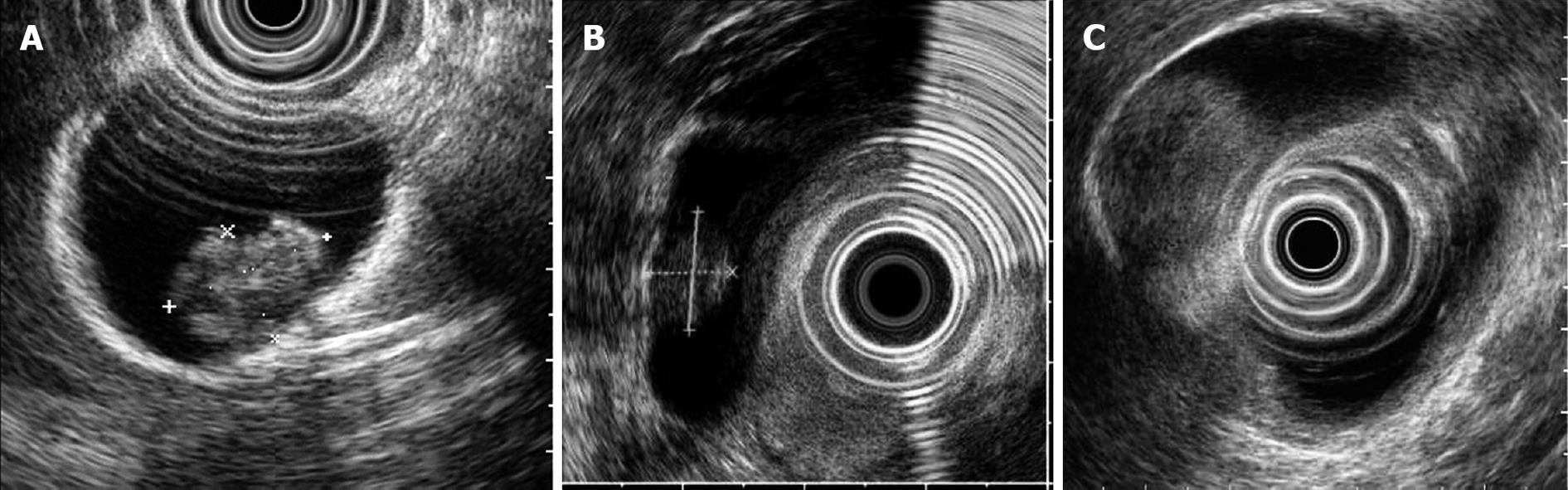
Cholesterol polyps (Figure 1A) are pedunculated lesions with a granular surface. The internal echo is hyperechoic to isoechoic with a tiny, spotty echo pattern. Relatively large polyps, those greater than 10 mm in diameter, may not give the typical image but may have a spotty echo area. Localized adenomyomatosis is imaged as a sessile echogenic mass containing multiple microcysts or with a comet tail artifact. Neoplastic polyps (adenoma, Figure 1B or carcinoma, Figure 1C) are pedunculated or sessile masses without echogenic spots, multiple microcysts, or comet tail artifacts; the internal echo is hypoechoic to isoechoic and almost homogeneous.
Transabdominal US was performed using a real-time scanner with a 3.5-MHz curved array transducer (SSD-2000; Aloka, Tokyo, Japan). EUS was performed using an echoendoscope with a 7.5-MHz or 12-MHz radial sector scan transducer (GF-UM2, UM3, UM20; Olympus Co., Tokyo, Japan). The GB was visualized from the duodenum and gastric antrum. For sedation, 5 mg of midazolam were administered intravenously.
The results were analyzed by Fisher’s exact probability test or the Wilcoxon test, as appropriate. Differences were considered significant at P < 0.05.
Of the 94 patients, 19 had neoplastic lesions and 75 had non-neoplastic lesions. The mean age of the patients with non-neoplastic polyps was 50 ± 12.5 years, and that of patients with neoplastic polyps was 51 ± 11.3 years. Most of the non-neoplastic polyps were cholesterol polyps (56/75, 74.7%). Seventeen polypoid lesions were adenomyomatosis, and two polyps were inflammatory polyps. Adenocarcinoma was found in two patients; and adenomas, in 17. Two of the 17 adenomas contained focal high-grade dysplasia. The prevalence of neoplastic lesions with a diameter of 5-10 mm was 17.2% (10/58); 11-15 mm, 15.4% (4/26), and 16-20 mm, 50% (5/10) (Table 1). The average size of non-neoplastic polyps was 9.8 ± 2.8 mm (5-18). Among neoplastic polyps, the average size of an adenoma without high grade dysplasia, adenoma with high grade dysplasia, and adenocarcinoma were 9.9 ± 3.6 mm (6-17), 12.0 mm (7 and 17), and 19.0 mm (13 and 25), respectively. The average size of neoplastic polyps including adenoma with high grade dysplasia and carcinoma tended to be larger than neoplastic polyps without high grade dysplasia and non-neoplastic polyps.
| Size (mm) | Cholesterol | Adenomyomatosis | Inflammatory | Adenoma | Cancer | Total |
| 5-10 | 39 | 9 | 0 | 10 | 0 | 58 |
| 11-15 | 14 | 7 | 1 | 3 | 1 | 26 |
| 16-20 | 3 | 1 | 1 | 4 | 1 | 10 |
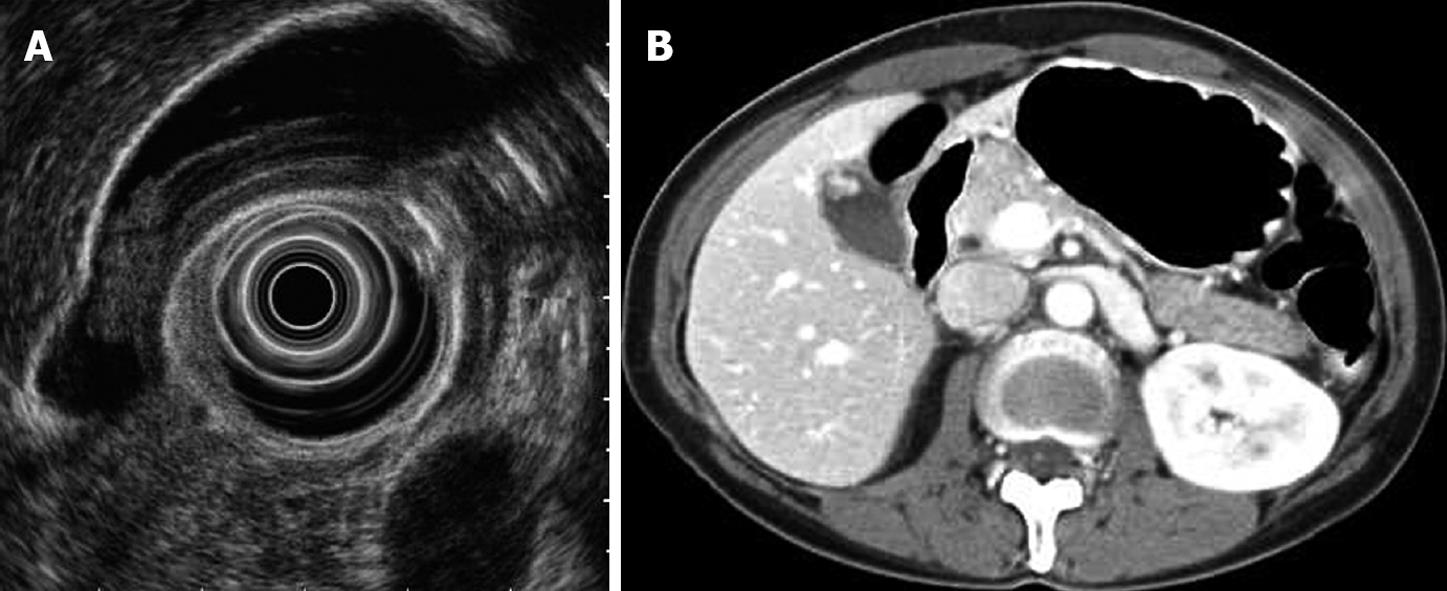
Differential diagnosis by EUS and US was successful in 70 (74.5%) and 54 (57.4%) of 94 patients, respectively; the difference between these rates was significant (P = 0.014). When the results of EUS were assessed according to the pathological results (Table 2), cholesterol polyps were correctly identified in 47 of 56 patients (83.9%). The unsuccessful diagnoses included nine cases that were misjudged as adenoma. Adenomyomatosis was correctly identified in 11 of 17 patients (64.7%). Among the six misdiagnosed cases, four were cholesterol polyps and two were neoplastic polyps. Neoplastic polyps were correctly identified in 12 of 19 patients (63.2%). The unsuccessful diagnoses included seven cases misjudged as cholesterol polyps. Five of the seven cases were less than 1.0 cm in size, and another of the cases was 17 mm in size before surgery. EUS showed a homogeneously isoechoic, pedunculated mass, and abdominal CT showed an enhanced polypoid mass of the GB in the arterial phase. Therefore, we diagnosed it as an early cancer. However, this polyp was confirmed to be a cholesterol polyp after cholecystectomy (Figure 2). Of the 19 neoplastic polyps, two were adenocarcinoma, with diameters of 10 and 19 mm, respectively.
| Pathologic diagnosis (n) | ||||
| Cholesterol polyp | Adenomyomatosis | Inflammatory polyp | Neoplastic lesions | |
| EUS diagnosis | ||||
| Cholesterol | 47 | 4 | 0 | 7 |
| Adenomayomatosis | 0 | 11 | 1 | 0 |
| Neoplastic lesion | 9 | 2 | 1 | 12 |
| US diagnosis | ||||
| Cholesterol | 41 | 8 | 0 | 10 |
| Adenomayomatosis | 0 | 4 | 1 | 0 |
| Neoplastic lesion | 15 | 5 | 1 | 9 |
Table 3 shows the EUS results categorized according to the size of the polypoid GB lesion (< 10 mm vs 10-20 mm). Of the 58 cases with a diameter less than 10 mm, EUS correctly distinguished 84.6% (33/39) of the cholesterol polyps, 36.4% (4/11) of the adenomyomatosis, and 50.0% (4/8) of the neoplastic lesions. Of the 36 cases with polyps greater than 10 mm in diameter, EUS correctly distinguished 82.4% (14/17) of the cholesterol polyps, 87.5% (7/8) of the adenomyomatosis, and 88.9% (8/9) of the neoplastic lesions. The accuracy of EUS in diagnosing neoplastic lesions tended to be lower for polyps greater than 10 mm (79.7%) than for polyps less than 10 mm (83.3%) (P = 0.12). There was no significant difference between EUS and US in the diagnosis of cholesterol polyps.
| Pathology (n) | ||||||
| Cholesterol | Adenomyomatosis | Neoplastic lesion | ||||
| Size (mm) | 5-10 | 11-20 | 5-10 | 11-20 | 5-10 | 11-20 |
| EUS | ||||||
| Cholesterol | 33 | 14 | 0 | 0 | 6 | 3 |
| Adenomyomatosis | 6 | 0 | 4 | 7 | 1 | 1 |
| Inflammatory | 0 | 0 | 0 | 1 | 0 | 1 |
| Neoplastic lesions | 4 | 1 | 0 | 0 | 4 | 8 |
| US | ||||||
| Cholesterol | 32 | 9 | 0 | 0 | 7 | 8 |
| Adenomyomatosis | 7 | 1 | 1 | 3 | 1 | 4 |
| Inflammatory | 0 | 0 | 0 | 1 | 0 | 1 |
| Neoplastic lesions | 8 | 2 | 0 | 0 | 2 | 7 |
Table 4 summarizes the results of differential diagnoses between neoplastic and benign polyps assessed by EUS and US. The overall sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for EUS (US) in the diagnosis of neoplastic lesions were 66.7% (47.5%), 84.2% (72.0%), 50.0% (30.0%), 91.4% (84.4%), and 80.9% (67.0%), respectively (Table 5). When the results for relatively smaller polyps (diameter, < 10 mm) and for larger polyps (diameter, > 10 mm) were considered separately, the sensitivity, specificity, PPV, NPV, and accuracy for US were all lower than the values for EUS in both groups. The values for EUS in polyps less than 1.0 cm in diameter were lower than those in polyps greater than 1.0 cm in diameter.
| Diagnosis by postoperative histological examination | ||
| Neoplastic polyp | Non-neoplastic polyp | |
| Diagnosis by EUS | ||
| Neoplastic polyps | 12 | 12 |
| Non-neoplastic polyps | 6 | 64 |
| Diagnosis by US | ||
| Neoplastic polyps | 9 | 21 |
| Non-neoplastic polyps | 10 | 54 |
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| EUS | |||||
| Overall | 66.7 | 84.2 | 50.0 | 91.4 | 80.9 |
| 5-10 mm | 44.4 | 86.0 | 36.4 | 89.6 | 79.7 |
| 11-20 mm | 88.9 | 81.5 | 61.5 | 95.7 | 83.3 |
| US | |||||
| Overall | 47.4 | 72.0 | 30.0 | 84.4 | 67.0 |
| 5-10 mm | 20.0 | 83.3 | 20.0 | 83.3 | 72.4 |
| 11-20 mm | 77.8 | 51.9 | 35.0 | 87.5 | 63.9 |
Owing to the widespread use of conventional US, an increasing number of polypoid lesions of the GB are being identified. However, it is difficult to make differential diagnoses of polypoid GB lesions by US, CT, and magnetic resonance imaging. In general, factors that increase the probability that a GB polyp will be malignant include age greater than 50 years, a solitary lesion, a polyp greater than 1.0 cm in size, the presence of gallstones, a sessile lesion, and a rapid change in lesion size on serial ultrasonography[89]. All of these factors should be taken into consideration when advising patients with a polypoid lesion of the GB (PLG). The correct surgical management of PLGs is controversial. Although it is widely agreed that patients with symptomatic PLGs should be offered cholecystectomy, preferably by the laparoscopic route, the best treatment for an asymptomatic patient is not clear. In cases with a high probability of a malignant lesion, such as a PLG larger than 2 cm, open surgery is preferred to reduce the risk of tumor seeding associated with laparoscopic surgery. For asymptomatic PLGs smaller than 1 cm, follow-up US every 6 to 12 mo is necessary to exclude a rapidly growing malignant tumor[3].
There are a number of reports suggesting that sessile lesions smaller than 1.0 cm have an increased incidence of malignancy compared with those with a stalk[10]. In this study, 10 of 19 (52.6%) neoplastic polyps were pedunculated lesions smaller than 1 cm. Sugiyama et al[11] reported that approximately 30% of polyps with a diameter of 11-15 mm were cholesterol polyps and that about 40% of neoplastic polyps were 6-10 mm in diameter. Kubota et al[12] found that 57% of cholesterol polyps, 75% of adenomas, and 13% of neoplastic polyps were less than 10 mm in diameter. Thus, for polyps less than 10 mm in diameter, criteria other than size, along with an aggressive work-up, are needed to discriminate between neoplastic and non-neoplastic polyps.
EUS is considered to be superior to conventional US for imaging GB lesions, because EUS can provide high-resolution images of small lesions at higher ultrasound frequencies (7.5-12 MHz vs 3.5-5 MHz). Many studies have investigated the relationship between the neoplastic nature of GB polyps and their morphological characteristics such as the number of polyps, the polyp shape, the diameter of the largest polyp, the echo level and internal echo pattern, and the polyp margin[12–14]. Among these variables, size is the most significant predictor of neoplastic polyps. However, the accuracy of EUS in identifying neoplastic lesions among polyps smaller than 10 mm in our study was only 44.4%, which was significantly lower than the identification rate among polyps greater than 1.0 cm (88.9%, P < 0.05).
To overcome the limitation of EUS in the differential diagnosis of neoplastic and non-neoplastic polypoid lesions less than 10-15 mm or less than 20 mm in size, an EUS scoring system has been adopted[715]. According to this system, the sensitivity, specificity, and accuracy of the risk for a neoplastic polyp were 81%, 86%, and 83.7%, respectively, for polyps with an EUS score of 6 or greater, whereas the sensitivity, specificity, and accuracy using a 10 mm cut-off diameter were 60%, 64%, and 62.7%, respectively[7]. Based on the EUS scoring system of Sadamoto et al[15], the sensitivity, specificity, and accuracy of the risk for neoplasia in polyps with scores of 12 or higher were 77.8%, 82.7%, and 82.9%, respectively. The EUS scoring system will be useful for differentiating between neoplastic and non-neoplastic polyps of the GB, however, the EUS variables used to calculate the score differ between the different EUS scoring systems.
The accuracy of EUS results tend to be lower for polyps smaller than 1 cm than for polyps greater than 1 cm in size. In our study, seven of 11 (63.6%) polyps less than 1.0 cm in size that were determined to be neoplastic by EUS before surgery were confirmed after surgery to be non-neoplastic lesions, including six cholesterol polyps and one adenomyomatosis. Using US, only two of 10 cases were determined to be neoplastic polyps after surgery. Thus, despite its higher accuracy compared with conventional US, EUS could not differentiate malignant from benign polyps smaller than 1.0 cm. No carcinoma was found in polyps less than 1.0 cm in size, but the prevalence of adenoma was 17.2% in our study.
Although EUS was more accurate than US, its accuracy for differentiating malignant from benign PLGs of less than 1.0 cm was low. EUS could not differentiate malignant lesions from benign polyps less than 1.0 cm in size, because such small polyps do not often show findings typical of cholesterol polyps, localized types of adenomyomatosis, or neoplastic lesions. Thus, EUS alone is not sufficient for determining a treatment strategy for PLGs of less than 1.0 cm. Polyps less than 1.0 cm in diameter without typical EUS or US findings should be followed-up by US at intervals of 6-12 mo. Changes in the size or structure of polypoid lesions should prompt reinvestigation with EUS and lead physicians to consider cholecystectomy.
The distinction between non-neoplastic, neoplastic and potentially malignant lesions is a major diagnostic dilemma and the therapeutic options for small polypoid lesions of the gallbladder remain controversial. Although endoscopic ultrasonography (EUS) was more accurate than ultrasonography (US), its accuracy for differentiating malignant from benign polypoid gallbladder lesions (PLGs) of less than 1.0 cm was low.
Among many variables, size is the most significant predictor of neoplastic polyps. Although EUS was more accurate than US, its accuracy for differentiating malignant from benign PLGs of less than 1.0 cm was low. Thus, EUS alone is not sufficient for determining a treatment strategy for PLGs of less than 1.0 cm.
Many studies have investigated the relationship between the neoplastic nature of gallbladder (GB) polyps and their morphological characteristics such as the number of polyps, the polyp shape, the diameter of the largest polyp, the echo level and internal echo pattern, and the polyp margin. Among these variables, size is the most significant predictor of neoplastic polyps. However, the accuracy of EUS results tends to be lower for polyps smaller than 1 cm than for polyps greater than 1 cm in size. To overcome the limitation of EUS in the differential diagnosis of neoplastic from benign PLGs, an EUS scoring system has been adopted. The EUS scoring system will be useful for differentiating between neoplastic and non-neoplastic polyps of the GB, however, the EUS variables used to calculate the score differ between the different EUS scoring systems. Thus, EUS alone is not sufficient for determining a treatment strategy for PLGs of less than 1.0 cm.
The study results suggest that EUS alone is not sufficient for determining a treatment strategy for PLGs of less than 1.0 cm. Thus, new diagnostic criteria of EUS or tools are needed to distinguish between non-neoplastic, neoplastic, and potentially malignant lesions in small PLGs.
Neoplastic gallbladder polyps: A neoplastic polyp has the properties of a neoplasm including adenoma and carcinoma. A non-neoplastic polyp does not have the properties of a neoplasm and includes cholesterol, inflammatory and fibrous polyps.
Although retrospective, this study involves a large series of patients and describes the role of EUS well. The results suggest that EUS alone is not sufficient for determining a treatment strategy for PLGs of less than 1.0 cm and a new diagnostic approach is needed.
| 1. | Segawa K, Arisawa T, Niwa Y, Suzuki T, Tsukamoto Y, Goto H, Hamajima E, Shimodaira M, Ohmiya N. Prevalence of gallbladder polyps among apparently healthy Japanese: ultrasonographic study. Am J Gastroenterol. 1992;87:630-633. |
| 2. | Chen CY, Lu CL, Chang FY, Lee SD. Risk factors for gallbladder polyps in the Chinese population. Am J Gastroenterol. 1997;92:2066-2068. |
| 4. | Muguruma N, Okamura S, Ichikawa S, Tsujigami K, Suzuki M, Tadatsu M, Kusaka Y, Okita Y, Yano M, Ito S. Endoscopic sonography in the diagnosis of gallbladder wall lesions in patients with gallstones. J Clin Ultrasound. 2001;29:395-400. |
| 5. | Azuma T, Yoshikawa T, Araida T, Takasaki K. Differential diagnosis of polypoid lesions of the gallbladder by endoscopic ultrasonography. Am J Surg. 2001;181:65-70. |
| 6. | Sugiyama M, Xie XY, Atomi Y, Saito M. Differential diagnosis of small polypoid lesions of the gallbladder: the value of endoscopic ultrasonography. Ann Surg. 1999;229:498-504. |
| 7. | Choi WB, Lee SK, Kim MH, Seo DW, Kim HJ, Kim DI, Park ET, Yoo KS, Lim BC, Myung SJ. A new strategy to predict the neoplastic polyps of the gallbladder based on a scoring system using EUS. Gastrointest Endosc. 2000;52:372-379. |
| 8. | Koga A, Watanabe K, Fukuyama T, Takiguchi S, Nakayama F. Diagnosis and operative indications for polypoid lesions of the gallbladder. Arch Surg. 1988;123:26-29. |
| 9. | Mainprize KS, Gould SW, Gilbert JM. Surgical management of polypoid lesions of the gallbladder. Br J Surg. 2000;87:414-417. |
| 10. | Ishikawa O, Ohhigashi H, Imaoka S, Nakaizumi A, Kitamura T, Sasaki Y, Shibata T, Wada A, Iwanaga T. The difference in malignancy between pedunculated and sessile polypoid lesions of the gallbladder. Am J Gastroenterol. 1989;84:1386-1390. |
| 11. | Sugiyama M, Atomi Y, Kuroda A, Muto T, Wada N. Large cholesterol polyps of the gallbladder: diagnosis by means of US and endoscopic US. Radiology. 1995;196:493-497. |
| 12. | Kubota K, Bandai Y, Noie T, Ishizaki Y, Teruya M, Makuuchi M. How should polypoid lesions of the gallbladder be treated in the era of laparoscopic cholecystectomy? Surgery. 1995;117:481-487. |
| 13. | Yang HL, Sun YG, Wang Z. Polypoid lesions of the gallbladder: diagnosis and indications for surgery. Br J Surg. 1992;79:227-229. |
| 14. | Collett JA, Allan RB, Chisholm RJ, Wilson IR, Burt MJ, Chapman BA. Gallbladder polyps: prospective study. J Ultrasound Med. 1998;17:207-211. |