Published online May 14, 2009. doi: 10.3748/wjg.15.2220
Revised: March 11, 2009
Accepted: March 18, 2009
Published online: May 14, 2009
AIM: To find a rapid and efficient analysis method of gastrointestinal microflora in Pi-deficient (spleen-deficient) rats and to evaluate traditional Chinese drugs.
METHODS: Enterobacterial repetitive intergenic consensus-PCR (ERIC-PCR) based assay was performed to examine changes of intestinal microflora in two Pi-deficienct animal models and to evaluate the efficacy of four traditional Chinese drugs as well as a probiotic recipe and another therapy in Pi-deficient rats.
RESULTS: A molecular marker was identified for Pi-deficiency in rats. The pharmacodynamic evaluation system, including identified molecular markers (net integral area and abundance of DNA bands), Shannon’s index for diversity of intestinal microflora, and Sorenson’s pairwise similarity coefficient, was established. The four major clinical recipes of traditional Chinese drugs for Pi-deficiency in rats, especially at their medium dose (equivalence to the clinical dose), produced more pronounced recovery activities in Pi-deficient rats, while higher doses of these recipes did not show a better therapeutic effect but some toxic effects such as perturbation deterioration of intestinal microflora.
CONCLUSION: Both fingerprint analysis and identified marker can show Pi-deficiency in rats and its difference after treatment. The identified molecular marker may be applied in screening for the active compounds both in relative traditional Chinese drugs and in pharmacodynamic study of Pi-deficiency in rats.
- Citation: Peng Y, Wang Z, Lu Y, Wu CF, Yang JY, Li XB. Intestinal microflora molecular markers of spleen-deficient rats and evaluation of traditional Chinese drugs. World J Gastroenterol 2009; 15(18): 2220-2227
- URL: https://www.wjgnet.com/1007-9327/full/v15/i18/2220.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2220
Pi-deficiency (spleen deficiency), a common clinical syndrome in traditional Chinese medicine (TCM), is described as symptoms such as epigastralgia, flatulence after meal, lack of appetite, wilted complexion, loose stool, lassitude, fatigue, etc. The “Pi” here is the Chinese spelling of “spleen” in TCM, which relates to the functions of digestion, absorption and nutrition, differs from the “spleen” in Western medicine that belongs to the blood and immune systems. Pi-deficiency in TCM is one of the most common digestive diseases and usually the patients’ equilibrium of gastrointestinal microflora is broken, which plays an important role in the growth, development and performance of the host[1]. Therefore, more clinical interests are arising in monitoring changes of intestinal microflora in intestinal disease and its consequent treatment with TCM therapies. It has been found that some traditional Chinese drugs have curative effects on Pi-deficiency by regulating the equilibrium of intestinal microflora and therefore promote the recovery of Pi-deficiency[2–4].
However, methods of monitoring the intestinal flora are quite limited, not only because of the complexity of its constitution, but also the difficulty in culturing most gastrointestinal bacteria in vitro. Recent development in molecular biology techniques provides various possibilities of illustrating microbial biodiversity without in vitro culture of bacteria[56]. Enterobacterial repetitive intergenic consensus (ERIC) sequences are non-coding sequences of highly conserved 127 bp that are repeated multiple times through the genome of most bacterial species[7]. Variation in the number and location of ERIC sequences between different populations of microbes will result in differences between strains in the number and size of PCR products by ERIC primers. Based on this, ERIC-PCR has been used to investigate the diversity of bacteria[8–11].
In this study, we introduced ERIC-PCR fingerprinting in study of Pi-deficiency syndrome, used molecular markers to detect changes of intestinal microflora in Pi-deficient rats, and evaluated the therapeutic effects of traditional Chinese drugs.
Wistar rats (200 ± 20 g) of either sex were obtained from the Experimental Animal Centre of Shenyang Pharmaceutical University (Shenyang, China). The rats were kept under standard environmental conditions with free access to rodent diet and water. All animal experiments were performed in accordance with the Guidelines for Use of Experimental Animals established by Shenyang Pharmaceutical University.
Plant materials including Radix and Rhizoma Rhei, Folium Sennae, etc, used in the study, were purchased from a local TCM apothecary in Shanghai, China (Table 1), and identified by Dr. Meng-Yue Wang, Department of Pharmacognosy, School of Pharmacy, Shanghai Jiao Tong University.
| Prescription | Composition | Dose (g crude plants/kg) | Effecta |
| Banxia Houpu Tang | Rhizoma Pinelliae, Poria, Cortex Magnoliae Officinalis, Folium Perillae, Rhizoma Zingiberis Recens (4:4:3:3:2) | 4.3 (clinical dose) | N↓, A↓, C↓, H↓ |
| Si Junzi Tang | Radix Ginseng, Rhizoma Atractylodis Macrocephalae, Poria, Radix Glycyrrhizae (10:9:9:6) | 1.2 (triplicate of clinical dose) | N↓1, A↓1, C↑, H↑ |
| 3.5 (clinical dose) | N↓1, A↓1, C↑1, H↑ | ||
| 10.5 (triplication of clinical dose) | N↓1, A↓, C↑, H↓ | ||
| Lizhong Tang | Radix Codonopsis, Rhizoma Atractylodis Macrocephalae, Rhizoma Zingiberis, Radix Glycyrrhiza preparata (1:1:1:1) | 1.8 (triplicate of clinical dose) | N↓, A↓, C↑, H↑ |
| 5.4 (clinical dose) | N↓1, A↓1, C↑, H↑ | ||
| 16.2 (triplication of clinical dose) | N↓1, A↓, C↑, H↑ | ||
| Buzhong Yiqi Tang | Radix Astragali, Radix Ginseng, Radix Angelicae Sinensis, Rhizoma Atractylodis Macrocephalae, Radix Glycyrrhiza preparata, Radix Bupleuri, Rhizoma Cimicifugae, Pericarpium Citri Reticulatae (6:1:1:1:3:2:2:2) | 1.6 (triplicate of clinical dose) | N↓1, A↓1, C↑, H↑ |
| 4.8 (clinical dose) | N↓1, A↓1, C↑1, H↑ | ||
| 14.4 (triplication of clinical dose) | N↓1, A↓, C↑, H↓ | ||
| Yiwei Tang | Radix Glehniae, Raidix Ophiopogonis, Rehmannia Dride Rhizome, Rhizoma Polygonati Odorati, rock candy (3:3:3:1:3) | 1.9 (triplicate of clinical dose) | N↓1, A↓1, C↑, H↓ |
| 5.8 (clinical dose) | N↓1, A↓1, C↑, H↓ | ||
| 17.4 (triplication of clinical dose) | N↓, A↓, C↑, H↓ |
One hundred milliliters aqueous decoction was prepared with 100 g of each crude Radix, Rhizoma Rhei and Folium Sennae. For the preparation of decoction of traditional Chinese drug recipes, the crude drugs were mixed first according to the ratio as prescribed, and then decocted 3 times in 10 volumes of distilled water for 30 min, finally the solution was filtered and concentrated. The ratios and concentrations are shown in Table 1.
Rats were randomly divided into 16 groups (n = 8). Rats in group 1 received distilled water only (10 mL/kg, po) during the whole experiment. Rats in groups 2-16 were intragastrically given Radix and Rhizoma Rhei extract, 10 mL/kg, twice a day for the first 10 d to induce Pi-deficiency[12]. Rats in group 2 (model group) received distilled water only, once a day for 10 d. Rats in group 3 received Entrocoordinatibiogen (16.2 mg/kg, po), once a day for 10 d. Rats in group 4 received Banxia Houpu Tang (Decoction of Pinellia and Magnolia Bark, 4.3 g crude drug/kg, po), once a day for 10 d. Rats in groups 5-7 were treated with Si Junzi Tang (Decoction of Four Noble Drugs, 1.2, 3.5 and 10.5 g of each crude drug/kg, po), once a day for 10 d. Rats in groups 8-10 received Lizhong Tang (Decoction for Regulating the Function Of Middle Jiao, 1.8, 5.4 and 16.2 g of each crude drug/kg, po), once a day for 10 d. Rats in groups 11-13 received Buzhong Yiqi Tang (Decoction for Regulating the Function Of Middle Jiao and Supplementing Qi, 1.6, 4.8 and 14.4 g of each crude drug/kg, po), once a day for 10 d. Rats in groups 14-16 received Yiwei Tang (Decoction for Nourishing the Stomach, 1.9, 5.8 and 17.4 g of each crude drug/kg, po), once a day for 10 d. Banxia Houpu Tang is a recipe for tussis but not for Pi-deficiency. Si Junzi Tang, Lizhong Tang, Buzhong Yiqi Tang and Yiwei Tang are commonly used clinical recipes for Pi-deficiency. Another experiment was performed as described above, except that 10 mL/kg Folium Sennae was given instead of Radix and Rhizoma Rhei to induce Pi-deficiency[1314].
Three or four pieces of fecal pellets (about 1 g) per rat were directly collected from the anus into sterile plastic tubes and stored at -20°C immediately. Fecal pellets were collected 5 d before induction of Pi-deficiency and then every two days.
Total DNA was isolated from the fecal samples as previously described[10] with some modifications. Each sample (0.2 g) was suspended in 1 mL sterile 0.05 mol/L PBS (pH 7.4) followed by vortexing for 5 min in a 2 mL tube. The suspension was centrifuged at 200 ×g for 6 min and the supernatant was transferred to a new tube. Then 1 mL sterile PBS was added to the pellets and vortexed for 5 min, the suspension was centrifuged and the supernatant was transferred to the new tube as well. Combination of the two sets of supernatant was then centrifuged at 300 ×g for 6 min to remove coarse particles. The cells in the supernatant were collected and washed twice with PBS by centrifuging at 10 000 r/min for 6 min. The washed cell pellets were resuspended in 300 &mgr;L of solution I containing 150 mmol/L NaCl, 50 mmol/L Na2EDTA (pH 8.0). The suspension was gently mixed with 100 &mgr;L lysozyme solution (100 mg/mL) and 20 &mgr;L RNase (10 mg/mL), pre-warmed in 37°C water bath for 30 min and then combined with 300 &mgr;L of solution II containing 100 mmol/L NaCl, 50 mmol/L Tris base (pH 8.0). The cell suspension was gently mixed with 100 &mgr;L of 10% SDS and 50 &mgr;L of 20% PVP, and incubated on ice for 5 min. DNA was then purified by sequential extraction with Tris-equilibrated phenol and chloroform-isoamyl alcohol (v/v/v, 25:24:1), and chloroform isoamyl alcohol (v/v, 24:1) followed by precipitation with 2 volumes of ethanol and 50 &mgr;L of 3 mol/L sodium acetate. DNA was collected by centrifugation and washed once with 70% ethanol, air dried and dissolved in 50 &mgr;L of sterile distilled water. The DNA was checked for integrity first by electrophoresis analysis on 1% agarose gel (compared with size-known Hind III digested bacteriophage λ DNA), and then quantified.
ERIC-PCR was performed on a MJ Research PTC-100 thermal cycler (MJ Research, Inc., Waltham, USA) using the ERIC primers (ERIC1R: 5’-ATGTAAGCTCCTGGGGATTCAC-3’, ERIC2: 5’-AAGTAAGTGACTGGGGTGAGCG-3’)[7]. The reaction system was optimized and determined with orthogonal array design and statistic analysis method as previously described[15]. PCR consisted of 2.5 &mgr;L 10 × buffer, 200 &mgr;mol/L dNTP, 2.5 mmol/L Mg2+, 0.4 &mgr;mol/L primer, 1U HotstarTaq DNA polymerase and 2 &mgr;L DNA template (or correspondingly 2 &mgr;L sterile distilled water in controls) in a total 25 &mgr;L volume. PCR conditions were as follows: an initial denaturation at 94°C for 5 min, followed by 35 cycles of denaturing at 95°C for 50 s, annealing at 49°C for 30 s, at 46°C for 30 s, extension at 72°C for 3 min, and a final extension at 72°C for 9 min. PCR products were separated by electrophoresis on 2% agarose gel (Agarose LE, Mdbio, Inc.) containing 0.5 &mgr;g/mL ethidium bromide and observed under UV light by Tannon GIS2010 Image System Ver. 3.73 (Tanon, Inc., Shanghai, China). The size and quantity of the amplified fragments were determined using 1 kb plus DNA makers (Tiangen, Inc., Beijing, China).
ERIC-PCR profiles were analyzed using the Gel Compare function of Tannon GIS2010 Image System Ver. 3.73 and transformed to data sets by taking into account the relative square root of the area under each PCR peak and abundance of each peak. Similarities between samples and their temporal stability were determined by calculating Sorenson’s pairwise similarity coefficient (Cs), which is commonly used to compare the species composition of different ecosystems. Two identical profiles create a value of 100%, whereas two completely different profiles result in a value of 0%.
Cs (%) = (2 ×j)/(a+b) × 100%
where ‘a’ is the number of total bands in the ERIC-PCR pattern for one sample, ‘b’ is the number for the other, and ‘j’ is the number of the common bands shared by both samples[16].
Shannon’s index (H’), which originally refers to the community richness, was also employed to measure the distribution of PCR bands in our study. We used it to describe the quantitative difference in intestinal microflora under different conditions, although each ERIC-PCR band does not have to stand for one individual bacterial species.
H’ = -∑(Pi)(lnPi)
where Pi is the relative abundance of each band, calculated as the proportion of the ith band in the fringerprint[16–18].
Results were described as mean ± SE. The statistical significance (P < 0.05) of difference between means was determined using paired-samples t test or ANOVA with SPSS version 11.5 (SPSS Inc., Chicago, USA), where appropriate.
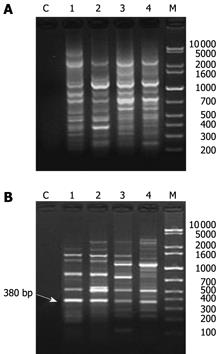
ERIC-PCR profiles of total fecal DNA were obtained for samples collected from naive rats before induction. Repeats of fingerprints showed that there were 9-12 fragments ranging 120-3000 bp with various intensities. There was a considerable variation of ERIC-PCR profiles between individual rats, in which only approximately 50% similarity was seen (Figure 1A). However, samples collected on different days from the same rat showed much a better consistency, with a similarity (Cs) ranging 63%-88% (data not shown). The occurrence of each fragment was calculated using Tannon GIS2010 Image System. Two fragments (590 bp and 300 bp) showed a higher occurrence of 83% and 74% respectively (occurrence > 70%) among the fingerprints of 128 rats, indicating that these two predominant bands are likely to be populations-associated naive rat gastrointestine.
Symptoms of Pi-deficiency[1–4], including humped back, narrow eyes, watery stools, listlessness, lack of appetite and weight loss[14] occurred in the rats that received Radix and Rhizoma Rhei or Folium Sennae. A remarkable difference in ERIC-PCR profile was found between rats with Pi-deficiency induced by Radix and Rhizoma Rhei or Folium Sennae and normal rats (Figures 1 and 2). Shannon’s index (H’) of the rat Pi-deficiency model (1.77 ± 0.03, n = 200) was significantly lower than that of normal ones (2.02 ± 0.02, P < 0.05, n = 200), indicating that altered profiles and lesser diversities of ERIC-PCR fingerprints are in the status of Pi-deficiency. The similarity (Cs) of ERIC-PCR fingerprints of the same rat before and after Pi-deficiency induction decreased to approximately 39% in groups 2-16, whereas 62% in control group that received distilled water only (P < 0.05), suggesting that the constitution of intestinal bacterial community in Pi-deficiency rats is significantly different from that in normal rats.
Analysis of ERIC-PCR profiles for 100 rats with Pi-deficiency induced by Radix and Rhizoma Rhei implied that three fragments (590, 380 and 300 bp) showed that Radix and Rhizoma Rhei administration can induce significant changes in abundance and band net integral area (P < 0.05), as well as the occurrence of those fragments (Table 2). The 590 bp and 300 bp fragments, especially the 300 bp fragment, were shown in most normal rats, but in much fewer rats after Pi-deficiency induction (Figure 1B). Different from the 590 bp and 300 bp fragments, the 380 bp fragment was not seen in normal rats, but in most Pi-deficient rats, indicating that Radix and Rhizoma Rhei administration can induce great changes in the proportion of individual bacterial species. The rest fragments were randomly detected in either normal or Pi-deficient rats, with no correlation between the presence of fragments and Pi-deficiency. Therefore, these fragments (590, 380 and 300 bp) were selected as preliminary biomarkers for intestinal microflora ERIC-PCR fingerprints of rats with Pi-deficiency induced by Radix and Rhizoma Rhei.
| Fragments | Occurrence (%) | Net integral area | Abundance | |||||
| Beforeadministration(healthy) | Afteradministration(Pi-deficiency) | Beforeadministration(healthy) | Afteradministration(Pi-deficiency) | Range | Before administration (healthy) | Afteradministration(Pi-deficiency) | Range | |
| 590 bp | 79 | 53 | 851.65 ± 68.00 | 385.76 ± 37.63a | Decrease 55% | 18.49 ± 1.36 | 11.69 ± 1.05a | Decrease 37% |
| 380 bp | 33 | 94 | 98.61 ± 18.09 | 563.64 ± 32.94a | Increase 470% | 2.74 ± 0.58 | 19.16 ± 1.30a | Increase 590% |
| 300 bp | 75 | 10 | 604.93 ± 46.67 | 70.87 ± 23.12a | Decrease 88% | 12.70 ± 0.94 | 2.31 ± 0.71a | Decrease 82% |
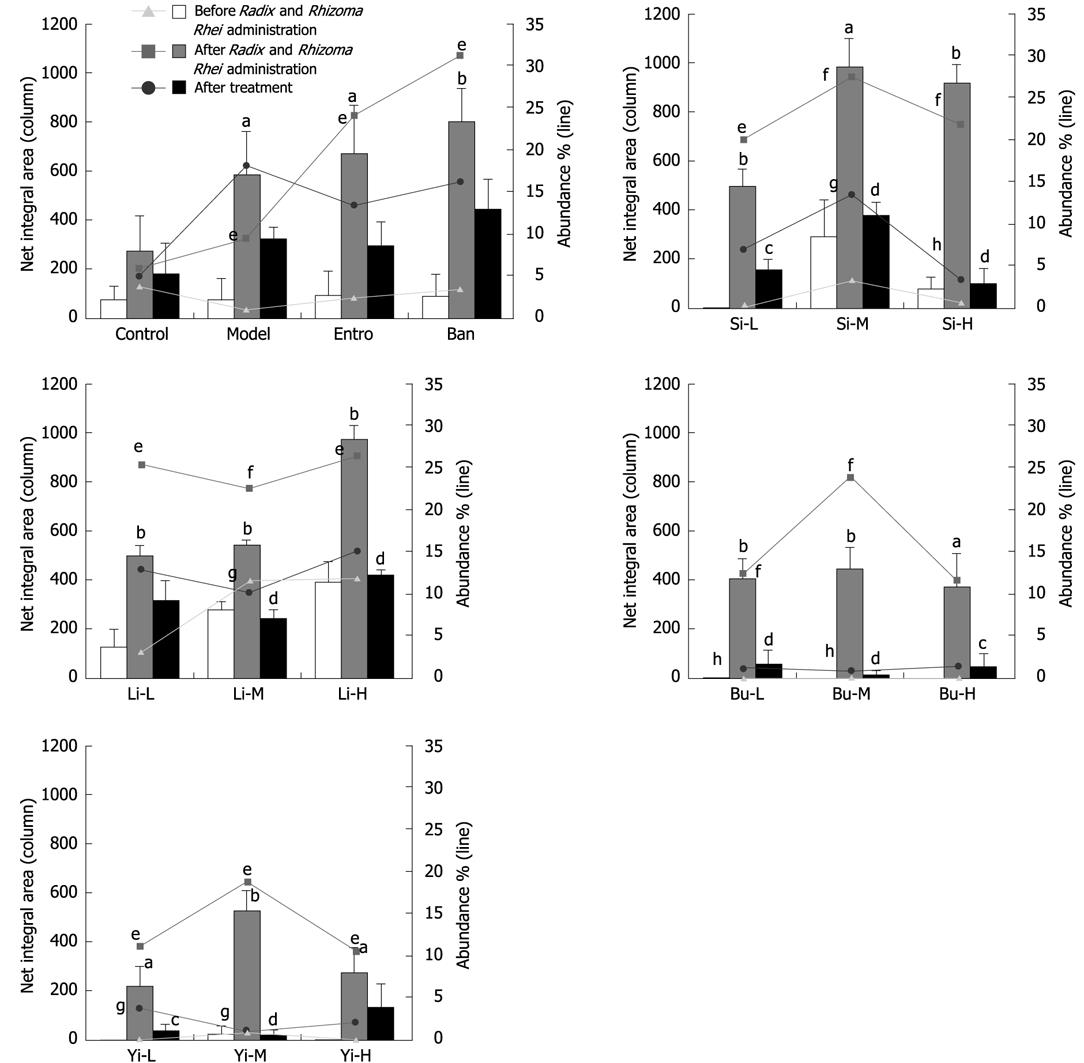
In order to further identify the optimal biomarker of ERIC-PCR fingerprints for rats with Pi-deficiency, the profile of preliminary biomarkers (590, 380 and 300 bp) in different groups of Pi-deficient rats that received TCM treatment was also investigated. As a result, 4 TCM recipes restored the net integral area and abundance of 380 bp fragment to a certain extent. However, changes in 590 bp and 300 bp fragments were not as significant as in 380 bp fragment (data not shown). Given the data above, the 380 bp fragment was identified as the biomarker of ERIC-PCR fingerprints for rats with Pi-deficiency induced by Radix and Rhizoma Rhei, the net integral area and abundance of the 380 bp fragment could therefore be used as parameters to evaluate the therapeutic effects of TCM on Pi-deficiency.
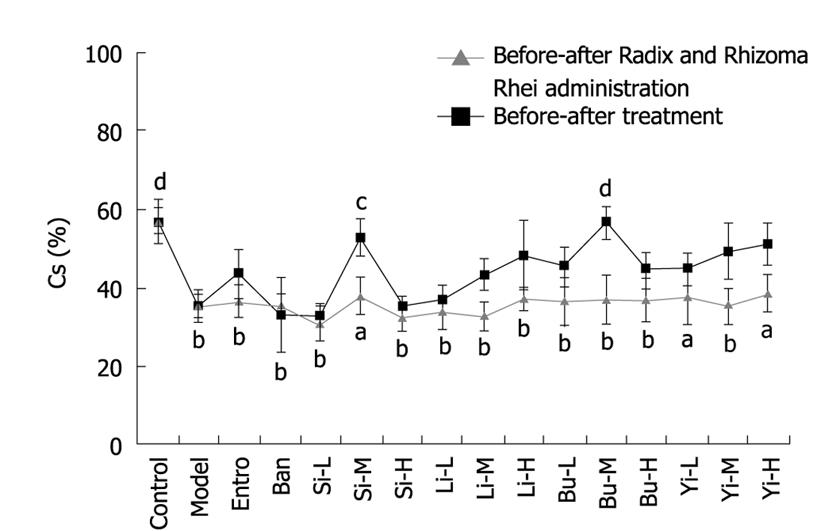
Additionally, in 7 out of 12 Pi-deficient groups that received TCM recipes, The Shannon’s index (H’) of ERIC-PCR fingerprints was restored (Figure 3). A similar trend of the similarity (Cs) of ERIC-PCR fingerprints was seen in those groups that received TCM recipes (Figure 4), indicating that Shannon’s index (H’) and Sorenson’s pairwise similarity coefficient (Cs) can also be considered as biomarkers for Pi-deficiency and used to evaluate the therapeutic effects of TCM on Pi-deficiency.
As shown in Figures 3–5 and Table 1, Si Junzi Tang reduced the net integral area and abundance of the 380 bp fragment and increased the Sorenson’s pairwise similarity coefficient (Cs) in a dose-dependent manner. The effects were most significant at the dose of 3.5 g crude drug/kg. However, the Shannon’s index (H’) increased after treatment with Si Junzi Tang at the dose of 3.5 g crude drug/kg, but decreased after treatment with Si Junzi Tang at a higher dose of 10.5 g crude drug/kg.
The net integral area and abundance of the 380 bp were significantly different before and after treatment with Lizhong Tang at the dose of 5.4 g. The Sorenson’s pairwise similarity coefficient (Cs) increased in a dose-dependent manner and the Shannon’s index (H’) also increased at the three doses with no significant difference.
Moreover, Buzhong Yiqi Tang also significantly decreased the 380 bp fragment and increased the Sorenson’s pairwise similarity coefficient (Cs) in a dose-dependent manner, in which the maximal and significant effects were shown at the dose of 4.8 g crude drug/kg. Buzhong Yiqi Tang increased and decreased the Shannon’s index (H’) at the doses of 1.6 and 4.8 g crude drug/kg, and 14.4 g crude drug/kg, respectively.
The net integral area and abundance of the 380 bp were decreased after treatment with Yiwei Tang, indicating that the effect is statistically significant at the dose of 1.9/5.8 g crude drug/kg. Yiwei Tang increased the Sorenson’s pairwise similarity coefficient (Cs) in a dose-dependent manner. However, the Shannon’s index (H’) was lower than that before treatment.
The H’ and Cs values as well as the net integral area and abundance of the 380 bp fragment were decreased in rats that received water, which might be due to the long-term Pi-deficiency. A similar trend was seen in the group that received Banxia Houpu Tang. Entrocoordinatibiogen increased the Shannon’s index (H’) and Sorenson’s pairwise similarity coefficient (Cs) (P < 0.05), but had no significant effects on the net integral area and abundance of the 380 bp fragment.
The four biomarkers (H’, Cs, net internal area and abundance of the 380 bp fragment) that were identified in rats with Pi-deficiency induced by Radix and Rhizoma Rhei were also proved to be valid for Folium Sennae induced Pi-deficiency. The four recipes (Si Junzi Tang, Lizhong Tang, Buzhong Yiqi Tang and Yiwei Tang) for Pi-deficiency significantly reduced the net integral area and abundance of the 380 bp fragment at smaller and medium doses, but significantly increased the Shannon’s index (H’) and Sorenson’s pairwise similarity coefficient (Cs) (data not shown).
This study reported the changes of intestinal microflora in rats with Pi-deficiency induced by Radix and Rhizoma Rhei or Folium Sennae. ERIC-PCR fingerprinting system is highly reproducible when it is used to examine the status of intestinal microflora in rats. In this study, fingerprints of the Sorenson’s pairwise similarity coefficient (Cs) from the same DNA extraction were over 95%, respectively.
The dominating intestinal microbial population may vary in subjects due to changed physiological conditions. The replicates (collected on different days) of PCR fingerprints from the same rat showed a high reproducibility (Cs > 75%). However, this value decreased to 57% after water administration, indicating that water administration can affect the intestinal physiology after intragastric operation. These results indicate that ERIC-PCR is a sensitive tool for examining the structure of fecal bacterial community.
Entrocoordinatibiogen (Shenyang No. 1 Pharmaceutical Factory, Shenyang, China) consisting of bacillus licheniformis, a kind of probiotics and a biotherapeutic agent modulating microdysbiosis of intestine, is used in treatment of acute bacillary dysentery. Based on the analysis of ERIC-PCR intestinal microflora molecular markers, the rats that received Entrocoordinatibiogen had a certain extent of recovery as indicated by the increased Shannon’s index (H’) and Sorenson’s pairwise similarity coefficient (Cs). However, neither the net integral area nor the abundance of 380 bp fragment significantly decreased. The 4 major clinical recipes for Pi-deficiency recovered the activities of Pi-deficient rats, especially Si Junzi Tang and Buzhong Yiqi Tang at their medium dose (equivalent to the clinical dose). These results strongly support the rationale behind the current common use of these two recipes for Pi-deficiency[121419]. However, it should be noted that the higher dose of these recipes did not show a better therapeutic effect on Pi-deficiency in the present study. A possible explanation of this phenomenon might be that the recipes have some anti-microorganism actions on Pi-deficiency. It was reported that Si Junzi Tang and some TCM recipes have certain modulating functions in intestinal flora[20–22].
The pathogenesis of Pi-deficiency induced by Folium Sennae or by Radix and Rhizoma Rhei is similar. Folium Sennae, Radix and Rhizoma Rhei, classified as “bitter-cold” in terms of taste and properties, can simulate the intestinal motility and secretion to induce diarrhea, which results in Pi-deficiency. However, the diarrhea-inducing action of Folium Sennae is weaker than that of Radix and Rhizoma Rhei. That is why the four recipes showed a better recovery profile for Folium Sennae-induced Pi-deficiency than that for Radix and Rhizoma Rhei-induced Pi-deficiency, in the present study.
In conclusion, Pi-deficiency, one of the most common digestive system diseases, is generally caused by the change in intestinal microflora. Although the underlying mechanism of action of TCM is not completely understood, it has been known that TCM has positive effects on some syndromes including Pi-deficiency. ERIC-PCR fingerprints can be used to screen changes in composition of bacterial communities associated with the development of intestinal disease, and to investigate the pharmacodynamic effect of TCM on intestinal microflora or intestinal diseases such as Pi-deficiency.
Pi-deficiency, a clinical syndrome in traditional Chinese medicine (TCM), is one of the most common digestive system diseases and generally considered to be associated with abnormalities of gastrointestinal microflora.
Although the underlying mechanism of action of TCM is not completely understood, it has been known that TCM has positive effects on some syndromes including Pi-deficiency. It was reported that some TCM have certain modulating functions in intestinal flora.
In this study, the authors used the molecular markers in study of Pi-deficiency syndrome, changes in intestinal microflora, and evaluation of the therapeutic effects of traditional Chinese drugs. This is the first study reporting the changes in intestinal microflora of Pi-deficient rats using the enterobacterial repetitive intergenic consensus-PCR (ERIC-PCR) fingerprint profiles.
ERIC-PCR fingerprints can be used to screen changes in the composition of bacterial communities associated with the development of intestinal disease, and to investigate the pharmacodynamic effect of TCM on intestinal microflora or intestinal diseases including Pi-deficiency.
ERIC-PCR is a PCR-based technique in which DNA is isolated from a mixed sample and amplified using conserved ERIC primers targeting short repetitive sequences which are dispersed throughout various bacterial genomes.
The authors identified the molecular markers of intestinal microflora by modified ERIC-PCR in rats with Pi-deficiency induced by administration of Radix and Rhizoma Rhei. In addition, data on the effect of several decoctions on P-deficiency induced by Radix and Rhizoma Rhei are interesting and seem reliable. The study is interesting and well-designed.
| 1. | Kong J, Li XB, Wu CF. A molecular biological method for screening and evaluating the traditional Chinese medicine used in Pi-deficiency therapy involving intestinal microflora. Yazhou Chuantong Yiyao. 2006;1:1-6. |
| 2. | Zhu S. Experimental research on the effects of Jianpizhixie granules on the intestinal flora and small intestine mucosa in mice with diarrhea of splenic deficiency type [Chinese]. Beijing Zhongyiyao Daxue Xuebao. 2003;26:28-30. |
| 3. | Hu J, Yang XD, Xia QP, Yuan XH, Cai ZW. Research regulation of traditional Chinese drugs SHENQU to alteration of intestinal flora mice and the intestines protective function [Chinese]. Zhongguo Wei Shengtaixue Zazhi. 2004;16:208-211. |
| 4. | Ding WJ, Zhou BJ, Zhai MD, Bai H. Influence of Shenlinbaizhu Powder in enteric bacteria flora in mouse model with spleen-insufficiency syndrome [Chinese]. Beijing Zhongyiyao Daxue Xuebao. 2006;29:530-533. |
| 5. | Daly K, Stewart CS, Flint HJ, Shirazi-Beechey SP. Bacterial diversity within the equine large intestine as revealed by molecular analysis of cloned 16S rRNA genes. FEMS Microbiol Ecol. 2001;38:141-151. |
| 6. | Vaahtovuo J, Toivanen P, Eerola E. Bacterial composition of murine fecal microflora is indigenous and genetically guided. FEMS Microbiol Ecol. 2003;44:131-136. |
| 7. | Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19:6823-6831. |
| 8. | Gillings M, Holley M. Repetitive element PCR fingerprinting (rep-PCR) using enterobacterial repetitive intergenic consensus (ERIC) primers is not necessarily directed at ERIC elements. Lett Appl Microbiol. 1997;25:17-21. |
| 9. | Di Giovanni GD, Watrud LS, Seidler RJ, Widmer F. Fingerprinting of mixed bacterial strains and BIOLOG gram-negative (GN) substrate communities by enterobacterial repetitive intergenic consensus sequence-PCR (ERIC-PCR). Curr Microbiol. 1999;38:217-223. |
| 10. | Wei G, Pan L, Du H, Chen J, Zhao L. ERIC-PCR fingerprinting-based community DNA hybridization to pinpoint genome-specific fragments as molecular markers to identify and track populations common to healthy human guts. J Microbiol Methods. 2004;59:91-108. |
| 11. | Van Driessche E, Houf K, Vangroenweghe F, De Zutter L, Van Hoof J. Prevalence, enumeration and strain variation of Arcobacter species in the faeces of healthy cattle in Belgium. Vet Microbiol. 2005;105:149-154. |
| 12. | Zheng XW, Wang Y, Song H. Experimental study on effect of Buzhong Yiqi decoction on serum gastrin in spleen-qi deficiency rats [Chinese]. Zhonghua Zhongyiyao Zazhi. 2006;21:393-395. |
| 13. | Qiu JF, Liu YH, Ye ZY, Huang YL, Ye BF. Establishment of animal model of spleen deficiency in rats and therapeutic effects of traditional China medicine [Chinese]. Shiyan Dongwu Kexue Yu Guanli. 2006;23:13-15. |
| 14. | Wang XM, Yi J, Liao SX, Pu TF, Sen H, Li DX. Objective evaluation on spleen deficiency syndrome animal models [Chinese]. Zhonghua Zhongyiyao Zazhi. 2006;21:406-408. |
| 15. | Peng Y, Jin J, Wu C, Yang J, Li X. Orthogonal array design in optimizing ERIC-PCR system for fingerprinting rat's intestinal microflora. J Appl Microbiol. 2007;103:2095-2101. |
| 16. | Scanlan PD, Shanahan F, O'Mahony C, Marchesi JR. Culture-independent analyses of temporal variation of the dominant fecal microbiota and targeted bacterial subgroups in Crohn's disease. J Clin Microbiol. 2006;44:3980-3988. |
| 17. | McCracken VJ, Simpson JM, Mackie RI, Gaskins HR. Molecular ecological analysis of dietary and antibiotic-induced alterations of the mouse intestinal microbiota. J Nutr. 2001;131:1862-1870. |
| 18. | Li F, Hullar MA, Lampe JW. Optimization of terminal restriction fragment polymorphism (TRFLP) analysis of human gut microbiota. J Microbiol Methods. 2007;68:303-311. |
| 19. | Liu YZ, Wang CJ, Liu J, Zhou JL, Liu ZZ, Ou ZS, Jin Y. Si-Jun-Zi decoction repairs mitochondrial damage of cells of liver myocardium, gastric mucosa and skeletal muscle in rats with spleen asthenia. Zhongguo Linchuang Kangfu. 2006;10:170-173. |
| 20. | Ju BL, Bi L, Yang JY. Study regulation of Chinese drugs Si Junzi Tang to alteration of intestinal flora mouse [Chinese]. Mudanjiang Yixueyuan Xuebao. 2003;24:4-6. |
| 21. | Shi Q, Xue YH, Zhao GY, Yang JY, Ma SX, Li J, Li LQ, Nie Q, Liu JX, Shi ZK. Screening the traditional Chinese medicine with modulating function in rat intestinal flora [Chinese]. Heilongjiang Yiyao Kexue. 2005;28:28-30. |
| 22. | Yang CJ, Su DW, Yang LY, Wang CM, Cui G, Li LQ. Study regulation of traditional Chinese medicine Sijunzitong on intestinal flora of radiated mouse [Chinese]. Heilongjiang Yiyao Kexue. 2006;29:49-50. |